
Race, age at diagnosis and histological characteristics of lung cancer in never-smokers (LCINS) and ever-smokers in low-dose computed tomography (LDCT) screening: a systematic review and meta-analysis
Highlight box
Key findings
• This study found that among normal-risk individuals, lung cancer in never-smokers (LCINS) had a significantly higher likelihood to be diagnosed among Asians than non-Asians (baseline incidence 0.62% vs. 0.27%, P=0.001), predominantly manifesting as adenocarcinoma (ADC) (96.58% vs. 70.37%, P=0.001) and diagnosed approximately 2 years younger than ever-smokers (ES) (mean age difference of −1.98 years (95% confidence interval: −3.38 to −0.58).
What is known and what is new?
• LCINS predominantly manifests as ADC.
• The lung cancer detected by low-dose computed tomography (LDCT) screening among general population reveals a 1.98-year younger age for lung cancer diagnosed among LCINS compared to those in ES. Additionally, the risk of LCINS is higher among Asians than non-Asians, with a baseline incidence of 0.62% compared to 0.27%.
What is the implication, and what should change now?
• As LCINS has a higher baseline incidence among Asians and as lung cancer is diagnosed at a younger age among LCINS, thus LDCT screening for lung cancer among Asian never-smokers (NS) would have a higher number of life-year gained per lung cancer death averted than estimated by the MIcrosimulation SCreening ANalysis in NS based on Western population data. Also, the age limit to initiate lung cancer screening in NS may be set lower compared to ES.
Introduction
The prevalence of lung cancer deaths has surged by nearly 30% since 2007 (1). Lung cancer in never-smoker (LCINS) is estimated to be the 5th leading cancer cause of death globally in 2020. With the steady decrease in smoking prevalence, conceivably LCINS could become the most common type of lung cancer (2). The ability to detect LCINS at an early stage will be important going forward. Asia has the highest burden of lung cancer worldwide, accounting for 60% of incidence and 62% of deaths (3). Notably, the proportion of lung cancers among never-smokers (NS) women in Asia exceeds 60%, varying from 60% to 95%.
The implementation of low-dose computed tomography (LDCT) lung cancer screening programs has demonstrated a mortality benefit in high-risk individuals, by enabling the detection of lung cancer at an earlier, more treatable stage. LDCT screening for lung cancer has been recommended for high-risk current and former smokers in the United States since 2013 with the current guidelines of age 50–80 years, 20 pack-years, and current smoker or quit within the past 15 years (4). However, the disparities still exist as majority of Asian and African-American women diagnosed with lung cancer are still ineligible for lung cancer screening under the 2021 U.S. Preventive Services Task Force (USPSTF) guideline (5). A modeling of LDCT screening in NS, MIcrosimulation SCreening ANalysis (MISCAN) has been performed which compared to the National Lung Screening Trial (NLST) ever-smokers (ES) criteria based on Western population data [the Surveillance, Epidemiology, and End Results (SEER) Program and the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial] showed a higher risk reduction of lung cancer deaths among NS (37.2% vs. 32%). However, the modeling projected an older age of diagnosis for NS, resulting in a lower number of life-year gained per lung cancer death averted than the USPSTF eligible cohort and did not result in any recommendation of LDCT screening in NS (6). However, the difference of age at lung cancer diagnosis among NS and ES is inconclusive, particularly in Asian population.
We previously reported in a meta-analysis of 14 observational studies in LDCT screening for lung cancer in general population that LDCT lung cancer screening is as efficacious among Asian female NS as among Asian male ES [risk ratio (RR) =1.22; 95% confidence interval (CI): 0.89–1.68] and about two times higher among female NS over male NS (RR =1.78; 95% CI: 1.41–2.24) (7). Additionally, of the lung cancer diagnosed by LDCT among NS; 88.5% was stage 1 and 95.41% detected at the baseline scan, with subsequent significant decrease in lung-cancer-specific death (RR =0.27; P<0.001) and 5-year all-cause death (RR =0.13; P<0.001) among NS when compared to ES. In this study, investigated if race (Asian vs. non-Asian), age, and histology further differentiate between lung cancer diagnosed by LDCT screening among ES and NS. We present this article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-816/rc).
Methods
We conducted a subgroup analysis based on data from the 14 studies with 15 publications identified in our prior meta-analysis (8-22). The criteria for eligible studies included: (I) conducting LDCT screening for lung cancer or health checkup; (II) including both ES (former and current) and general NS (participants not limited to those with high-risk factors such as family history of cancer or chronic obstructive pulmonary disease); (III) being published in English; and (IV) having more than or equal to 10 total cases of diagnosed lung cancer. The literature search strategy was detailed in the previous study (7) and a glossary of search terms was noted in Table S1. Two investigators (N.T. and S.H.I.O.) independently evaluated the abstracts of the studies. We pursued discordant evaluations by discussion to reach consensus.
For each included study, one investigator (N.T.) extracted relevant data about the population, screening protocols and settings, smoking status, and outcomes, and a second investigator (S.H.I.O.) reviewed this information for completeness and accuracy. Disagreements were resolved by discussion. We regarded those studies conducted in the same hospital by the same authors during the same study period, either partially or entirely, as identical studies. In cases where the same data were reported in multiple relevant articles, the most recently published study was referenced and data abstracted. The quality of the 14 studies was assessed independently by two investigators using a modified Newcastle-Ottawa Scale (NOS) and was included in the supplemental materials in our previous primary analysis (7). The studies and participant’s characteristics and modified NOS were summarized and presented in Table S2. Funnel plots were generated to assess for publication bias (Figures S1-S4). NS were defined as having smoked fewer than 100 cigarettes in their lifetime in six studies (10,12,14,18-20), while the rest of the studies did not mention on the definition.
The primary outcome measure was to identify characteristics unique to LCINS including race-specific risk, age at lung cancer diagnosis, and histology as compared to ES. Data for race-specific risk included number of lung cancer diagnosed and screened participants among Asians and non-Asians. Data for age at diagnosis and prevalence of lung cancer by age groups included inclusion age, number of screened participants, and lung cancer diagnosed in three age groups: 40–49, 50–59, and 60–69 years in ES and NS. Data for histological characteristics included NSCLC subtypes [adenocarcinoma (ADC) and squamous cell carcinoma (SqCC)], and small cell lung carcinoma (SCLC) of lung cancer diagnosed per patient and per lesion in ES and NS. Findings in each outcome were summarized and displayed in tabular, figures and narrative format (Tables 1-3, Figure 1).
Table 1
| Author (ref.) | Inclusion age (years) | NS | ES | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | LC cases | Mean age (years) | SD (years) | Total | LC cases | Mean age (years) | SD (years) | |||
| Sone (10) | 40–74 | 3,040 | 29 | 64.11 | 6.86 | 2,440 | 31 | 65.03 | 7.25 | |
| Henschke (8) | 40–90 | 12,368 | 52 | 63.08 | 10.93 | 49,756 | 616 | 65.23 | 7.55 | |
| Kakinuma (20) | >40 | 6,021 | 66 | 60.60 | 8.30 | 6,090 | 66 | 62.70 | 8.40 | |
| Nojo† (11) | 40–59 | 9,405 | 8 | 53.00 | 4.31 | 19,282 | 16 | 55.30 | 3.18 | |
| Total | – | 30,834 | 155 | – | – | 77,568 | 729 | – | – | |
†, Nojo et al. reported data of male NS and male ES. Ref., reference; NS, never-smokers; ES, ever-smokers; LC, lung cancer; SD, standard deviation.
Table 2
| Author (ref.) |
NS (patients) | ES (patients) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADC | SqCC | LC, NOS | SCLC | Total cases | Total screened | ADC | SqCC | LC, NOS | SCLC | Total cases | Total screened | ||
| Sone (10) | 28 | 0 | 1 | 0 | 29 | 3,040 | 20 | 6 | 1 (LCC) | 4 | 31 | 2,440 | |
| Wu (16) | 22 | 0 | 0 | 0 | 22 | 1,256 | 2 | 0 | 0 | 0 | 2 | 507 | |
| Kim (19) | 82 | 1 | 1 | 0 | 84 | 17,968 | 96 (2 AdSqCC) |
15 | 8 | 4 | 123 | 19,468 | |
| Shan (22) | 15 | 4 | 0 | 1 | 20 | 4,102 | 16 | 16 | 0 | 2 | 34 | 4,982 | |
| Nojo† (11) | 7 | 0 | 0 | 0 | 7 | 9,405 | 13 | 1 | 3 (1 LCC) | 0 | 17 | 19,282 | |
| Total | 154 | 5 | 2 | 1 | 162 | 35,771 | 147 | 38 | 12 | 10 | 207 | 46,679 | |
†, Nojo et al. reported data of male NS and male ES. Ref., reference; NS, never-smokers; ES, ever-smokers; ADC, adenocarcinoma; SqCC, squamous cell carcinoma; LC, lung cancer; NOS, not otherwise specified; SCLC, small cell lung carcinoma; LCC, large cell carcinoma; AdSqCC, adenosquamous cell carcinoma.
Table 3
| Author (ref.) | NS (lesions) | ES (lesions) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADC | SqCC | LC, NOS | SCLC | Lesions | Cases | Stage I (%) | Screened | ADC | SqCC | LC, NOS | SCLC | Lesions | Cases | Stage I (%) | Screened | ||
| Sone (14) | 160 (1 AdSqCC) | 0 | 9 | 0 | 169 | 155 | 90.3 | 27,881 | 78 | 19 | 24 (5 LCC) | 5 | 126 | 112 | 71.3 | 21,905 | |
| Kakinuma (20) | 75 | 0 | 2 | 0 | 77 | 66 | 100 | 6,021 | 61 (1 AdSqCC) | 8 | 1 | 2 | 72 | 66 | 84.8 | 6,090 | |
| Yi† (15) | 30 | 0 | 0 | 0 | 30 | 20 | 94.1‡ | 955 | 10 | 5 | 1 (LCC) | 2 | 18 | 14 | 72.0§ | 903 | |
| Total | 265 | 0 | 11 | 0 | 276 | 241 | 93.4 | 34,857 | 149 | 32 | 26 | 9 | 216 | 192 | 76.0 | 28,898 | |
†, Yi et al. reported data of female NS compared to high-risk ES (at least 20 pack-years); ‡, stage I–II in non-smokers and non-high-risk smokers (ex-smoker <20 pack-years); §, stage I–II in high-risk smokers (at least 20 pack-years). Ref., reference; NS, never-smokers; ES, ever-smokers; ADC, adenocarcinoma; SqCC, squamous cell carcinoma; LC, lung cancer; NOS, not otherwise specified; SCLC, small cell lung carcinoma; AdSqCC, adenosquamous cell carcinoma; LCC, large cell carcinoma.
Statistical analysis
Meta-analyses were conducted to assess RR of lung cancer diagnosed of ES over NS among Asian and non-Asian individuals, mean difference in age at lung cancer diagnosis, RRs of histology subtypes per patient and per lesion between the ES and NS, and pooled weighted estimates of proportion of histology subtypes per patient and per lesion between the two groups. Certainty assessment for the outcomes was evaluated using a 95% CI. The prevalence of lung cancer per 100,000 participants with specific subgroups (P) was calculated by this formula: prevalence (P) = [numbers of individuals with lung cancer diagnosed among screened participants in the specific subgroup (N)/total number of screened participants in the specific subgroup (T)] × 100,000.
All combined effects were estimated using random-effects models. Results were pooled and used to assess the heterogeneity between the studies by using both the I2 and the Q statistics. We considered I2>75% signifying substantial heterogeneity and considered to perform meta-regressions or subgroup analysis to identify any potential sources of the heterogeneity. A P value less than 0.05 was considered statistically significant except for the test for heterogeneity Q statistics which was considered statistically significant at P value less than 0.10. All meta-analyses, forest, and funnel plots were analyzed and generated via Stata.
Results
The selection of the articles is depicted in a PRISMA flowchart (Figure 1). A total of 14 studies from 15 publications were included in our previous meta-analysis with modified NOS scores ranging from 7 to 9 (Table S2) (7).
We investigated race-specific RR of lung cancer diagnosis, prevalence of lung cancer in different age groups, mean difference in age at lung cancer diagnosis, and histology of lung cancer diagnosis between ES and NS from the 12 publications that reported the relevant data and summarized the relevant findings in each study in Tables 1-3.
Race-specific RR of lung cancer diagnosis
Twelve out of 14 studies reported the number of lung cancer diagnosed by LDCT screening among men and women. 11 studies were conducted in Asia including Japan, Korea, China, and Taiwan (China), and one multi-cohort study from the United States, Spain, Japan, and China. The majority of the participants in the multi-cohort study were White, accounting for 84% (41,830/49,756) of ES and 48% (5,940/12,368) of NS. While Asian participants (Chinese and Japanese) accounted for 7.5% of ES and 47% of NS. All 11 Asian studies reported total numbers of lung cancers diagnosed in their studies and five studies also reported the number of lung cancers diagnosed at the baseline screening. The multi-cohort study reported the number of lung cancers diagnosed at the baseline screening.
To compare the race-specific RR of lung cancer diagnosis, we focused only on the baseline incidence of lung cancer diagnosed by LDCT from the five Asian studies and one multi-cohort study. Among all Asian participants, 409 lung cancer cases were diagnosed among 65,811 screened NS, and 345 lung cancer cases were diagnosed among 61,442 screened ES. Among non-Asian participants, 18 lung cancer cases were diagnosed among 6,568 screened NS, and 580 lung cancer cases were diagnosed among 46,003 screened ES. The race-specific subgroup analysis showed Asian ES had no significant difference in risk of lung cancer diagnosed at baseline screening as NS (RR =0.96; 95% CI: 0.74–1.24), but among non-Asian race ES had a 4.56 times significantly higher risk than NS (RR =4.56; 95% CI: 2.85–7.28) [Figure 2A, Figure S1 (funnel plot)]. The overall incidence of lung cancer diagnosed among Asians from the 12 studies demonstrated 0.95% (95% CI: 0.74–1.18%) in ES and 0.90% (95% CI: 0.63–1.21%) in NS with RR =1.13 (95% CI: 0.89–1.42).
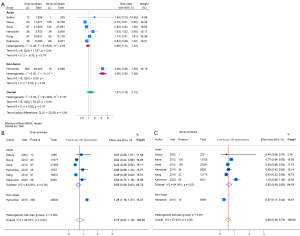
Proportional meta-analysis (metaprop) of the proportion of participants with lung cancer diagnosed by LDCT at baseline screening and screened participants demonstrated that the highest baseline incidence was observed in non-Asian ES (1.26%, 95% CI: 1.16–1.37%), followed by Asian ES (0.65%, 95% CI: 0.49–0.83%), Asian NS (0.62%, 95% CI: 0.45–0.82%), and non-Asian NS (0.27%, 95% CI: 0.17–0.43%) (Figure 2B,2C). The baseline incidence of lung cancer diagnosed was significantly 2.3 times higher among Asian NS compared to non-Asian NS (incidence 0.62% vs. 0.27%, P of heterogeneity between groups =0.001) (Figure 2C). On the other hand, the baseline incidence of lung cancer diagnosed among Asian ES baseline was about half of non-Asian ES (incidence 0.65% vs. 1.26%, P of heterogeneity between groups <0.001) (Figure 2B).
Prevalence of lung cancer in different age groups by smoking exposure
Three studies with total of 273 lung cancer patients in 46,270 screened NS, and 794 lung cancer patients in 77,751 screened ES reported relevant data for prevalence of lung cancer in different age groups (8,14,20). Of the three, two studies were conducted in Japan (one reported baseline prevalence and another reported accumulated prevalence), and the other multi-cohort study reported baseline prevalence. The prevalence of lung cancer per 100,000 participants increased with age. The Japanese studies showed higher prevalence among NS aged 40–69 years, particularly at age 40–49 years (14,20). While the multi-cohort study indicated higher prevalence among ES, particularly at age 60–69 years (Figure 3A).
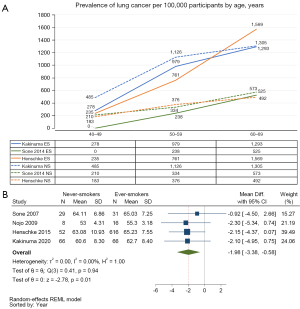
Mean difference in age at lung cancer diagnosis by smoking exposure
Four studies with a total of 77,568 screened ES and 729 lung cancer patients diagnosed, and 30,834 screened NS and 155 lung cancer patients diagnosed reported the age or age range at diagnosis (Table 1) (8,10,11,20). Three studies were conducted in Japan (10,11,20) and one was multi-cohort study (8). All studies reported data of both male and female lung cancer patients except Nojo (11) reported only data of male. All of the four studies recruited participants with a minimum age of 40 years but the maximum age varied from 59 years to no limit (Table S2).
Mean age at diagnosis varied from 53.00 to 64.11 years among LCINS, and 55.30–65.23 among lung cancer in ES across the four studies. Meta-analysis of mean difference in age at lung cancer diagnosis consistently showed across these studies that LCINS was diagnosed at a younger age compared to ES, with a significant average age difference of 1.98 years (95% CI: −3.38 to −0.58; I2=0.00%) [Figure 3B, Figure S2 (funnel plot)].
Histology of lung cancer diagnosed by LDCT by smoking exposure
Among the 14 studies, eight studies, totaling 403 lung cancer patients in 70,628 screened NS, and 399 lung cancer patients in 75,577 screened ES, reported histology data. This included five studies reporting histology data per patient (10,11,16,19,22) and three studies reported histology data per lesion (14,15,20). Three screening cohorts with total of 46,278 screened participants reported interval cancer data. Two cohorts screened annually (10,11) and one cohort recommended screening again 5 years after the baseline screening, with the option to undergo additional annual screening according to the participant’s wishes (20). Notably, three patients in the ES group were diagnosed with interval lung cancer, consisting of two SCLC cases and one poorly differentiated ADC. None of the NS were diagnosed with interval lung cancer.
Lung cancer patients analyzed by histology
Five studies reported histology of lung cancer per patient, with 162 lung cancer patients among 35,771 NS screened and 207 lung cancer patients among 46,679 ES screened. Among the total of 162 LCINS, there were 154 ADC, five SqCC, one SCLC, and two lung cancer, not otherwise specified (LC NOS) cases. In contrast, among the 207 lung cancer patients in ES, there were 147 ADC [included two adenosquamous cell carcinoma (AdSqCC)], 38 SqCC, 10 SCLC, and 12 LC NOS [included two large cell carcinoma (LCC)] cases (Table 2).
Patients with ADC
Metaprop of the proportion of patients with ADC lung cancer to total lung cancer diagnosed revealed substantial heterogeneity (I2=86.8%) and subgroup analysis demonstrated NS had a significantly higher proportion (96.58%; 95% CI: 88.34–100.00%; I2=61.01%) of ADC compared to ES (70.37%; 95% CI: 54.02–84.79%; I2=68.88%) with P value of heterogeneity between groups =0.001 (Figure 4A). Meta-analysis of RR of patients with ADC lung cancer demonstrates the RR of ADC in NS was 1.16 (95% CI: 0.98–1.38; I2=0.00%), indicating a 16% increased risk compared to ES [Figure 4B, Figure S3A (funnel plot)].
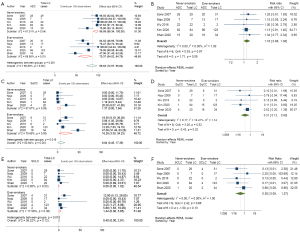
Patients with SqCC
Metaprop of patients with SqCC showed substantial heterogeneity (I2=83.64%) and subgroup analysis demonstrated the proportion of SqCC was significantly lower in NS (1.37%; 95% CI: 0.00–7.84%; I2=57.97%) compared to ES (16.28%; 95% CI: 3.30–34.23%; I2=78.68%) with P value of heterogeneity between groups =0.018 (Figure 4C). Meta-analysis of RR of patients with SqCC lung cancer demonstrates the consistent finding across the studies that LCINS has a lower risk of SqCC compared to those in ES, with the RR for SqCC in NS 0.31 (95% CI: 0.12–0.80; I2=12.41%), indicating a 69% lower risk compared to ES [Figure 4D, Figure S3B (funnel plot)].
Patients with SCLC
The number of SCLC among NS was extremely low accounting for only one case out of 162 lung cancer patients identified compared to 10 SCLC cases among 207 lung cancer patients among ES. Metaprop of patients with SCLC revealed NS had a minimal proportion of SCLC (0%; 95% CI: 0.00–1.02%), while ES showed a proportion of 1.44% (95% CI: 0.00–5.65%) (Figure 4E). Meta-analysis of RR of patients with SCLC consistently demonstrated across the studies that LCINS have a lower risk of SCLC compared to those in ES, with a significant 64% lower risk of SCLC (RR =0.36; 95% CI: 0.09–1.37; I2=0.00%) [Figure 4F, Figure S3C (funnel plot)].
Lung lesions analyzed by histology
Three studies reported on the histology of lung cancer lesion with 276 lung cancer lesions in 241 lung cancer patients diagnosed among 34,857 screened NS and 216 lung cancer lesions in 192 lung cancer patients diagnosed among 28,898 screened ES. Among the total of 276 lung cancer lesions in NS, 265 were ADC (included one AdSqCC) and 11 LC NOS with no instances of SqCC or SCLC. Conversely, among the 216 lung cancer lesions in ES, there were 149 ADC (included one AdSqCC), 32 SqCC, nine SCLC, and 26 LC NOS (included LCC) cases (Table 3).
ADC lesions
Metaprop of the proportion of diagnosed ADC lung cancer lesions to total lung cancer lesions diagnosed showed substantial heterogeneity (I2=94.26%) and subgroup analysis demonstrated NS had a significantly higher proportion (96.87%; 95% CI: 93.62–99.12%) of ADC lesions compared to ES (69.39%; 95% CI: 49.46–86.28%) with a highly significant difference (P value of heterogeneity between groups <0.001) (Figure 5A). In terms of RR, the analysis showed that LCINS had a 20% significantly increased risk of ADC compared to ES (RR =1.20; 95% CI: 1.03–1.40; I2=0.00%) [Figure 5B, Figure S4A (funnel plot)].
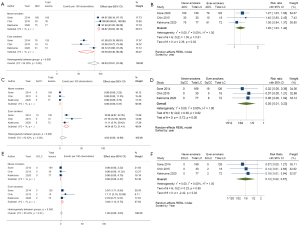
SqCC and SCLC lesions
Metaprop of the proportion of diagnosed SqCC and SCLC lesions to total lung cancer lesions diagnosed showed substantial heterogeneity (I2=92.02%), and moderate heterogeneity (I2=68.24%), respectively. There were no cases of SqCC or SCLC diagnosed among NS in the three studies. The metaprop showed 0% (95% CI: 0.00–0.47%) for both SqCC and SCLC. In contrast, among ES, the proportions were significantly higher, with 14.54% (95% CI: 8.73–21.41%) for SqCC (Figure 5C). Meta-analysis of RR indicated a significant 95% lower risk of SqCC lesions in NS compared to ES (RR =0.05; 95% CI: 0.01–0.23; I2=0.00%) [Figure 5D, Figure S4B (funnel plot)].
The metaprop showed 0% (95% CI: 0.00–0.47%) also for SCLC in NS. In contrast, among ES, the proportions were 3.48% (95% CI: 1.12–6.75%) for SCLC (Figure 5E). Meta-analysis of RR indicated an 88% lower risk of SCLC lesions in NS compared to ES (RR =0.12; 95% CI: 0.02–0.67; I2=0.00%) [Figure 5F, Figure S4C (funnel plot)].
Discussion
The recent publication of the TALENT trial conducted in Taiwan of LDCT screening in NS indicated the detection rate of lung cancer was similar to if not higher than the expected detection rate among ES from the NLST. Female sex, positive family history of lung cancer, age ≥60 years old are associated with increased risk of lung cancer detected (23). However, 37.4% of the invasive lung cancer were detected in participants without any family history of lung cancer (23). Hence to improve potential LDCT lung cancer screening programs among NS, it is crucial to consider race-specific lung cancer risk to assess if LDCT screening program should be implemented outside Asia or enroll only Asian participants, and the age at lung cancer diagnosed and hence at what age to screen, as it plays a pivotal role in determining the eligibility of screening and number of life-years gained per lung cancer death averted. Furthermore, from our clinical experience, LCINS is usually associated with lung nodules and ground glass opacities hence histologic characteristics of the lung cancers diagnosed between ES and NS in LDCT screening study are important to discern. In light of this, our sub-group analysis aimed to investigate race-specific lung cancer risk, the mean difference of age at lung cancer diagnosis, and the histological characteristics of lung cancer in LDCT screening studies involving both ES and NS meeting the same inclusion criteria.
Our first major finding indicated that the incidence of lung cancer diagnosed at baseline was 2.3 times higher among Asian NS than non-Asian NS (Figure 2C). On the other hand, the incidence of lung cancer diagnosed at baseline among Asian ES was about half of non-Asian ES (Figure 2B). Our data indicated LDCT lung cancer screening for NS may not be as effective outside of Asia or for non-Asian participants.
Air pollution is the second leading cause of lung cancer (1). The global proportion of lung cancer deaths attributable to ambient air pollution with fine particulate matter 2.5 microns or less in diameter (PM2.5) was 14.1%, particularly high in Asia and highest in China (20.5%) (1). Additionally, PM2.5 enhances lung cancer formation via promoting growth of EGFR mutated/KRAS mutated lung cancer cells (24). The high risk of first-degree relative with history of lung cancer (RR =1.75; 95% CI: 1.37–2.24) in TALENT study in the absence of germline analysis may represent environmental exposure. Correlative data showing no increased risk of lung cancer detected among participants with a second degree relative with history of lung cancer (RR =0.63; 95% CI: 0.26–1.52) in the TALENT trial which argues against germline mutation but common environmental exposure (24). Our results that LDCT screening for lung cancer among general population, Asian ES had no significant difference on risk of lung cancer diagnosed as Asian NS (RR =1.13; 95% CI: 0.89–1.42) consistent with the findings of the TALENT study and that LDCT lung cancer screening in NS may be effective in regions of high air pollution.
The difference of age at diagnosis at LCINS when compared to lung cancer in ES is inconclusive. A retrospective review from a cancer unit in Portugal reported LCINS were more likely to be older (67 vs. 66 years old, P=0.019) (25). While a retrospective study of patients with stage I NSCLC in the United States (26), and a screening cohort in Japan (20) reported LCINS were more likely to be younger (66.0 vs. 69.0, P<0.04, and 60.6 vs. 62.7, P=0.27, respectively). Our meta-analysis demonstrated that LCINS in the LDCT screening cohorts were diagnosed at an average age approximately 2 years younger than lung cancer in ES and that the stage at diagnosis is primarily stage 1. While this difference of only 2 years may not seem significant, it implies a higher number of life-year gained per lung cancer death averted than previously estimated by MISCAN modeling that based on Western population data and indicated a higher risk reduction in lung cancer deaths for LDCT screening in NS compared to ES. The youngest age participants can enter to the majority of the studies in our meta-analysis was 40 years hence our results suggest the age limit to initiate lung cancer screening in NS could be set to 40 years or above. The incidence of LCINS among 30–39 years old requires further investigations to see if these younger NS participants should also be included in future LDCT screening studies for NS.
Not surprisingly, our third major finding was that ADC is the dominant histology identified in LCINS (2). We demonstrate that nearly all LCINS cases (97%) are characterized by ADC, with multifocal ADC being frequently observed (more numbers of ADC lesions than numbers of patients diagnosed with LCINS with majority of early-stage lung cancers) (Table 3). All our observations are consistent with the TALENT trial where all lung cancers diagnosed were ADC except one out of 318 cases was AdSqCC, and 18.9% had multiple primary lung cancers (24). Many times, with multi-focal lesions, the decision at multi-disciplinary tumor board is whether to biopsy one or more or all of the lesions. It is noted that a higher relative risk (RR) when analyzing per lesion (RR =1.20; 95% CI: 1.03–1.40; I2=0.00%) compared to per patient (RR =1.16; 95% CI: 0.98–1.38) was observed. Our subgroup analysis indicates once the ADC diagnosis is established, the other nodules are likely to be ADC although molecular profiling will still be required to definitively distinguish between metastasis vs. synchronous primaries.
There are limitations of our meta-analysis. Firstly, the meta-analysis is not based on randomized trials since there is no randomized LDCT lung cancer screening trial that has ever been performed in NS. Hence the “certainty of the evidence” is based on observation studies. Secondly, 13 out of the fourteen included studies were conducted in East Asia, featuring Asian participants, and given only one study had enrolled non-Asian NS participants, the observation of race-specific relative risk is at best hypothesis-generating and the generalizability of our findings beyond Asia or Asian populations requires further studies. Thirdly, variations in study periods and differences in study protocols, smoking pack-year and quit years of ES, and lung cancer staging classification among the included studies could potentially impact the incidence and outcomes observed. For example, in earlier studies that used the term formerly known as bronchioloalveolar carcinoma (BAC) that we managed non-solid nodules the same as solid nodule, there would be a potential impact leading to over diagnosis.
Despite these limitations, our analysis provides valuable insights into the race-specific lung cancer risk, age at which lung cancer is diagnosed by LDCT screening and its histological characteristics among the studies that included simultaneously both ES and general NS, (not restricted to participants with high risk, i.e., family history of cancer, chronic obstructive pulmonary disease). These results shed light on the intricate epidemiology of lung cancer and emphasize the importance of considering Asian and a younger age at diagnosis when considering implementing LDCT screening in NS. Further research is needed to validate and expand upon our findings in diverse settings and populations, ultimately enhancing our ability to combat lung cancer and reduce its impact on public health.
Conclusions
In conclusion, Asian NS have a 2.3 times increase in baseline incidence of lung cancer than non-Asian NS arguing for caution in implementing LDCT lung cancer screening among non-Asian NS. Asian NS have a similar risk of lung cancer diagnosed by LDCT as Asian ES, consistent with the results from the TALENT study and argue for LDCT lung cancer screening in Asian NS. LCINS was diagnosed at an average age approximately 2 years younger than in ES. LCINS was predominantly ADC and if multiple lesions are identified in LCINS almost of the lesions are also likely to be ADC. Hence a biopsy of a contralateral lesion (if present) followed by molecular profiling will be important to distinguish metastasis vs. synchronous primary.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-816/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-816/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-816/coif). S.H.I.O. serves as an Associate Editor-in-Chief of Translational Lung Cancer Research from August 2023 to July 2025. N.T. got payment or honoraria for lectures or presentations from AstraZeneca, Roche, and Thoracic Society of Thailand under Royal Patronage (TST) in the past 36 months; got support for attending meetings from AstraZeneca, Roche, TST, IASLC, and I-ELCAP in the past 36 months. M.N. received consulting fees from Pfizer, Lilly, Novartis, Jassen, Daiichi Sankyo, BMS, AnHeart Therapeutics, J Inst Bio and BluePrint Medicines within the past 36 months; got payment or honoraria for lectures or presentations from Pfizer, Janssen, Dava Oncology LLP, EMD Sereno, OncLive, Takeda, Caris Life Science and Mirati within the past 36 months; got support for attending meetings from AnHeart Therapeutics within the past 36 months; and has stock ownership in MBrace Therapeutics within the past 36 months. E.S. received research grant from Delfi Diganostics within the last 36 months; received consulting fees from Astra Zeneca, Boerhinger Ingelheim, Janssen, and Genentech within the last 36 months; and received lecture fees from OncLive within the past 36 months. S.H.I.O. received consulting fees from Pfizer, Lilly, Jassen, Daiichi Sankyo, BMS, AnHeart Therapeutics, J Inst Bio, and Bayer within the past 36 months; got payment or honoraria for lectures or presentations from Pfizer, Janssen, Dava Oncology LLP, Caris Life Science and OncLive within the past 36 months; has received payment for scientific advisory board from Elevation Oncology within the past 36 months; and has stock ownership in MBrace Therapeutics, BlossomHill Therapeutics, Nuvalent, Lilly, Turning Point Therapeutics, and Elevation Oncology within the past 36 months. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berg CD, Schiller JH, Boffetta P, et al. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J Thorac Oncol 2023;18:1277-89. [Crossref] [PubMed]
- LoPiccolo J, Gusev A, Christiani DC, et al. Lung cancer in patients who have never smoked - an emerging disease. Nat Rev Clin Oncol 2024;21:121-46. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Force UPST. Final Recommendation Statement. Lung Cancer: Screening 2021. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- Osarogiagbon RU, Yang PC, Sequist LV. Expanding the Reach and Grasp of Lung Cancer Screening. Am Soc Clin Oncol Educ Book 2023;43:e389958. [Crossref] [PubMed]
- Ten Haaf K, de Koning HJ. Should Never-Smokers at Increased Risk for Lung Cancer Be Screened? J Thorac Oncol 2015;10:1285-91. [Crossref] [PubMed]
- Triphuridet N, Zhang SS, Nagasaka M, et al. Low-Dose Computed Tomography (LDCT) Lung Cancer Screening in Asian Female Never-Smokers Is as Efficacious in Detecting Lung Cancer as in Asian Male Ever-Smokers: A Systematic Review and Meta-Analysis. J Thorac Oncol 2023;18:698-717. [Crossref] [PubMed]
- Henschke CI, Yip R, Boffetta P, et al. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung Cancer 2015;88:42-7. [Crossref] [PubMed]
- Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911-20. [Crossref] [PubMed]
- Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. [Crossref] [PubMed]
- Nojo T, Imanaka Y, Ishizaki T, et al. Lung cancer incidence in middle-aged men estimated by low-dose computed tomography screening. Lung Cancer 2009;65:56-61. [Crossref] [PubMed]
- Liu X, Liang M, Wang Y, et al. The outcome differences of CT screening for lung cancer pre and post following an algorithm in Zhuhai, China. Lung Cancer 2011;73:230-6. [Crossref] [PubMed]
- Nawa T, Nakagawa T, Mizoue T, et al. Long-term prognosis of patients with lung cancer detected on low-dose chest computed tomography screening. Lung Cancer 2012;75:197-202. [Crossref] [PubMed]
- Sone S, Kondo R, Ishii K, et al. Performance of low-dose CT screening for detecting lung cancer at the early stage and the estimated tumor growth rate according to the smoking status/age. Haigan 2014;54:937-46.
- Yi CA, Lee KS, Shin MH, et al. Low-dose CT screening in an Asian population with diverse risk for lung cancer: A retrospective cohort study. Eur Radiol 2015;25:2335-45. [Crossref] [PubMed]
- Wu FZ, Huang YL, Wu CC, et al. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin Lung Cancer 2016;17:e45-56. [Crossref] [PubMed]
- Hsu HT, Tang EK, Wu MT, et al. Modified Lung-RADS Improves Performance of Screening LDCT in a Population with High Prevalence of Non-smoking-related Lung Cancer. Acad Radiol 2018;25:1240-51. [Crossref] [PubMed]
- Kang HR, Cho JY, Lee SH, et al. Role of Low-Dose Computerized Tomography in Lung Cancer Screening among Never-Smokers. J Thorac Oncol 2019;14:436-44. [Crossref] [PubMed]
- Kim YW, Kang HR, Kwon BS, et al. Low-dose chest computed tomographic screening and invasive diagnosis of pulmonary nodules for lung cancer in never-smokers. Eur Respir J 2020;56:2000177. [Crossref] [PubMed]
- Kakinuma R, Muramatsu Y, Asamura H, et al. Low-dose CT lung cancer screening in never-smokers and smokers: results of an eight-year observational study. Transl Lung Cancer Res 2020;9:10-22. [Crossref] [PubMed]
- Zhang Y, Jheon S, Li H, et al. Results of low-dose computed tomography as a regular health examination among Chinese hospital employees. J Thorac Cardiovasc Surg 2020;160:824-831.e4. [Crossref] [PubMed]
- Shan W, Chen Z, Wei D, et al. Lung cancer screening with low-dose computed tomography at a tertiary hospital in Anhui, China and secondary analysis of trial data. Br J Radiol 2021;94:20200438. [Crossref] [PubMed]
- Chang GC, Chiu CH, Yu CJ, et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: a prospective cohort study. Lancet Respir Med 2024;12:141-52. [Crossref] [PubMed]
- Hill W, Lim EL, Weeden CE, et al. Lung adenocarcinoma promotion by air pollutants. Nature 2023;616:159-67. [Crossref] [PubMed]
- Dias M, Linhas R, Campainha S, et al. Lung cancer in never-smokers - what are the differences? Acta Oncol 2017;56:931-5. [Crossref] [PubMed]
- Adler S, Yip R, Chan H, et al. Comparison of lung cancer aggressiveness in patients who never smoked compared to those who smoked. Lung Cancer 2022;171:90-6. [Crossref] [PubMed]



