
Predicting MET exon 14 skipping mutation in pulmonary sarcomatoid carcinoma by whole-tumour texture analysis combined with clinical and conventional contrast-enhanced computed tomography features
Highlight box
Key findings
• This study proposed a noninvasive and efficient method to predict MET exon 14 (METex14) skipping mutation in pulmonary sarcomatoid carcinoma (PSC) patients by whole-tumour texture analysis combined with clinical and conventional contrast-enhanced computed tomography (CECT).
What is known and what is new?
• Previous studies have attempted to predict epidermal growth factor receptor, anaplastic lymphoma kinase, and Kirsten rat sarcoma viral oncogene homolog mutations in non-small cell lung cancer patients through radiomics or conventional imaging features.
• We applied a similar approach to predict METex14 skipping mutation in PSC patients and proposed PSC patient-specific CECT imaging features, such as pseudocapsular or annular enhancement.
What is the implication, and what should change now?
• Using our predictive model to estimate METex14 skipping mutation in PSC patients can have considerable clinical benefits. Future studies should focus on validating these results in larger populations and exploring the potential application of these findings.
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare, highly malignant type of non-small cell lung cancer (NSCLC) with a poor prognosis; it has a 0.3–1% incidence rate of all lung cancers (1-3). Although surgery is currently considered the primary treatment for PSC and some conventional chemotherapy regimens can be applied, their efficacy is limited (3-5). Excitingly, NSCLC has recently seen tremendous clinical benefits and improved patient outcomes from targeted therapy (6-9), which also provides new opportunities for therapeutic strategies in PSC. In recent studies, the frequency of MET exon 14 skipping (METex14 skipping) mutations appears to be much more prevalent in PSC (approximately 13–22%) (10-12). At the same time, several high-quality clinical studies have suggested that targeted drugs for METex14 skipping mutation (e.g., savolitinib, capmatinib, tepotinib, and crizotinib) can have considerable clinical benefits (1,13-15). Therefore, the accurate judgement of METex14 skipping mutations in PSC patients is critical for individualized treatment selection.
Advanced unresectable or fragile PSC patients require a biopsy to identify the pathological type and METex14 skipping mutation status, but PSC is usually large in size and mixed in components. Biopsy may not be performed, and false negative results may also be obtained (12,16-18). Meanwhile, the occurrence of METex14 skipping mutation is low, and the diverse compositions of the METex14 splice sites and their variable locations in intronic regions require examination by high-throughput sequencing or genotyping technologies (12). These are cost-inefficient for both hospitals and patients. Therefore, a noninvasive, convenient, and more reliable way of detecting METex14 skipping mutations in PSC patients is needed.
Computed tomography (CT) is the most used tool for detecting and diagnosing lung cancer, and contrast-enhanced computed tomography (CECT) can better reflect the tumour blood supply and contrast between tissues (19,20). Recent studies have investigated some clinical features or conventional CT/CECT features associated with METex14 skipping mutations in NSCLC (11,21,22), but only a very small number of PSC patients were included in these studies. Meanwhile, texture analysis or radiomics signatures, which can deeply reflect the heterogeneity of tumours, have been widely used in predicting gene mutations in lung cancer, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and Kirsten rat sarcoma viral oncogene homolog (KRAS) (23-27); however, none have been used for METex14 skipping mutations. Therefore, based on more PSC patients, our study tried to predict METex14 skipping mutations by whole-tumour texture analysis combined with clinical and CECT features. To the best of our knowledge, there have been no imaging studies on PSC in a large number of patients. Our study findings will also have implications for diagnosing PSC using conventional imaging. We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-56/rc).
Methods
Patient information
In this study, we reviewed the imaging data of all patients (n=77) based on a previously published study (12) and excluded patients (n=33) without CECT images or standard images. We reviewed the imaging data of all patients (n=70) diagnosed with PSC at the Cancer Hospital, Chinese Academy of Medical Sciences from January 2017 to October 2021 and excluded patients (n=58) without METex14 skipping mutation tests or CECT images. Thus, 56 patients were included in this study and divided into two groups: the METex14 skipping mutation group and the nonmutation group.
The following inclusion criteria were applied: (I) pathological diagnosis of PSC; (II) METex14 skipping mutation status was detected by using targeted DNA- and RNA-based next-generation sequencing (NGS); (III) no clinical treatments, such as chemotherapy, were administered prior to the METex14 skipping mutation test; and (IV) CECT was performed before surgery or treatment, and the image quality was good. The exclusion criteria were as follows: (I) no METex14 skipping mutation test; (II) missing CECT images before surgery or other treatment; and (III) poor image quality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethical Committee of the Cancer Hospital, Chinese Academy of Medical Sciences approved this study approved this retrospective study (No. 18-224/1782) and waived the requirement for obtaining informed consent from patients, as data were de‑identified, involving no potential risk to patients and no link between the patients and researchers. The flow diagram of the study cohort is shown in Figure 1.

METex14 skipping mutation analysis
All patients in the study received diagnoses of PSC confirmed through independent evaluations of hematoxylin and eosin (H&E) stained sections by two pathologists. The tissue samples were preserved using formalin fixation and embedded in paraffin for analysis. DNA- and RNA-based NGS was performed to test METex14 skipping mutations in tumour samples. Briefly, DNA and RNA extractions were performed using TIANamp Genomic DNA Kit (Tiangen, Beijing, China) and RecoverAllTM Total Nucleic Acid Isolation Kit for FFPE (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Qubit quantitation platform was used to quantitate DNA and RNA concentration.
NGS based on DNA was conducted utilizing a tailor-made gene panel that includes the MET gene along with 17 additional oncogenes. DNA libraries were prepared and targeted NGS was performed as previously reported (12). A custom-crafted probe panel encompassing the junction of MET exons 13 to 15 was employed for the execution of RNA-based NGS. RNA libraries were prepared and the amplicon libraries were sequenced with an Ion Torrent Systems Proton system as previously reported (12). Representative H&E morphology and DNA-based NGS results of one METex14 skipping mutation case and one nonmutation case are shown in Figure 2.

Image acquisition
The image acquisition is described in supplementary material (Appendix 1).
Collection of clinical data and evaluation of conventional CECT features
Clinical data were collected through the Hospital Information System (HIS), including the following 12 characteristics: sex, age, smoking history, T stage, N stage, M stage, American Joint Committee on Cancer (AJCC) stage, location-lobe, location-distribution, translobar growth, long-axis diameter, and short-axis diameter. The clinical stage was determined according to the 8th edition of the AJCC guidelines for NSCLC staging.
All CECT images were evaluated by two radiologists (with three and 10 years of thoracic imaging diagnosis experience) who were blinded to the clinical data. The results were then confirmed by a chief physician specializing in thoracic imaging diagnosis. In cases of disagreement, a consensus was reached through consultation. Twenty CECT features were assessed, including shape, lobulation, margin, spiculation, texture, calcification, air bronchogram, bubble-like lucency, cystic change or necrosis, obstructive change, enhancement heterogeneity, pseudocapsular or annular enhancement, overall CECT value, CECT value of back muscle in the same slice, enhancement relative ratio, enhancement degree, pleural attachment, pleural retraction, pleural effusion, and lymphadenopathy. The definitions and scoring rules of the conventional CECT features are described in Table S1.
Tumour segmentation
The whole tumour was manually delineated slice-by-slice by two radiologists (with 3 and 10 years of lung cancer imaging diagnosis experience) in the lung window setting [width 1,200 Hounsfield unit (HU), level −500 HU] and mediastinal window (width 350 HU, level 50 HU) by using ITK-SNAP (version 2.2.0, www.itksnap.org) software and reviewed by an expert radiologist with 20 years of lung cancer imaging diagnosis experience. The radiologists were blinded to the patients’ clinical and pathological details. The volume of interest (VOI) was drawn according to the tumour-lung interface, excluding vasculature, bronchus, atelectasis, lymph nodes, consolidation or obstructive changes in the lung, and other adjacent normal tissues as much as possible.
Feature extraction
First, all CECT images were preprocessed by resampling to 1×1×1 (mm3) before VOI delineation to eliminate differences between images with different slice thicknesses. Then, all CECT images were normalized with Z scores to obtain a standard normal distribution of image intensities. Texture features were extracted from the VOIs by using AK software (Analysis Kit, GE Healthcare, Chicago, IL, USA). Each image had 1,316 texture features, including 14 shape features (3D), 18 first-order features, 24 grey level co-occurrence matrix (GLCM) features, 16 grey level run length matrix (GLRLM) features, 16 grey level size zone matrix (GLSZM) features, 14 grey level dependence matrix (GLDM) features, 5 neighbouring grey tone difference matrix (NGTDM), 744 wavelet transform features (using the PyWavelets package), 186 Laplacian of Gaussian (LoG) features (based on SimpleITK functionality), and 279 local binary patterns (LBPs). All texture features were obtained from open-source PyRadiomics (http://www.radiomics.io/pyradiomics.html) as recommended by the Image Biomarker Standardisation Initiative (IBSI) (28). The processes of tumour segmentation and feature extraction are shown in Figure 3.
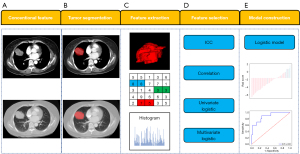
Feature selection and model construction
Data reduction and feature selection processes were performed to select the most relevant features for constructing the models. First, the correlation test between features was assessed. Some features with high correlation (r>0.8) were further evaluated for the diagnostic performance of predictive targets, and the features with higher area under the curve (AUC) were retained. Based on the above methods, the texture features with high diagnostic efficacy and low correlation between features (r<0.8) can be screened out. Second, we used univariate logistic regression to eliminate irrelevant features, and features with P<0.05 were selected. Finally, multivariate logistic regression analysis was used to select the most significant features, and the texture model was established. The differences in clinical/conventional features between the METex14 skipping mutation and nonmutation groups were compared using univariate analysis, and features with P<0.05 were selected. Multivariate logistic regression analysis was used to select the most significant features, and the conventional model was established. The combined model was established by combining the texture model and the conventional model by using multivariable logistic regression. The Radscore, which included the conventional_score, texture_score and combined_score was calculated via the logistic regression linear combination of the selected features weighted by their respective coefficients for each patient. Conventional_score, texture_score and combined_score were the corresponding formulas for calculating the above three models (conventional model, texture model and combined model).
Statistical analysis
The data were analysed using R software (version 3.6.1; http://www.Rproject.org) and MedCalc (ver. 10.3.0.0, MedCalc software). The Kolmogorov-Smirnov test was utilized to evaluate whether all sets of data adhered to a normal distribution. The t test or the Mann-Whitney U test was used to compare the differences between continuous variables, while the chi-squared test or Fisher’s exact test was used for categorical variables. The agreement between different observers was evaluated through the application of the intraclass correlation coefficient (ICC). The strength of the association among the groups was assessed using the Pearson correlation coefficient, and r>0.8 indicated a high correlation. The Mann-Whitney U test was used to evaluate the difference in the Radscore between the METex14 skipping mutation group and the nonmutation group for each model. The three models were evaluated with 10-fold cross-validation. The performance of each model in predicting METex14 skipping mutation was evaluated by receiver operating characteristic curve (ROC) analysis. The DeLong test was used to compare differences among the three models. The benefit of the model was evaluated by decision curve analysis (DCA).
Results
Clinical and conventional CECT features
Fifty-six patients (40 males and 16 females; mean age, 58.14±8.7 years) were included in this study, of which 10 patients (17.9%) had METex14 skipping mutations.
The clinical features are listed in Table 1. T stage, long-axis diameter, and short-axis diameter were significantly different between the two groups (P=0.02, P=0.02, and P=0.01, respectively). The results of conventional CECT features are listed in Table 2. Pseudocapsular or annular enhancement was significantly different between the two groups (P=0.03). Mutations were found more frequently in (I) those who had smaller long- or short-axis diameters (P=0.02, P=0.01); (II) those who had lower T stages (stage I or II) (P=0.02); and (III) those with pseudocapsular or annular enhancement (P=0.03).
Table 1
| Characteristic | All patients | METex14 skipping mutation | Non-mutation | P value |
|---|---|---|---|---|
| Sex | 0.62 | |||
| Female | 16 (28.6) | 4 (40.0) | 12 (26.1) | |
| Male | 40 (71.4) | 6 (60.0) | 34 (73.9) | |
| Age, years | 58.14±8.74 | 60.30±9.13 | 57.67±8.68 | 0.34 |
| Size, cm | ||||
| Long axis diameter | 5.26±1.97 | 3.95±1.35 | 5.54±1.98 | 0.02 |
| Short axis diameter | 3.97±1.75 | 2.75±1.04 | 4.24±1.77 | 0.01 |
| Smoking history | 0.39 | |||
| No | 24 (42.9) | 6 (60.0) | 18 (39.1) | |
| Yes | 32 (57.1) | 4 (40.0) | 28 (60.9) | |
| Location (distribution) | 0.92 | |||
| Central | 26 (46.4) | 4 (40.0) | 22 (47.8) | |
| Peripheral | 30 (53.6) | 6 (60.0) | 24 (52.2) | |
| Location (translobar) | 0.94 | |||
| No | 48 (85.7) | 9 (90.0) | 39 (84.8) | |
| Yes | 8 (14.3) | 1 (10.0) | 7 (15.2) | |
| Location (lobe) | 0.57 | |||
| Right upper lobe | 9 (16.1) | 2 (20.0) | 7 (15.2) | |
| Right middle lobe | 4 (7.1) | 0 | 4 (8.7) | |
| Right lower lobe | 11 (19.6) | 1 (10.0) | 10 (21.7) | |
| Left upper lobe | 18 (32.1) | 4 (40.0) | 14 (30.4) | |
| Left lower lobe | 14 (25.0) | 3 (30.0) | 11 (23.9) | |
| T stage | 0.02 | |||
| 1 | 7 (12.5) | 2 (20.0) | 5 (10.9) | |
| 2 | 21 (37.5) | 7 (70.0) | 14 (30.4) | |
| 3 | 20 (35.7) | 1 (10.0) | 19 (41.3) | |
| 4 | 8 (14.3) | 0 | 8 (17.4) | |
| N stage | 0.79 | |||
| 0 | 32 (57.1) | 6 (60.0) | 26 (56.5) | |
| 1 | 11 (19.6) | 2 (20.0) | 9 (19.6) | |
| 2 | 11 (19.6) | 2 (20.0) | 9 (19.6) | |
| 3 | 2 (3.6) | 0 | 2 (4.3) | |
| M stage | 0.51 | |||
| 0 | 50 (89.3) | 10 (100.0) | 40 (87.0) | |
| 1 | 6 (10.7) | 0 | 6 (13.0) | |
| AJCC stage | 0.13 | |||
| I | 15 (26.8) | 5 (50.0) | 10 (21.7) | |
| II | 18 (32.1) | 2 (20.0) | 16 (34.8) | |
| III | 17 (30.4) | 3 (30.0) | 14 (30.4) | |
| IV | 6 (10.7) | 0 | 6 (13.0) |
Values are n (%) or mean ± SD. METex14 skipping, MET exon 14 skipping; AJCC, American Joint Committee on Cancer; SD, standard deviation.
Table 2
| Features | All patients | METex14 skipping mutation | Non-mutation | P value |
|---|---|---|---|---|
| Shape | 0.43 | |||
| 0 | 5 (8.9) | 2 (20.0) | 3 (6.5) | |
| 1 | 24 (42.9) | 4 (40.0) | 20 (43.5) | |
| 2 | 27 (48.2) | 4 (40.0) | 23 (50.0) | |
| Lobulation | 0.14 | |||
| 0 | 4 (7.1) | 2 (20.0) | 2 (4.3) | |
| 1 | 52 (92.9) | 8 (80.0) | 44 (95.7) | |
| Margin | 0.92 | |||
| 0 | 47 (83.9) | 9 (90.0) | 38 (82.6) | |
| 1 | 9 (16.1) | 1 (10.0) | 8 (17.4) | |
| Spiculation | 0.95 | |||
| 0 | 4 (7.1) | 1 (10.0) | 3 (6.5) | |
| 1 | 30 (53.6) | 5 (50.0) | 25 (54.4) | |
| 2 | 22 (39.3) | 4 (40.0) | 18 (39.1) | |
| Bubble-like lucency | 0.42 | |||
| 0 | 42 (75.0) | 6 (60.0) | 36 (78.3) | |
| 1 | 14 (25.0) | 4 (40.0) | 10 (21.7) | |
| Cystic change or necrosis | 0.68 | |||
| 0 | 34 (60.7) | 5 (50.0) | 29 (63.0) | |
| 1 | 22 (39.3) | 5 (50.0) | 17 (37.0) | |
| Obstructive change | 0.92 | |||
| 0 | 30 (53.6) | 6 (60.0) | 24 (52.2) | |
| 1 | 26 (46.4) | 4 (40.0) | 22 (47.8) | |
| Enhancement heterogeneity | 0.59 | |||
| 0 | 12 (21.4) | 1 (10.0) | 11 (23.9) | |
| 1 | 44 (78.6) | 9 (90.0) | 35 (76.1) | |
| Pseudocapsular or annular enhancement | 0.03 | |||
| 0 | 45 (80.4) | 5 (50.0) | 40 (87.0) | |
| 1 | 11 (19.6) | 5 (50.0) | 6 (13.0) | |
| Overall CT value (HU) | 42.09±15.18 | 38.30±16.65 | 42.91±14.91 | 0.39 |
| CT value of back muscle (HU) | 57.57±10.30 | 53.00±12.90 | 58.57±9.53 | 0.12 |
| Enhancement relative ratio | 0.74±0.25 | 0.72±0.26 | 0.74±0.24 | 0.83 |
| Enhancement degree | 0.67 | |||
| 0 | 33 (58.9) | 7 (70.0) | 26 (56.5) | |
| 1 | 23 (41.1) | 3 (30.0) | 20 (43.5) | |
| Pleural attachment | 0.68 | |||
| 0 | 11 (19.6) | 2 (20.0) | 9 (19.6) | |
| 1 | 45 (80.4) | 8 (80.0) | 37 (80.4) | |
| Pleural retraction | 0.76 | |||
| 0 | 12 (21.4) | 2 (20.0) | 10 (21.7) | |
| 1 | 44 (78.6) | 8 (80.0) | 36 (78.3) | |
| Pleural effusion | 0.24 | |||
| 0 | 46 (82.1) | 10 (100.0) | 36 (78.3) | |
| 1 | 10 (17.9) | 0 | 10 (21.7) | |
| Lymphadenopathy | 0.98 | |||
| 0 | 31 (55.4) | 6 (60.0) | 25 (54.3) | |
| 1 | 25 (44.6) | 4 (40.0) | 21 (45.7) | |
| None calcification | 56 (100.0) | 10 (100.0) | 46 (100.0) | >0.99 |
| None air bronchogram | 56 (100.0) | 10 (100.0) | 46 (100.0) | >0.99 |
| Solid texture | 56 (100.0) | 10 (100.0) | 46 (100.0) | >0.99 |
Values are n (%) or mean ± SD. CECT, contrast-enhanced computed tomography; METex14 skipping, MET exon 14 skipping; CT, computed tomography; HU, Hounsfield unit; SD, standard deviation.
There are some implications for the diagnosis of PSC in conventional CECT features. In our study, PSCs were all solid masses (100%), mostly with some irregularities or irregular in shape (91.1%), with lobulation (92.9%), spiculation (92.9%), and poorly defined margins (83.9%). In some tumours, cystic changes or necrosis (39.3%) could be seen, and there may be obstructive changes (46.4%). The tumours were mostly heterogeneously enhanced (78.6%) on CECT, and some showed pseudocapsular or annular enhancement (19.6%). The overall CT value of PSC was 42.09 HU on average, which was lower than or equal to the back muscles at the same level (100%). Most of them had a pleural attachment (80.4%) or retraction (78.6%), and enlarged lymph nodes were seen in the mediastinum or hilum (44.6%). Meanwhile, bubble-like lucency (25%) was rare within the tumour, usually without pleural effusion (82.1%), no calcification (100%), and air bronchogram (100%).
Feature selection and model construction
All parameters showed good interobserver agreement (ICC >0.8). First, 44 features were retained after the correlation test. Second, 16 features were selected after univariate analysis. Finally, after multivariate logistic regression analysis, three features (wavelet_LowHighLow_GLDM_LowGrayLevelEmphasis, lbp_3D_m2_GLRLM_RunLengthNonUniformityNormalized, and wavelet_LowLowHigh_NGTDM_Strength) remained, and the corresponding texture model was established.
For the conventional model, two features (pseudocapsular or annular enhancement and short-axis diameter) remained after multivariate logistic regression analysis, and the corresponding model was established.
The combined model integrated the conventional model and texture model and was constructed by using the conventional_score and texture_score. The details of the multivariate logistic regression models are listed in Table 3. Radscore was calculated as follows:
Table 3
| Model | OR | 95% CI | β coefficient | P value | Model fit |
|---|---|---|---|---|---|
| Conventional model | 0.184 | ||||
| Pseudocapsular or annular enhancement | 2.493 | 1.214–5.121 | 0.914 | 0.01 | |
| Short axis diameter | 0.205 | 0.056–0.759 | −1.58 | 0.02 | |
| Texture model | 0.643 | ||||
| wavelet_LowHighLow_GLDM_LowGrayLevelEmphasis | 3.072 | 1.246–7.576 | 1.122 | 0.02 | |
| lbp_3D_m2_GLRLM_RunLengthNonUniformityNormalized | 4.001 | 1.221–13.141 | 1.388 | 0.02 | |
| wavelet_LowLowHigh_NGTDM_Strength | 2.280 | 1.048–4.959 | 0.824 | 0.04 | |
| Combined model | 0.643 | ||||
| Conventional_score | 6.532 | 1.875–22.759 | 1.045 | 0.15 | |
| Texture_score | 8.552 | 2.186–33.456 | 1.574 | 0.04 |
The goodness-of-fit of the logistic regression model was assessed using the Hosmer-Lemeshow test, and a model with P>0.05 was considered to be well fitted. OR, odds ratio; CI, confidence interval.
Conventional_score = −2.34 + (0.914 × pseudocapsular or annular enhancement − 1.58 × short-axis diameter);
Texture_score = 2.65 + (1.122 × wavelet_LowHighLow_GLDM_LowGrayLevelEmphasis + 1.388 × lbp_3D_m2_GLRLM_RunLengthNonUniformityNormalized + 0.824 × wavelet_LowLowHigh_NGTDM_Strength);
Combined_score = −2.82 + (1.045 × conventional_score + 1.574 × texture_score).
Model comparison and evaluation
The differences in the Radscore between the METex14 skipping mutation group and the non-mutation group for each model are shown in Figure 4. The performance and ROC curves of each model are shown in Table 4 and Figure 5. The AUCs of the conventional model, texture model, and combined model for predicting METex14 skipping mutation were 0.83 [95% confidence interval (CI): 0.669–0.985], 0.88 (95% CI: 0.766–0.994), and 0.89 (95% CI: 0.785–0.997), respectively. The DCA curves showed that all three models had better net returns than the None model and the All model over a wide range of risk thresholds between 0.1 and 1.0 (Figure 6).

Table 4
| Model | AUC | 95% CI | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Conventional model | 0.83 | 0.669–0.985 | 50.0 | 97.8 | 89.3 |
| Pseudocapsular or annular enhancement | |||||
| Short axis diameter | |||||
| Texture model | 0.88 | 0.766–0.994 | 80.0 | 73.9 | 75.0 |
| wavelet_LowHighLow_GLDM_LowGrayLevelEmphasis | |||||
| lbp_3D_m2_GLRLM_RunLengthNonUniformityNormalized | |||||
| wavelet_LowLowHigh_NGTDM_Strength | |||||
| Combined model | 0.89 | 0.785–0.997 | 70.0 | 82.6 | 80.4 |
| Conventional_score | |||||
| Texture_score | |||||
METex14 skipping, MET exon 14 skipping; PSC, pulmonary sarcomatoid carcinoma; AUC, area under the curve; CI, confidence interval.

Discussion
This study aimed to predict METex14 skipping mutation status through clinical characteristics, conventional imaging features, and texture features, and it is also the PSC imaging study with the largest sample size. In this study, we constructed three models (conventional model, texture model, and combined model) based on CECT image-derived texture parameters, conventional features and clinical features to predict METex14 skipping mutation status in PSC patients. All the three models performed well, among which the combined model had a higher AUC (0.89). Regarding the conventional features, mutations were found more frequently in (I) those who had a smaller long- or short-axis diameter (P=0.02, P=0.01); (II) those who had lower T stage (P=0.02); (III) those with pseudocapsular or annular enhancement (P=0.03).
PSC is a rare type of NSCLC with a high degree of malignancy, a poor prognosis, and limited efficacy in conventional surgical treatment (1). Fortunately, PSC has an “Achilles’ heel”: approximately 10% to 20% of patients have a METex14 skipping mutation. Some clinical trials have demonstrated that through targeted therapy (e.g., savolitinib, capmatinib, tepotinib and crizotinib), inhibition of METex14 mutations can bring clinical benefits. Therefore, the accurate judgement and early prediction of METex14 skipping mutation are significant for PSC. On the basis of previous research (12), our study included new PSC patients diagnosed in Cancer Hospital, Chinese Academy of Medical Sciences from 2017 to 2021, reviewed the CECT images of all patients, and then established three prediction models (conventional model, texture model, and combined model) to predict the METex14 skipping mutation status of PSC patients in a noninvasive way. From the clinical data, the proportion of METex14 skipping mutation in this study was 17.9%, which was basically consistent with that in previous studies (12,29). The median age of patients with PSC was 58 years, and the median tumour size (long-axis diameter) was 5.26 cm. The patients were more commonly male (71.4%), smokers (57.1%), and in stage 2–3 of the AJCC staging system (62.5%) at the time of initial diagnosis. These are consistent with previous studies (3,30,31). Our real-world clinical data help advance and complement PSC research. It is worth noting that in the conventional model, only the odds ratio (OR) value of short-axis diameter is less than 1, indicating a negative correlation with the predicted outcome. Indicating that the lesions in patients with mutations are relatively small, we suspect that the necrosis of smaller lesions is relatively mild, and the tumor parenchyma is relatively large, making it easier to detect tumor mutations. We also discussed imaging features about METex14 skipping mutation in previous studies, as shown in supplementary material (Appendix 1).
Texture analysis or radiomics can exploit the heterogeneity within the tumour to extract high-throughput image features from image data, and on this basis, the relationship between these deep image features and tumour gene expression or mutation can be analysed (32-34). Many studies have proven that the gene expression levels or gene mutations of lung cancer can be predicted by texture analysis or radiomics. Liu et al. (35), Jia et al. (24), Hong et al. (19), and Shiri et al. (36) predicted the EGFR mutation status; Weiss et al. (37) and Velazquez et al. (38) predicted the KRAS mutation status; and Kim et al. (39) and Choi et al. (40) predicted the ALK mutation status. At the same time, adding clinical or conventional imaging features on the basis of texture analysis or radiomics is more helpful for the diagnosis and prediction of the model. Song et al. (25) used radiomics to predict ALK mutation status in lung adenocarcinoma and added clinical and conventional CT features on this basis, which ultimately improved the diagnostic performance of the primary model (AUC =0.83–0.88, P=0.01). There are many similar studies that incorporated clinical, conventional imaging features and texture features (41-43). Three prediction models were established in our study, all of which can predict METex14 skipping mutation status to a certain extent. Among them, the combined model performed the best. Three features were incorporated into the construction of the texture model, including two wavelet transform features. The wavelet transform is particularly suited for analyzing nonstationary signals and elementary functions that exhibit variation in both the frequency domain and spatial extent. It is crafted to deliver superior frequency resolution for the low-frequency elements, representing the overall image intensity, and excellent temporal resolution for the high-frequency elements, which correspond to the image's edge details (44). These three features all can reflect the tumour’s intensity and textural features surrounding and within the high-intensity CT voxels, providing the possibility to predict gene mutations.
This study has some limitations. First, these data are based on a single-centre retrospective study, which will inevitably lead to selection bias. The number of cases with METex14 skipping mutations included in the study was relatively small, which may cause overfitting of the model. Nonetheless, this study is the largest imaging study of PSC ever. Second, the VOI and conventional imaging features were manually determined. These human factors may cause errors in feature extraction and conventional feature judgement. In future work, we hope to build a more advanced system by referring to the work of Tomassini et al. (45), to determine the METex14 skipping mutation by segmentation free CT scans, coupled with an on-cloud decision-support system. Third, due to the small amount of data in this study, we did not have a validation group to validate the model.
Conclusions
In conclusion, our findings highlight the feasibility of noninvasively predicting METex14 skipping mutations in PSC patients using an integrated model that combines clinical and conventional CECT features and texture features.
Acknowledgments
We would like to thank the investigators at all participating study sites.
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-56/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-56/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-56/coif). Y.W. is an employee of GE Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethical Committee of the Cancer Hospital, Chinese Academy of Medical Sciences approved this study (No. 18-224/1782) and waived the requirement for obtaining informed consent from patients, as data were de-identified, involving no potential risk to patients and no link between the patients and researchers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med 2021;9:1154-64. [Crossref] [PubMed]
- Yang Z, Xu J, Li L, et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat Commun 2020;11:4878. [Crossref] [PubMed]
- Zeng Q, Li J, Sun N, et al. Preoperative systemic immune-inflammation index predicts survival and recurrence in patients with resected primary pulmonary sarcomatoid carcinoma. Transl Lung Cancer Res 2021;10:18-31. [Crossref] [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Pécuchet N, Vieira T, Rabbe N, et al. Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 2017;28:1597-604. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Vuong HG, Ho ATN, Altibi AMA, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - A systematic review and meta-analysis. Lung Cancer 2018;123:76-82. [Crossref] [PubMed]
- Li Y, Gao L, Ma D, et al. Identification of MET exon14 skipping by targeted DNA- and RNA-based next-generation sequencing in pulmonary sarcomatoid carcinomas. Lung Cancer 2018;122:113-9. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med 2020;26:47-51. [Crossref] [PubMed]
- Li X, Wu D, Liu H, et al. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol 2020;12:1758835920950207. [Crossref] [PubMed]
- Boland JM, Mansfield AS, Roden AC. Pulmonary sarcomatoid carcinoma-a new hope. Ann Oncol 2017;28:1417-8. [Crossref] [PubMed]
- Yang ZY, Liu ZY, Mu CY, et al. Letter: Exploring the accuracy of biopsy in diagnosis of pulmonary sarcomatoid carcinoma. Lung Cancer 2021;151:97. [Crossref] [PubMed]
- Hong D, Xu K, Zhang L, et al. Radiomics Signature as a Predictive Factor for EGFR Mutations in Advanced Lung Adenocarcinoma. Front Oncol 2020;10:28. [Crossref] [PubMed]
- Sosa E, D'Souza G, Akhtar A, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: A systematic review. CA Cancer J Clin 2021;71:299-314. [Crossref] [PubMed]
- Watari N, Yamaguchi K, Terada H, et al. Characteristic computed tomography features in mesenchymal-epithelial transition exon14 skipping-positive non-small cell lung cancer. BMC Pulm Med 2022;22:260. [Crossref] [PubMed]
- Digumarthy SR, Mendoza DP, Zhang EW, et al. Clinicopathologic and Imaging Features of Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. Cancers (Basel) 2019;11:2033. [Crossref] [PubMed]
- Le NQK, Kha QH, Nguyen VH, et al. Machine Learning-Based Radiomics Signatures for EGFR and KRAS Mutations Prediction in Non-Small-Cell Lung Cancer. Int J Mol Sci 2021;22:9254. [Crossref] [PubMed]
- Jia TY, Xiong JF, Li XY, et al. Identifying EGFR mutations in lung adenocarcinoma by noninvasive imaging using radiomics features and random forest modeling. Eur Radiol 2019;29:4742-50. [Crossref] [PubMed]
- Song L, Zhu Z, Mao L, et al. Clinical, Conventional CT and Radiomic Feature-Based Machine Learning Models for Predicting ALK Rearrangement Status in Lung Adenocarcinoma Patients. Front Oncol 2020;10:369. [Crossref] [PubMed]
- Agazzi GM, Ravanelli M, Roca E, et al. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol Med 2021;126:786-94. [Crossref] [PubMed]
- Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441-6. [Crossref] [PubMed]
- Zwanenburg A, Vallières M, Abdalah MA, et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020;295:328-38. [Crossref] [PubMed]
- Han S, Fang J, Lu S, et al. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco Targets Ther 2019;12:7323-8. [Crossref] [PubMed]
- Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg 2018;53:828-34. [Crossref] [PubMed]
- Mehrad M, Roy S, LaFramboise WA, et al. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology 2018;73:207-14. [Crossref] [PubMed]
- van Timmeren JE, Cester D, Tanadini-Lang S, et al. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging 2020;11:91. [Crossref] [PubMed]
- Bodalal Z, Trebeschi S, Nguyen-Kim TDL, et al. Radiogenomics: bridging imaging and genomics. Abdom Radiol (NY) 2019;44:1960-84. [Crossref] [PubMed]
- Liu Z, Wu K, Wu B, et al. Imaging genomics for accurate diagnosis and treatment of tumors: A cutting edge overview. Biomed Pharmacother 2021;135:111173. [Crossref] [PubMed]
- Liu Y, Kim J, Balagurunathan Y, et al. Radiomic Features Are Associated With EGFR Mutation Status in Lung Adenocarcinomas. Clin Lung Cancer 2016;17:441-448.e6. [Crossref] [PubMed]
- Shiri I, Maleki H, Hajianfar G, et al. Next-Generation Radiogenomics Sequencing for Prediction of EGFR and KRAS Mutation Status in NSCLC Patients Using Multimodal Imaging and Machine Learning Algorithms. Mol Imaging Biol 2020;22:1132-48. [Crossref] [PubMed]
- Weiss GJ, Ganeshan B, Miles KA, et al. Noninvasive image texture analysis differentiates K-ras mutation from pan-wildtype NSCLC and is prognostic. PLoS One 2014;9:e100244. [Crossref] [PubMed]
- Rios Velazquez E, Parmar C, Liu Y, et al. Somatic Mutations Drive Distinct Imaging Phenotypes in Lung Cancer. Cancer Res 2017;77:3922-30. [Crossref] [PubMed]
- Kim TJ, Lee CT, Jheon SH, et al. Radiologic Characteristics of Surgically Resected Non-Small Cell Lung Cancer With ALK Rearrangement or EGFR Mutations. Ann Thorac Surg 2016;101:473-80. [Crossref] [PubMed]
- Choi CM, Kim MY, Hwang HJ, et al. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015;275:272-9. [Crossref] [PubMed]
- Xiao G, Hu YC, Ren JL, et al. MR imaging of thymomas: a combined radiomics nomogram to predict histologic subtypes. Eur Radiol 2021;31:447-57. [Crossref] [PubMed]
- Seo M, Choi MH, Lee YJ, et al. Evaluating the added benefit of CT texture analysis on conventional CT analysis to differentiate benign ovarian cysts. Diagn Interv Radiol 2021;27:460-8. [Crossref] [PubMed]
- Ye R, Weng S, Li Y, et al. Texture Analysis of Three-Dimensional MRI Images May Differentiate Borderline and Malignant Epithelial Ovarian Tumors. Korean J Radiol 2021;22:106-17. [Crossref] [PubMed]
- Bhattacharjee S, Kim CH, Park HG, et al. Multi-Features Classification of Prostate Carcinoma Observed in Histological Sections: Analysis of Wavelet-Based Texture and Colour Features. Cancers (Basel) 2019;11:1937. [Crossref] [PubMed]
- Tomassini S, Falcionelli N, Bruschi G, et al. On-cloud decision-support system for non-small cell lung cancer histology characterization from thorax computed tomography scans. Comput Med Imaging Graph 2023;110:102310.



