
Neoadjuvant aumolertinib monotherapy for EGFR-mutant lung squamous cell carcinoma: a case report
Highlight box
Key findings
• A case report on neoadjuvant aumolertinib monotherapy for EGFR-mutant lung squamous cell carcinoma (LUSQm) is presented, and the pathological complete response was achieved.
What is known and what is new?
• Neoadjuvant targeted therapy for EGFR-mutated non-small cell lung cancer is common.
• At present, the incidence of EGFR mutation in LUSQ is relatively low, and there are few studies on the use of EGFR tyrosine kinase inhibitor (EGFR-TKI) in the treatment of LUSQm, and the effect of EGFR-TKI on LUSQm is not clear. The effect of neoadjuvant targeted therapy for LUSQ is also unclear. We tried neoadjuvant aumolertinib monotherapy for LUSQm.
What is the implication, and what should change now?
• Neoadjuvant aumolertinib may be feasible for locally advanced LUSQm.
Introduction
In China, lung cancer is the malignant tumor with high incidence and mortality (1,2), and more than 30% of non-small cell lung cancer (NSCLC) patients are in the locally advanced stage at the first diagnosis. The therapeutic regimens for locally advanced NSCLC are complex, however, the prognosis of this disease is still poor (2), especially for NSCLC with epidermal growth factor receptor (EGFR) mutations (NSCLCm).
Aumolertinib is a third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI). With the results of APOLLO and AENEAS registrational trial, aumolertinib has been approved in China for advanced NSCLCm as an effective and well-tolerated third-generation EGFR-TKI (3,4). However, the efficacy of EGFR-TKI for lung squamous cell carcinoma harboring EGFR mutation (LUSQm) remains unclear. In this case report, a LUSQm patient with stage IIIA (N2) was reported. We present this article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-47/rc).
Case presentation
The patient was a 65-year-old female without smoking history. The chest computed tomography (CT) illustrated a lesion located in the left lower lobe with enlarged para-aortic lymph nodes (LN). The positron emission tomography-CT (PET-CT) scan indicated heightened glucose metabolism at the left upper lobe mass and the enlarged para-aortic LN. The sample of primary tumor was obtained via CT-guided lung biopsy. LUSQ was confirmed by Hematoxylin-eosin staining and p40 staining (Figures 1,2). The patient was classified as stage IIIA (T2aN2M0) according to the tumor-node-metastasis (TNM) staging system (the 8th edition) (5). And EGFR mutation subtype (19del) was identified by polymerase chain reaction (PCR).

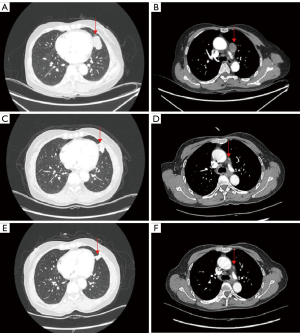
The patient was enrolled in a phase 2 clinical trial conducted in our center, registered at https://www.clinicaltrials.gov (NCT04685070). Inclusion criteria for the clinical trial were stage III to IV NSCLCm. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. From August 30, 2022, aumolertinib (110 mg/day, oral) was administrated to the patient for 4 cycles (16 weeks). The neoadjuvant therapeutic efficacy was assessed by chest CT scans on the last day of each treatment cycle. As Figure 3 showed, surveillance CT scans illustrated that the size of primary tumor (baseline 38.8 mm, and after treatment 9 mm) and metastatic LN (baseline 26.2 mm, and after treatment 6 mm) decreased significantly after 16 weeks aumolertinib monotherapy compared with the values at baseline. As shown in Figure 4, surveillance PET-CT scans demonstrated that the maximum standardized uptake value (SUVmax) of glucose metabolism in primary tumor (baseline 16.05, and after treatment 0.99) and metastatic LN (baseline 16.74, and after treatment 2.26) declined significantly after 16 weeks aumolertinib monotherapy compared with the values at baseline. After discontinuing neoadjuvant aumolertinib monotherapy 1 week, the patient received video-assisted left upper lobectomy combined with LN dissection. Postoperative pathological detection revealed that the patient achieved pathological complete response (pCR: the absence of residual viable tumor cells in all the pathological samples of the resected primary lung tumor and LN following neoadjuvant therapy). The patient received adjuvant aumolertinib monotherapy postoperatively. No recurrence was observed until April 1, 2024, the date of last follow-up. The patient was instructed to continue taking aumolertinib for 3 years after surgery.

All adverse events (AEs) related to neoadjuvant therapy were observed in grade I (decreased lymphocytes, elevated alanine aminotransferase, elevated aspartate aminotransferase, chest pain, diarrhea, and fatigue). Besides, no surgery-related complication was observed in this patient.
Discussion
From the first generation to the third generation, EGFR-TKIs have shown good efficacy on NSCLC harboring common subtypes of EGFR mutations. Therefore, EGFR-TKIs have been approved for the treatment of advanced NSCLCm. EGFR-TKIs have been gradually applied for neoadjuvant target-therapy for locally advanced NSCLCm (6).
Compared with traditional platinum-based chemotherapy, target-therapy has better therapeutic efficacy and lower treatment-related toxicity (7,8). After neoadjuvant target-therapy, tumor stage tends to decline, which may improve the prognosis of patients. It was also found that neoadjuvant target-therapy does not increase the risk of delayed surgery.
At present, the incidence of EGFR mutation in LUSQ is relatively low, and there are few studies on the use of EGFR-TKI in the treatment of LUSQm, and the effect of EGFR-TKI on LUSQm is not clear. The effect of neoadjuvant targeted therapy for LUSQ is also unclear.
Conclusions
This report provides experience of neoadjuvant aumolertinib treatment for locally advanced LUSQm. This suggests that neoadjuvant aumolertinib may be feasible for the treatment of locally advanced LUSQm.
Acknowledgments
We thank the patient and her family for their support on the study. We also thank the study teams from Jiangsu Hansoh Pharmaceutical Group Co., Ltd. for the drug donation.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-47/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-47/coif). X.Y. reports receiving funding from Shanghai Hospital Development Center (No. SHDC22022225). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Gao S, Li N, Wang S, et al. Lung Cancer in People's Republic of China. J Thorac Oncol 2020;15:1567-76. [Crossref] [PubMed]
- Lu S, Wang Q, Zhang G, et al. Efficacy of Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Results From the APOLLO Registrational Trial. J Thorac Oncol 2022;17:411-22. [Crossref] [PubMed]
- Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol 2022;40:3162-71. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Bian D, Sun L, Hu J, et al. Neoadjuvant Afatinib for stage III EGFR-mutant non-small cell lung cancer: a phase II study. Nat Commun 2023;14:4655. [Crossref] [PubMed]
- Zhong WZ, Chen KN, Chen C, et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J Clin Oncol 2019;37:2235-45. [Crossref] [PubMed]
- Sun L, Guo YJ, Song J, et al. Neoadjuvant EGFR-TKI Therapy for EGFR-Mutant NSCLC: A Systematic Review and Pooled Analysis of Five Prospective Clinical Trials. Front Oncol 2020;10:586596. [Crossref] [PubMed]



