S100B and S100B autoantibody as biomarkers for early detection of brain metastases in lung cancer
Introduction
The identification of brain metastases has important implications for treatment and prognosis. A total of 10–15% of patients with lung cancer have brain metastases at diagnosis. The brain is a frequent site for progression of lung cancer because the blood-brain barrier (BBB) shelters the central nervous system (CNS) from systemic treatment (1). Guidelines suggest obtaining brain imaging at presentation in asymptomatic lung cancer patients with advanced stage non-small cell lung cancer, all patients with small cell carcinoma, and anyone with symptoms that could be related to the presence of brain metastases (2,3). On the other side, brain imaging is not routinely performed in longitudinal follow-up (4,5). The appropriate selection of patients at high risk of having brain metastasis could reduce the amount of unnecessary brain imaging, decrease costs and improve patient care. An accurate, inexpensive blood based biomarker for the presence of brain metastases could be used as an upfront staging test, prompting further characterization with brain imaging when positive. Furthermore, the test could be used in longitudinal follow-up, measured at scheduled intervals, for the early detection of brain metastases.
S100B is an important member of a multigenic family of calcium-binding proteins of the EF-hand type (helix E-loop-helixF) which is highly expressed in astrocytes in the brain. It is also expressed in the Schwann cells of the peripheral nervous system, and outside the nervous system in melanocytes, adipocytes and chondrocytes (6,7). However, extracranial sources of S100B do not affect serum levels (8). S100B may be released into the blood when the neovascularization and growth of metastatic lesions compromises the integrity of the BBB (9). An association between elevated serum S100B and the presence of brain metastases has been reported (10,11).
Increased S100B levels may also be found in patients with cerebral small vessel disease (SVD) (11). The population of older patients who are at risk to develop cancer often has comorbidities and risk factors for cerebrovascular disease (e.g., smoking, diabetes, and hypertension). Therefore SVD is a frequent finding on brain imaging performed for lung cancer staging. It is important to distinguish the SVD from brain metastases. We hypothesize that S100B autoantibody levels may help to distinguish the elevation of serum S100B caused by SVD from that caused by brain metastases (generally longer BBB disruption in SVD disruption) since the production of antibodies may be affected by the duration of exposure to S100B.
The aim of our study was to verify that levels of S100B protein are elevated in lung cancer patients with brain metastases and determine if the addition of S100B autoantibody levels can improve the accuracy of this biomarker.
Methods
This cohort study enrolled patients seen at the Cleveland Clinic from 2010 and 2012. We included adult patients ≥18 years of age at the time of diagnosis of lung cancer, with or without neurological symptoms. Patients who were previously treated for lung cancer, had synchronous malignancies, or HIV infection were excluded. The study was approved by the Institutional Review Board of the Cleveland Clinic (IRB #10-521) and all patients signed an informed consent.
We collected information about smoking history, comorbidities, anthropometric measurements, serum creatinine and brain imaging from the electronic medical record. Creatinine clearance was calculated using the Cockcroft-Gault equation. Tumor histology was assessed by pathologists and staging was classified based on the American Joint Committee on Cancer 7th edition of tumor, node, metastasis staging criteria (12).
Serum S100B protein and anti-S100B immunoglobulin G (IgG) were measured in each patient. S100B protein was measured using a commercially available, monoclonal, 2-site immunoluminometric assay from Diasorin (Stillwater, MI, USA) (8,11,13).
An ELISA was developed to detect anti-S100B IgG (14). First, 96 well plates were coated with PBS solution containing S100B protein (human brain, catalog number 559291, EMD Chemicals). Optimization of this ELISA was achieved by testing two concentrations of S100B protein (1 or 5 µg per well). No significant differences were observed at these two concentrations of S100B coating. Plates were coated overnight at 4 °C with S100B protein (1 µg/well). Wells were then washed three times with PBS. Subsequently, 100 µL of a 1% BSA blocking solution was added in each well and incubated for 2 hours at room temperature. Wells were then washed three times using 200 µL of PBS containing 0.05% tween-20. Serum samples and standards were added and incubated for 1 hour at room temperature. An S100B monoclonal antibody (catalog number: Q86610M Meridian Life Science Inc.) was used as a standard to allow for the conversion of absorbance unit values into concentration. Standard curves were obtained with 100 µL of serially diluted S100B monoclonal antibody. A secondary antibody solution of 200 µL of horseradish-peroxidase (HRP) goat anti-mouse IgG and 200 µL of HRP goat anti-human were added to the standards and serum samples respectively. After 1 hour incubation at room temperature wells were washed three times with 200 µL of PBS containing 0.05% tween-20. Finally, 100 µL of OPD solution was added and the reaction incubated for 30 minutes at room temperature. The reaction was stopped by adding 100 µL of 2.5 M sulfuric acid. Samples were analyzed using an ELISA plate reader at 490 nm.
Brain imaging, magnetic resonance imaging (MRI) or computed tomography scan (CT) was performed as part of routine care. Images were interpreted independently by an expert neuroradiologist who was blinded to the S100B and S100B autoantibody levels, and clinical information. Brain scans were evaluated for the presence of brain metastases and for white matter changes related to SVD. The age related white matter changes (ARWMC) rating scale was used to grade the white matter changes (15). White matter changes on MRI were defined as ill-defined hyperintensities ≥5 mm on both T2 and PD/FLAIR images, and on CT as ill-defined and moderately hypodense areas of ≥5 mm. Lacunes were defined as well-defined areas of >2 mm with attenuation (on CT) or signal characteristics (on MRI) the same as cerebrospinal fluid. If lesions with these characteristics were ≤2 mm, they were considered perivascular spaces. Changes in the basal ganglia were rated in the same way (15).
Statistical analysis was performed using SPSS (version 13, Chicago, IL, USA). We determined the sensitivity, specificity and accuracy of S100B and S100B autoantibodies to detect brain metastasis in patients with lung cancer. Logistic regression and receiver operating characteristic (ROC) curve analysis were used to determine the optimal cutoff values. A staged classification model determined the final class. A difference with P<0.05 was considered statistically significant.
Results
Demographics and lung cancer characteristics
Table 1 shows the characteristics of the patients enrolled in this study. We enrolled 145 patients newly diagnosed with lung cancer. Of these, 128 (88.3%) were included in our analysis as 17 (11.7%) patients had incomplete clinical or radiographic information. One hundred and nine patients had brain imaging; 85 (78%) patients had an MRI and 24 (22%) had a CT scan. Most patients were male (60.2%), Caucasians (87.5%) who had a positive history of smoking (95.3%). The mean age was 67.9±10.5 years. Forty three (33.5%) patients were diagnosed at stage IIIA–B and 38 (29.7%) stage IV (Table 2). The most common histologic subtype was adenocarcinoma (47.7%).
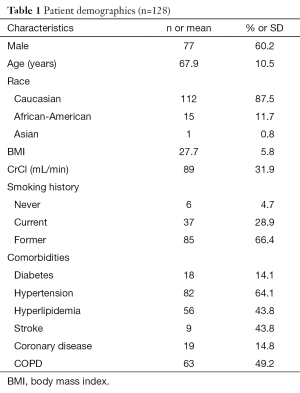
Full table
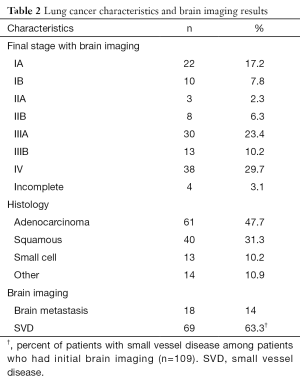
Full table
Brain imaging
Nineteen patients did not have brain imaging during initial staging. These patients either had negative brain imaging during follow up or completed a clinical follow-up of at least 2 years with no signs of recurrent or metastatic disease. Thus they were included in the group negative for brain metastases. Of these, 15 patients were diagnosed at stage I–II, and 4 patients at stage IIIA–B. These patients were not included in the analysis of SVD.
S100B, S100B autoantibodies and brain metastases
Of the patients with distant metastases, 18 had brain metastases. An ROC was generated (AUC 0.63) and we selected a threshold of S100B that yielded a clinically useful sensitivity, and then determined the specificity (Table 3) to detect brain metastasis. At a serum S100B level of 0.058 ng/mL, the sensitivity was 89% and the specificity was 43%. The positive predictive value (PPV) was 21.1% and the negative predictive value (NPV) was 96.2%. S100B levels were not associated with age, gender, body mass index (BMI), creatinine clearance, or histology in our stratified analyses or by logistic regression.
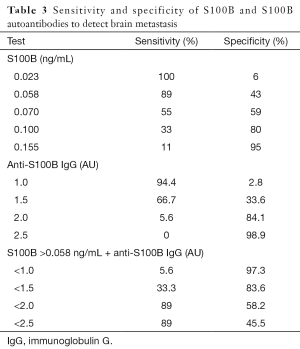
Full table
We selected an anti-S100B IgG threshold that would improve the specificity while maintaining a high sensitivity of S100B to detect brain metastases. An anti-S100B IgG level of 2.0 AU had a sensitivity of 5.6% and a specificity of 84.1%. We then used the anti-S100B IgG level <2.0 AU combined to S100B ≥0.058 ng/mL. The sensitivity remained at 89% while the specificity improved to 58.2%. The overall accuracy was 51% with S100B alone and it improved to 62.5% when combined with the antibody threshold.
There were a total of 46 patients in the hypothetical subgroup that would be diagnosed at stage I or II if they had not had brain imaging at diagnosis. Three of these patients had brain metastases. In this subgroup, S100B ≥0.058 ng/mL had a sensitivity of 100%, and NPV of 100% (Table 4). When combined with anti-S100B IgG <2.0 AU the sensitivity and NPV remained the same but specificity improved from 39.5% to 55.8% and PPV had a modest increased from 10.3% to 13.6%.

Full table
Sixty nine of the patients who had brain imaging (69/109) had evidence of SVD (Table 2). We analyzed the S100B and anti-S100B IgG levels according to the grading of white matter and basal ganglia lesions from SVD (Table S1), BMI (Figures S1,S2) and creatinine clearance (Figure S3). S100B levels or anti-S100B IgG levels did not significantly vary according to the severity of SVD in the white matter, BMI or creatinine clearance. There were only 7 patients with SVD in the basal ganglia and there was no variation in the S100B or anti-S100B IgG levels among the different grades.
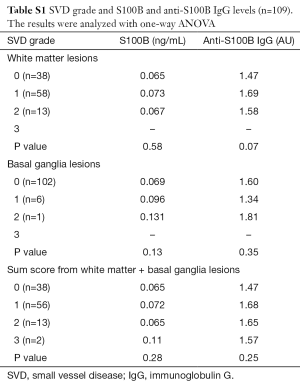
Full table
Discussion
This is a study investigating the accuracy of the S100B protein and S100B autoantibody for the detection of brain metastasis in patients with lung cancer. Our results confirm that elevated serum levels of S100B protein have a high sensitivity to detect brain metastases, and the addition of S100B autoantibody levels improves the specificity and overall accuracy.
Staging is an evaluation of the extent to which lung cancer has spread. Further investigation is necessary when a lesion suspicious for metastatic disease is identified. Dedicated imaging and/or a biopsy are performed until a pathological diagnosis or a very high probability of metastatic disease is obtained. The diagnosis of a brain metastasis in particular relies on the detailed radiographic characterization of a suspicious lesion as the potential morbidity limits the frequency with which a brain biopsy is performed. A biomarker could be used to identify those at highest and lowest risk of having brain metastases which could translate into more targeted use of brain imaging.
The properties of S100B are attractive for clinical use as a biomarker to detect brain metastases. When the BBB is disrupted by a metastatic brain lesion there is a leak of brain derived proteins, including S100B, into the blood stream. In our study, we used elevated serum levels of S100B as an indirect sign of metastatic growth in the brain and we found the combination of S100B and anti-S100B IgG resulted in improved specificity and accuracy while maintaining a high sensitivity. A serum S100B threshold level of 0.058 ng/mL had a sensitivity of 89% and when combined with anti-S100B IgG <2.00 AU the specificity improved from 43% to 58.2%, and the overall accuracy from 51% to 62.5%. In exploratory analysis, this relationship was maintained for those presenting with early stage disease (e.g., stage I or II before CNS staging). In this subgroup the sensitivity and NPVs were both 100%.
The use of S100B as a biomarker to select patients who would need brain imaging could impact the cost of initial staging. The United States is estimated to have 224,000 new cases of lung cancer in 2014 and approximately 33,600 (15%) patients will be diagnosed with localized disease (16). Fifty two percent of patients would have a negative result if we extrapolate the results of our study. Considering that the cost of an MRI of the brain is about US$3,000 and the cost of measuring S100B is US$50, the potential savings are more than 50 million dollars per year.
We have previously demonstrated that SVD is a common cause of BBB disruption and elevation of S100B levels in patients with lung cancer (11). However, for unclear reasons, we did not see a relationship between S100B and SVD in the current study. When S100B leaks into the bloodstream as a result of BBB disruption it is perceived as a non-self antigen by dendritic cells resulting in the production of autoantibodies against S100B. Multiple BBB disruptions measured by surges in serum S100B have been shown to trigger an autoimmune response characterized by elevated titers of anti-S100B IgGs in the blood (14). SVD is typically silent and may cause a long-term (months to years) and sustained BBB disruption. On the other hand, brain metastases that are detectable on brain imaging are likely present for a short time (weeks to months). Therefore, we hypothesized that autoantibody levels would be higher in SVD compared to brain metastases. We expected that brain metastasis would have an elevated serum S100B and a relatively low/normal autoantibody level while both S100B and autoantibody levels would be elevated in patients with SVD but no brain metastases.
Our study had limitations. Not all patients had brain imaging at initial staging. MRI and CT scans were obtained as part of routine care. Clinical practice may be heterogeneous in this aspect. Providers may choose not to obtain brain imaging when patients are presumed to have early stage lung cancer or when obvious extrathoracic metastatic disease is present and patients have no neurological symptoms. We only included patients who had brain imaging, and those who did not have brain imaging who were clinically metastases free after 2 years of follow-up in the analysis. Also, we observed that the serum S100B levels were overall lower compared to previous studies done by our group (11,17). We do not fully understand the reason for this variation but we attribute it to changes in the commercial kit. The test used in prior study was manufactured by Sangtec (Bromma, Sweden) and is no longer commercially available, and the surrogate ELISA used, by Diasorin, Inc. (Stillwater, MN, USA), may perform differently. In regard to autoantibodies, the measurement of anti-S100B IgG is not available commercially and it was developed in our laboratory (14). We did not observe a significant difference in the absorbance levels compared to previous study but further validation of the assay is necessary (11,17).
We have opportunities to continue to improve the diagnostic properties of S100B. It has the unique property to appear both as homo- or hetero-dimers (18,19). The homodimer S100B-B is the subtype released mainly by the brain, thus it may be more specific than the total S100B levels (which was measured in our study) in predicting BBB disruption and brain metastases in lung cancer (8). Further understanding of S100B physiology (e.g., half-life and clearance), immune response, and the different factors that might affect the serum levels will also contribute to the efforts to improve its specificity. S100B should also be studied in the longitudinal follow-up of patients with lung cancer after treatment in order to determine if it can accurately detect newly developed asymptomatic brain lesions.
In conclusion, the use of biomarkers to help identify CNS metastatic disease is promising. Serum S100B combined with S100B antibody levels are able to detect brain metastases from lung cancer with high sensitivity and reasonable specificity.
Acknowledgements
Funding: This work was supported by the National Institutes of Health (R01NS078307, R01NS43284, R41MH093302, R21NS077236, R42MH093302, UH2TR000491, R21HD057256 to D Janigro).
Footnote
Conflicts of Interest: D Janigro has two patents—Markers of Blood Barrier Disruption and Methods of using same (US 7,144,708) and Peripheral Marker of Blood Brain Barrier Permeability (US 6,884,591B2). D Janigro received 5 years ago income from a 2-year license of the S100B technology. We are not aware at this time of any organization which may benefit from the publication of this article. No diagnostic or use-related claims or data are contained herein. D Janigro holds two US patents on the use of S100B as a marker of BBB disruption. The Cleveland Clinic policy on conflict of interest has established a management plan to ensure compliance with NIH guidelines and Cleveland Clinic policies. The remainder authors report no conflicts of interest.
Ethical Statement: The study was approved by the Institutional Review Board of the Cleveland Clinic (No. 10-521) and written informed consent was obtained from all patients.
References
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005;75:5-14. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Ricciardi S, de Marinis F. Multimodality management of non-small cell lung cancer patients with brain metastases. Curr Opin Oncol 2010;22:86-93. [Crossref] [PubMed]
- Butler AR, Leo JS, Lin JP, et al. The value of routine cranial computed tomography in neurologically intact patients with primary carcinoma of the lung. Radiology 1979;131:399-401. [Crossref] [PubMed]
- Cole FH Jr, Thomas JE, Wilcox AB, et al. Cerebral imaging in the asymptomatic preoperative bronchogenic carcinoma patient: is it worthwhile? Ann Thorac Surg 1994;57:838-40. [Crossref] [PubMed]
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001;33:637-68. [Crossref] [PubMed]
- Heizmann CW, Fritz G, Schäfer BW. S100 proteins: structure, functions and pathology. Front Biosci 2002;7:d1356-68. [PubMed]
- Cai Q, Robertson ES. Ubiquitin/SUMO modification regulates VHL protein stability and nucleocytoplasmic localization. PLoS One 2010;5:e12636. [Crossref] [PubMed]
- Marchi N, Cavaglia M, Fazio V, et al. Peripheral markers of blood-brain barrier damage. Clin Chim Acta 2004;342:1-12. [Crossref] [PubMed]
- Kanner AA, Marchi N, Fazio V, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer 2003;97:2806-13. [Crossref] [PubMed]
- Vogelbaum MA, Masaryk T, Mazzone P, et al. S100beta as a predictor of brain metastases: brain versus cerebrovascular damage. Cancer 2005;104:817-24. [Crossref] [PubMed]
- Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e191S-210S.
- Georgiadis D, Berger A, Kowatschev E, et al. Predictive value of S-100beta and neuron-specific enolase serum levels for adverse neurologic outcome after cardiac surgery. J Thorac Cardiovasc Surg 2000;119:138-47. [Crossref] [PubMed]
- Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One 2013;8:e56805. [Crossref] [PubMed]
- Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318-22. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Marchi N, Mazzone P, Fazio V, et al. ProApolipoprotein A1: a serum marker of brain metastases in lung cancer patients. Cancer 2008;112:1313-24. [Crossref] [PubMed]
- Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 2008;41:755-63. [Crossref] [PubMed]
- Hasselblatt M, Mooren FC, von Ahsen N, et al. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology 2004;62:1634-6. [Crossref] [PubMed]




