
Lymph node dissection of station 8 improves the survival of T≤3 cmN0M0 lung adenocarcinoma patients
Highlight box
Key findings
• Station 8 lymph node dissection (8LND) improves the survival of T≤3 cmN0M0 lung adenocarcinoma patients, especially in male patients, smokers, patients with a pT2 tumor (≤3 cm), and patients with a poorly differentiated tumor.
What is known and what is new?
• 8LND is recommended by the National Comprehensive Cancer Network guideline for lung adenocarcinoma, but the dissection rate is low in real world clinical practice.
• We investigated the role of 8LND in 1,209 T≤3 cmN0M0 lung adenocarcinoma patients, and found patients with 8LND had better progression-free survival, overall survival, and cancer-specific survival than those not.
What is the implication, and what should change now?
• Lung adenocarcinoma is a tumor characterized by high metastasis rate. Our finding indicated that 8LND should be performed in lung adenocarcinoma patients, especially in male patients, smokers, patients with a pT2 tumor (≤3 cm), and patients with a poorly differentiated tumor, even the tumor size is ≤3 cm.
Introduction
Lobectomy and systemic lymph node (LN) dissection are the standard surgical procedure for lung cancer (1). LN dissection, especially mediastinal LN dissection, plays an important role in improving the survival (2,3). According to the guideline of National Comprehensive Cancer Network (NCCN) of U.S., an adequate N2 lymphadenectomy should include stations 2R, 4R, 7, 8, 9 for right-side cancers, and stations 4L, 5, 6, 7, 8, 9 for left-sided cancers. And the guideline emphasizes that at least three N2 stations should be dissected (4). But in the clinical practice, inadequate LN dissection is common (3,5), especially for station 8, of which the dissection rate is only around 20% (5,6). Nowadays, due to the wide application of low-dose computed tomography (CT), the frequency of small lung adenocarcinoma (≤3 cm) is increasing (7,8). Given that adenocarcinoma is a risk factor for mediastinal LN metastasis (9), and the gap between the clinical practice and the guideline for station 8 LN dissection (8LND) is obvious, we conducted this study to investigate the effect of 8LND on the survival of T≤3 cmN0M0 lung adenocarcinoma patients. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-184/rc).
Methods
Patients
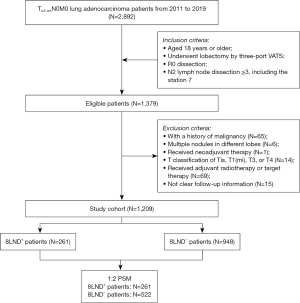
We retrospectively enrolled lung adenocarcinoma patients diagnosed between January 2011 and December 2019 from the West China Hospital. The inclusion criteria were: (I) aged 18 years or older; (II) pathologically diagnosed as T≤3 cmN0M0 lung adenocarcinoma; (III) underwent lobectomy by three-port video-assisted thoracoscopic surgery (VATS); (IV) R0 dissection; and (V) stations of mediastinal LNs dissection ≥3, including the subcarinal station. The exclusion criteria were: (I) with a history of malignancy before the diagnosis of lung adenocarcinoma; (II) multiple nodules in different lobes; (III) underwent neoadjuvant therapy; (IV) T classification of Tis, T1(mi), T3, or T4; (V) underwent adjuvant radiotherapy or target therapy, which strongly indicated non-R0 dissection in T≤3 cmN0M0 patients according to the NCCN guideline; and (VI) not clear follow-up information. A part of patients with a T2 tumor ≤3 cm was also included into our study because of the similar mediastinal lymphadenectomy strategies between those patients and patients with a T1 tumor. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of West China Hospital (No. 2023-1879), and the requirement for informed consent was waived.
Surgical procedure and follow up
The surgeries were performed by experienced surgeons. The entire lobe with the tumor was resected by three-port VATS and at least three stations of the mediastinal LNs, including station 7, were dissected in consistent with the LN map proposed in 2009 by the International Association for the Study of Lung Cancer (IASLC) (10). The pathological stage was determined according to the pathological findings of the tumor sample and the dissected LNs. All patients received a chest X-ray 1 month after the surgery and scheduled follow-up at 3- to 6-month intervals for the first 2 years, at 6-month intervals for the subsequent 3 years, and annually after 5 years were performed. During the follow-up, physical examinations, laboratory tests, CT scans of the chest and abdomen, CT scan or magnetic resonance imaging of the head, bone scintigraphy, or positron emission tomography/CT would be conducted when necessary.
Variables and outcomes
We collected the clinicopathological data of patients, including age at which the patient was diagnosed with lung adenocarcinoma, sex, smoking status, body mass index (BMI), primary site, tumor size, surgery details, pathological T classification, differentiation, adjuvant chemotherapy information, and follow-up data. The primary outcomes were progression-free survival (PFS) and overall survival (OS). The secondary outcome was cancer-specific survival (CSS). PFS was defined as the duration from surgery to the first event of relapse, metastasis, death because of any reason, or the last follow-up. OS was defined as the duration from surgery to the death because of any reason, or the last follow-up. CSS was defined as the duration from surgery to the death because of the diagnosed adenocarcinoma, or the last follow-up.
Statistical analysis
To reduce confounding effect and balance potential confounders, patients who underwent 8LND (8LND+ group) or not (8LND− group) were matched at 1:2 ratio using propensity score matching (PSM) method. Variables including age, sex, smoking status, BMI, primary site, tumor size, pathological T classification, differentiation, adjuvant chemotherapy status, and dissection status of other LN stations were matched with “nearest neighbor matching”. Univariable and multivariable analyses were used to evaluate the effect of 8LND on the survival in all enrolled patients. Variables with P<0.1 in the univariable analysis were included into the multivariable analysis. To further investigate the effect of 8LND, subgroup analysis was also conducted by performing a multivariable analysis on the survival outcomes using 8LND and the variable of interest. Additionally, sensitivity analysis was also conducted by excluding patients in certain subgroup.
Normally distributed continuous variables were described as mean and standard deviation (SD), and were also converted into categorical variables using the cutoffs based on the clinical meaning. Skewed continuous variables were described as median and the 25th and 75th percentiles. The continuous variables were analyzed using Student’s t-test, and the categorical variables were analyzed using Pearson chi-squared test. Kaplan-Meier method and log-rank test were used in the survival analyses. The Cox proportional hazards regression was used in the univariable analysis, multivariable analysis, subgroup analysis, and sensitivity analysis. Hazard ratio (HR) and 95% confidence interval (CI) were used to describe the effect of 8LND and the coefficients of the Cox proportional hazards regression model were tested by Wald test. The “MatchIt” R package was used in the PSM. The “jstable” R package was used to conduct subgroup analysis. The software used in this study was R (R-4.0.3). A P value <0.05 was considered significant and all P values were two-tailed.
Results
Clinicopathological characteristics of patients
A total of 1,209 lung adenocarcinoma patients were enrolled, of whom 261 (21.59%) underwent 8LND, 719 (59.5%) were female, and 892 (73.8%) were non-smokers (Table 1). The flowchart of the patient enrollment is shown in Figure 1. The mean age of the enrolled patients was 58.80±9.37 years old, the mean tumor size was 1.95±0.63 cm, and the mean number of dissected LNs were 12.70±5.19. Compared to 8LND− patients, 8LND+ patients had smaller BMI (22.95±2.89 vs. 23.40±3.02, P=0.03), and seemed to have larger proportion of male (45.6% vs. 39.1%, P=0.07). No significant difference was found in age, smoking status, primary site, tumor size, T classification, differentiation, or adjuvant chemotherapy between the groups.
Table 1
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| 8LND− patients (n=948) | 8LND+ patients (n=261) | P | 8LND− patients (n=522) | 8LND+ patients (n=261) | P | ||
| Age (years), n (%) | 0.84 | 0.84 | |||||
| <50 | 163 (17.2) | 39 (14.9) | 86 (16.5) | 39 (14.9) | |||
| 50–59 | 320 (33.8) | 90 (34.5) | 189 (36.2) | 90 (34.5) | |||
| 60–69 | 343 (36.2) | 99 (37.9) | 182 (34.9) | 99 (37.9) | |||
| ≥70 | 122 (12.9) | 33 (12.6) | 65 (12.5) | 33 (12.6) | |||
| Age (years), mean ± SD | 58.72±9.47 | 59.08±9.01 | 0.58 | 58.49±9.48 | 59.08±9.01 | 0.40 | |
| Sex, n (%) | 0.07 | 0.78 | |||||
| Female | 577 (60.9) | 142 (54.4) | 291 (55.7) | 142 (54.4) | |||
| Male | 371 (39.1) | 119 (45.6) | 231 (44.3) | 119 (45.6) | |||
| Smoking status, n (%) | 0.42 | 0.93 | |||||
| No | 705 (74.4) | 187 (71.6) | 377 (72.2) | 187 (71.6) | |||
| Yes | 243 (25.6) | 74 (28.4) | 145 (27.8) | 74 (28.4) | |||
| BMI (kg/m2), n (%) | 0.04 | 0.94 | |||||
| <24 | 554 (58.4) | 172 (65.9) | 341 (65.3) | 172 (65.9) | |||
| ≥24 | 394 (41.6) | 89 (34.1) | 181 (34.7) | 89 (34.1) | |||
| BMI (kg/m2), mean ± SD | 23.40±3.02 | 22.95±2.89 | 0.03 | 22.99±2.99 | 22.95±2.89 | 0.88 | |
| Primary site, n (%) | 0.20 | 0.99 | |||||
| Right upper lobe | 361 (38.1) | 86 (33.0) | 173 (33.1) | 86 (33.0) | |||
| Right middle lobe | 101 (10.7) | 29 (11.1) | 64 (12.3) | 29 (11.1) | |||
| Right lower lobe | 148 (15.6) | 56 (21.5) | 109 (20.9) | 56 (21.5) | |||
| Left upper lobe | 192 (20.3) | 54 (20.7) | 103 (19.7) | 54 (20.7) | |||
| Left lower lobe | 146 (15.4) | 36 (13.8) | 73 (14.0) | 36 (13.8) | |||
| Size, n (%) | 0.56 | >0.99 | |||||
| ≤1 cm | 101 (10.7) | 22 (8.4) | 44 (8.4) | 22 (8.4) | |||
| >1–2 cm | 535 (56.4) | 149 (57.1) | 296 (56.7) | 149 (57.1) | |||
| >2–3 cm | 312 (32.9) | 90 (34.5) | 182 (34.9) | 90 (34.5) | |||
| Size (cm), mean ± SD | 1.94±0.64 | 1.99±0.61 | 0.20 | 1.99±0.63 | 1.99±0.61 | 0.89 | |
| T classification, n (%) | 0.48 | 0.90 | |||||
| T1 | 447 (47.2) | 116 (44.4) | 236 (45.2) | 116 (44.4) | |||
| T2 | 501 (52.8) | 145 (55.6) | 286 (54.8) | 145 (55.6) | |||
| Differentiation, n (%) | 0.77 | 0.87 | |||||
| Well differentiated | 284 (30.0) | 73 (28.0) | 151 (28.9) | 73 (28.0) | |||
| Moderately differentiated | 464 (48.9) | 134 (51.3) | 271 (51.9) | 134 (51.3) | |||
| Poorly differentiated | 200 (21.1) | 54 (20.7) | 100 (19.2) | 54 (20.7) | |||
| Adjuvant chemotherapy, n (%) | >0.99 | 0.97 | |||||
| No | 848 (89.5) | 233 (89.3) | 468 (89.7) | 233 (89.3) | |||
| Yes | 100 (10.5) | 28 (10.7) | 54 (10.3) | 28 (10.7) | |||
| Dissected N1 stations, mean ± SD | 2.25±0.94 | 2.35±0.84 | 0.10 | 2.38±0.84 | 2.35±0.84 | 0.67 | |
| Dissected N1 LNs, mean ± SD | 5.02±3.24 | 5.07±3.05 | 0.83 | 5.20±3.18 | 5.07±3.05 | 0.58 | |
| Dissected N2 stations, mean ± SD | 3.57±0.65 | 4.70±0.71 | <0.001 | 3.68±0.67 | 4.70±0.71 | <0.001 | |
| Dissected N2 LNs, mean ± SD | 7.47±3.72 | 8.39±3.88 | 0.001 | 7.01±3.27 | 8.39±3.88 | <0.001 | |
| Dissected total stations, mean ± SD | 5.82±1.14 | 7.06±1.07 | <0.001 | 6.06±1.06 | 7.06±1.07 | <0.001 | |
| Dissected total LNs, mean ± SD | 12.49±5.21 | 13.45±5.03 | 0.008 | 12.21±4.78 | 13.45±5.03 | 0.001 | |
PSM, propensity score matching; 8LND, station 8 lymph node dissection; SD, standard deviation; BMI, body mass index; LN, lymph node.
With respect to LN dissection, no difference was found in dissected N1 LN stations or LNs number between the groups. But about one more N2 LN station (4.70±0.71 vs. 3.57±0.65, P<0.001) and one more N2 LN (8.39±3.88 vs. 7.47±3.72, P=0.001) were dissected in 8LND+ group as compared to 8LND− group. Similar differences as the N2 were also found in total dissected LN stations and LNs number. The detailed information of the dissection status of other LN stations between the groups is also demonstrated in Figure 2 and Table S1.

Survival outcomes
The median follow-up duration was 36 (the 25th to 75th percentiles: 25–53) months. Before PSM, 8LND+ patients had better PFS (P=0.03), OS (P=0.03), and CSS (P=0.05) (Figure 3A-3C). The 3- and 5-year PFS rates were 94.71%, 91.34% in 8LND+ patients and 90.63%, 88.03% in 8LND− patients. The 3- and 5-year OS rates were 99.04%, 97.10% in 8LND+ patients and 97.34%, 92.78% in 8LND− patients. The 3- and 5-year CSS rates were 99.62%, 97.67% in 8LND+ patients and 98.18%, 93.59% in 8LND− patients.

After PSM, the clinicopathological characteristics and dissection status of other LN stations were balanced between groups (Table 1 and Table S1). In the matched patients, 8LND was still associated with better PFS (P=0.006), OS (P=0.01), and CSS (P=0.03) (Figure 4A-4C). In the multivariable analysis of the enrolled patients, 8LND significantly reduced the risk of disease progression (HR: 0.46; 95% CI: 0.26–0.80; P=0.007) (Table 2), the risk of overall death [0.33 (0.13–0.85), P=0.02] (Table 3), and the risk of cancer-specific death [0.35 (0.12–1.00), P=0.05] (Table S2).

Table 2
| Characteristics | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||
| <50 | Reference | ||||
| 50–59 | 0.86 (0.49–1.50) | 0.60 | |||
| 60–69 | 0.64 (0.36–1.13) | 0.13 | |||
| ≥70 | 1.35 (0.73–2.52) | 0.34 | |||
| Sex | |||||
| Female | Reference | ||||
| Male | 1.37 (0.93–2.03) | 0.11 | |||
| Smoking status | |||||
| No | Reference | ||||
| Yes | 1.23 (0.80–1.87) | 0.34 | |||
| BMI (kg/m2) | |||||
| <24 | Reference | ||||
| ≥24 | 1.13 (0.76–1.67) | 0.56 | |||
| Primary site | |||||
| Right upper lobe | Reference | ||||
| Right middle lobe | 0.98 (0.47–2.06) | 0.96 | |||
| Right lower lobe | 1.06 (0.58–1.93) | 0.86 | |||
| Left upper lobe | 1.53 (0.92–2.56) | 0.10 | |||
| Left lower lobe | 1.39 (0.77–2.50) | 0.28 | |||
| Size | |||||
| ≤1 cm | Reference | Reference | |||
| >1–2 cm | 2.98 (0.92–9.59) | 0.07 | 2.55 (0.79–8.23) | 0.12 | |
| >2–3 cm | 5.67 (1.77–18.13) | 0.003 | 3.57 (1.10–11.55) | 0.03 | |
| T classification | |||||
| T1 | Reference | Reference | |||
| T2 | 2.71 (1.69–4.35) | <0.001 | 1.80 (1.10–2.96) | 0.02 | |
| Differentiation | |||||
| Well differentiated | Reference | Reference | |||
| Moderately differentiated | 3.78 (1.72–8.32) | 0.001 | 3.07 (1.38–6.81) | 0.006 | |
| Poorly differentiated | 7.54 (3.38–16.83) | <0.001 | 5.00 (2.19–11.39) | <0.001 | |
| Adjuvant chemotherapy | |||||
| No | Reference | Reference | |||
| Yes | 2.59 (1.66–4.06) | <0.001 | 1.79 (1.12–2.85) | 0.01 | |
| Station 2 dissection | |||||
| No | Reference | Reference | |||
| Yes | 0.67 (0.45–0.99) | 0.04 | 0.83 (0.35–1.95) | 0.67 | |
| Station 3 dissection | |||||
| No | Reference | ||||
| Yes | 0.97 (0.55–1.72) | 0.93 | |||
| Station 4 dissection | |||||
| No | Reference | Reference | |||
| Yes | 0.62 (0.41–0.93) | 0.02 | 0.69 (0.38–1.27) | 0.23 | |
| Station 5 dissection | |||||
| No | Reference | Reference | |||
| Yes | 1.43 (0.96–2.13) | 0.08 | 0.91 (0.40–2.06) | 0.81 | |
| Station 6 dissection | |||||
| No | Reference | ||||
| Yes | 1.32 (0.86–2.05) | 0.21 | |||
| Dissected station 2 LNs | |||||
| ≤2 | Reference | ||||
| >3 | 0.85 (0.55–1.32) | 0.48 | |||
| Station 8 dissection | |||||
| No | Reference | Reference | |||
| Yes | 0.54 (0.31–0.96) | 0.04 | 0.46 (0.26–0.80) | 0.007 | |
| Station 9 dissection | |||||
| No | Reference | ||||
| Yes | 1.32 (0.88–1.99) | 0.18 | |||
| Station 10 dissection | |||||
| No | Reference | Reference | |||
| Yes | 1.68 (0.92–3.07) | 0.09 | 1.53 (0.83–2.82) | 0.17 | |
| Station 11 dissection | |||||
| No | Reference | ||||
| Yes | 1.00 (0.61–1.63) | >0.99 | |||
| Station 12 dissection | |||||
| No | Reference | ||||
| Yes | 0.85 (0.57–1.26) | 0.42 | |||
| Station 13 dissection | |||||
| No | Reference | Reference | |||
| Yes | 0.45 (0.22–0.93) | 0.03 | 0.42 (0.20–0.86) | 0.02 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; LN, lymph node.
Table 3
| Characteristics | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||
| <50 | Reference | Reference | |||
| 50–59 | 1.92 (0.63–5.89) | 0.25 | 1.58 (0.51–4.88) | 0.43 | |
| 60–69 | 1.42 (0.46–4.40) | 0.55 | 1.13 (0.36–3.54) | 0.83 | |
| ≥70 | 5.66 (1.89–16.93) | 0.002 | 3.52 (1.15–10.76) | 0.03 | |
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 2.56 (1.40–4.67) | 0.002 | 2.49 (1.35–4.60) | 0.004 | |
| Smoking status | |||||
| No | Reference | ||||
| Yes | 1.47 (0.80–2.71) | 0.22 | |||
| BMI (kg/m2) | |||||
| <24 | Reference | ||||
| ≥24 | 1.02 (0.56 - 1.84) | 0.96 | |||
| Primary site | |||||
| Right upper lobe | Reference | ||||
| Right middle lobe | 1.99 (0.78–5.07) | 0.15 | |||
| Right lower lobe | 1.23 (0.48–3.13) | 0.66 | |||
| Left upper lobe | 1.64 (0.73–3.66) | 0.23 | |||
| Left lower lobe | 1.7 (0.67–4.32) | 0.27 | |||
| Size | |||||
| ≤1 cm | Reference | Reference | |||
| >1–2 cm | 4.39 (0.59–32.69) | 0.15 | 4.18 (0.55–31.54) | 0.17 | |
| >2–3 cm | 6.55 (0.89–48.48) | 0.07 | 4.45 (0.59–33.57) | 0.15 | |
| T classification | |||||
| T1 | Reference | Reference | |||
| T2 | 1.8 (0.91–3.56) | 0.09 | 1.37 (0.67–2.8) | 0.39 | |
| Differentiation | |||||
| Well differentiated | Reference | Reference | |||
| Moderately differentiated | 1.45 (0.54–3.90) | 0.46 | 1.2 (0.44–3.26) | 0.73 | |
| Poorly differentiated | 4.59 (1.73–12.20) | 0.002 | 3.15 (1.14–8.70) | 0.03 | |
| Adjuvant chemotherapy | |||||
| No | Reference | ||||
| Yes | 0.81 (0.34–1.92) | 0.63 | |||
| Station 2 dissection | |||||
| No | Reference | ||||
| Yes | 0.63 (0.35–1.13) | 0.12 | |||
| Station 3 dissection | |||||
| No | Reference | ||||
| Yes | 1.39 (0.69–2.82) | 0.36 | |||
| Station 4 dissection | |||||
| No | Reference | ||||
| Yes | 0.6 (0.32–1.11) | 0.10 | |||
| Station 5 dissection | |||||
| No | Reference | ||||
| Yes | 1.34 (0.73–2.43) | 0.34 | |||
| Station 6 dissection | |||||
| No | Reference | ||||
| Yes | 1.33 (0.70–2.54) | 0.38 | |||
| Dissected station 2 LNs | |||||
| ≤2 | Reference | ||||
| >3 | 0.89 (0.47–1.70) | 0.73 | |||
| Station 8 dissection | |||||
| No | Reference | Reference | |||
| Yes | 0.38 (0.15–0.96) | 0.04 | 0.33 (0.13–0.85) | 0.02 | |
| Station 9 dissection | |||||
| No | Reference | ||||
| Yes | 0.95 (0.52–1.75) | 0.88 | |||
| Station 10 dissection | |||||
| No | Reference | ||||
| Yes | 1.42 (0.60–3.36) | 0.43 | |||
| Station 11 dissection | |||||
| No | Reference | ||||
| Yes | 0.94 (0.47–1.87) | 0.86 | |||
| Station 12 dissection | |||||
| No | Reference | ||||
| Yes | 0.92 (0.49–1.73) | 0.79 | |||
| Station 13 dissection | |||||
| No | Reference | ||||
| Yes | 0.43 (0.13–1.38) | 0.16 | |||
OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; LN, lymph node.
Subgroup analyses and sensitivity analyses
To test whether the effect of 8LND was consistent within different patient subgroups, we performed subgroup analyses with interaction testing. We found that apart from the age <50 years subgroup, 8LND appeared to be associated with improved PFS and OS across all subgroups, although the differences were not significant in some subgroups (Figures S1,S2). In certain subgroups, the HR could not be evaluated because all patients in 8LND+ group were still alive until the last follow-up. Significant interactions between 8LND and sex on PFS and OS were found (P for interaction: 0.01 for PFS and 0.04 for OS). 8LND was associated with significant survival benefit in male patients [PFS: 0.16 (0.05–0.52), P=0.002; OS: 0.09 (0.01–0.66), P=0.02] but not in female patients [PFS: 0.94 (0.45–1.95), P=0.86; OS: 1.00 (0.28–3.57), P>0.99]. For other variables, no significant interactions on PFS or OS were found. But significant PFS benefit was found in smokers [0.19 (0.04–0.80), P=0.02], in patients with BMI <24 kg/m2 [0.26 (0.10–0.65), P=0.004], in T2 patients [0.41 (0.21–0.78), P=0.007], and in patients who did not receive adjuvant chemotherapy [0.46 (0.24–0.90), P=0.02]. And for patients with a poorly differentiated tumor, although the effect of 8LND on PFS was not significant [0.36 (0.12–1.06), P=0.06], the effect of 8LND on OS was significant [0.11 (0.01–0.84), P=0.03].
In the sensitivity analyses, in most cases, the effect of 8LND on PFS and OS were unchanged. After excluding male patients, smoking patients, patients whose BMI <24 kg/m2, T2 patients, or patients with a poorly differentiated tumor, the evaluated HR significantly changed, but the HRs were still ≤1 (Table S3).
Discussion
Lung adenocarcinoma of small size is more and more common in the past decades (7,8). In this study, we investigated the effect of 8LND on the prognosis of T≤3 cmN0M0 lung adenocarcinoma. Our result demonstrated that 8LND was associated with better PFS, OS, CSS both before and after PSM, especially in male patients, smokers, patients with a pT2 tumor (≤3 cm), and patients with a poorly differentiated tumor.
8LND is recommended by the NCCN guideline of non-small cell lung cancer, but in real world clinical practice, the dissection rate of station 8 is low. In the study by Edwards et al., the reported rates of 8LND were 18% in left-side-tumor patients and 21% in right-side-tumor patients (5). In the study by van der Woude et al., the rates of 8LND were less than 20% in patients with upper/middle lobe located lung cancer, and around 50% in patients with lower lobe located lung cancer (11). Yoshimura et al. reported the detailed LN dissection information of 12 patients with LN metastasis in their study, and only two patients underwent 8LND (12). In our study, the rate of 8LND was 21.59%. These evidences showed that the inadequate dissection of station 8 LN is very common.
We tried to explain the reasons behind the low rate of 8LND. One might be that the reported metastasis rate of station 8 is lower as compared to other N2 stations recommended to be dissected (13,14). The other might be that a randomized clinical trial by Darling et al. found that in N0/N1 patients without LN metastasis to station 2R, 4R, 7, 10 (right-sided tumor) or to station 5, 6, 7, 10 (left-sided tumor), further LN dissection of station 8, 9 did not bring about significant survival benefit (15). This high-grade evidence has been included into the NCCN guideline, and the NNCN guideline cautiously states “systematic LN sampling is appropriate during pulmonary resection; one or more nodes should be sampled from all mediastinal stations” (4). The difference in the effect of 8LND on survival between their study and ours might be that we only focused on adenocarcinoma patients but the proportion of adenocarcinoma patients in their study was only 41.3% (15). Studies have reported that adenocarcinoma was a risk factor for N2 metastasis (9,16,17). In the study by Bille et al., they enrolled cT1–2aN0M0 lung cancer patients and found that adenocarcinoma was a risk factor for occult pN2 disease (16). Several studies have also found that adenocarcinoma was a risk factor for LN micrometastasis, including N2 stations (18-22). In the study by Rena et al., they found that the rate of LN micrometastasis was 26.3% (10/38) in pT1–2N0M0 adenocarcinoma patients, while 5.6% (2/36) in squamous cell carcinoma patients (19). These findings might explain the survival benefit of 8LND in adenocarcinoma patients in our study.
In our study, we only included T≤3 cmN0M0 patients. The favorable effect of 8LND might result from the therapeutic effect of the dissection of potential micrometastasis (23,24) and the precise staging and adequate postoperative treatment (4). The 8LND might remove the lymphatic micrometastases and isolated tumor cells, thus preventing the local recurrence and distant metastasis (17). In our study, the PFS of patients who received 8LND was better than that of patients who did not. This result supported the effect of 8LND on removing lymphatic micrometastases. Previous studies have reported that male, smoking, and poor differentiation are risk factors for metastasis in lung cancer patients (25-28). Consistently, in our study, the subgroup analyses showed that 8LND brought significant survival benefit in male patients, smokers, and patients with a poorly differentiated tumor, but not in female patients, non-smokers, or patients with a well differentiated tumor. Studies have found that in N0 lung cancer patients, dissection of more LN stations and more LNs are associated with better survival (29,30). These findings together indicated the therapeutic effect of LN dissection in N0 patients.
According to the American Joint Committee on Cancer staging system and NCCN guideline, adequate LN dissection and postoperative pathological assessment are very important for precise staging of the tumor and affect the determination of postoperative treatment strategy (4,31,32). More LN dissection could help with more accurate pathological staging. Stage of patients who did not receive adequate LN dissection might be underestimated and thus the adjuvant treatment of those patients might be inadequate (33). In our study, patients who did not received 8LND might suffer inadequate postoperative treatment due to the underestimated stage, resulting in the survival difference as compared to patients who received 8LND.
Based on the lymph drainage regularity of LNs, lobe-specific N2 LN dissection has been proposed and conducted in clinical practice for small early-stage lung cancer (34,35). The rate of 8LND was higher in tumor of lower lobe than upper lobe in the study by van der Woude et al. (11). In our study, the rate of 8LND in tumors of upper lobe, middle lobe, and lower lobe was similar. In the subgroup analyses, there was a trend that 8LND was associated with better PFS in patients with a tumor in right upper or lower lobe. No benefit of 8LND was found in patients with a tumor in left upper/lower lobe with regard to PFS or OS. Survival benefit of 8LND was also found in T2 patient with regard to PFS and OS. And no survival benefit of 8LND was found in T1 patients. These results indicated that 8LND might improve the survival of patients with right-sided tumor or T2 tumor, but more studies are needed to investigate the effect.
Although our study was the first to investigate the effect of 8LND in T≤3 cmN0M0 lung adenocarcinoma, there were still some limitations in our study. First, our study was a retrospective study, thus selection bias and potential unobserved confounding effect might exist in our study, though we utilized PSM to balance the clinicopathological characteristics between groups. We expect better-designed prospective randomized studies to validate our finding. Second, as the subject in our study was T≤3 cmN0M0 (T1, T2) lung adenocarcinoma patients and the follow-up duration was not that long, the number of the event of death was small, and further follow-up should be continued to investigate the long-term effect of 8LND on the prognosis of those patients. Third, due to the small number of the event of death and recurrence, there was a probability of overfitting in the multivariable analysis, though the result of multivariable analysis was in consistent with the result of survival analysis both before and after PSM in our study.
Conclusions
Our study found that 8LND could improve the PFS, OS, CSS in T≤3 cmN0M0 lung adenocarcinoma patients. We recommend that 8LND should be regularly performed in those patients, especially in male, smokers, patients with a pT2 tumor (≤3 cm), and patients with a poorly differentiated tumor.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-184/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-184/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-184/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-184/coif). L.L. reports that this work was supported by the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21002). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of West China Hospital (No. 2023-1879), and the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg 2013;96:1178-89. [Crossref] [PubMed]
- Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines. Accessed May 26th, 2023. Available online: https://www.nccn.org/guidelines/category_1
- Edwards T, Balata H, Elshafi M, et al. Adequacy of Intraoperative Nodal Staging during Surgical Resection of NSCLC: Influencing Factors and Its Relationship to Survival. J Thorac Oncol 2017;12:1845-50. [Crossref] [PubMed]
- Evison M, Edwards T, Balata H, et al. Prevalence of nodal metastases in lymph node stations 8 & 9 in a large UK lung cancer surgical centre without routine pre-operative EUS nodal staging. Lung Cancer 2018;115:127-30. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Zhang Y, Jheon S, Li H, et al. Results of low-dose computed tomography as a regular health examination among Chinese hospital employees. J Thorac Cardiovasc Surg 2020;160:824-831.e4. [Crossref] [PubMed]
- Liang RB, Yang J, Zeng TS, et al. Incidence and Distribution of Lobe-Specific Mediastinal Lymph Node Metastasis in Non-small Cell Lung Cancer: Data from 4511 Resected Cases. Ann Surg Oncol 2018;25:3300-7. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- van der Woude L, Wouters MWJM, Hartemink KJ, et al. Completeness of lymph node dissection in patients undergoing minimally invasive- or open surgery for non-small cell lung cancer: A nationwide study. Eur J Surg Oncol 2021;47:1784-90. [Crossref] [PubMed]
- Yoshimura R, Deguchi H, Tomoyasu M, et al. Assessment of lymph node metastasis of ≤20 mm non-small cell lung cancer originating from superior segment compared to basal segment. Thorac Cancer 2023;14:304-8. [Crossref] [PubMed]
- Fang L, Xu J, Ye B, et al. Is lobe specific lymph node dissection adequate for cN0-1 non-small cell lung cancer? J Cardiothorac Surg 2020;15:46. [Crossref] [PubMed]
- Fang L, Wang L, Wang Y, et al. Predictors and survival impact of station 4L metastasis in left non-small cell lung cancer. J Cancer Res Clin Oncol 2019;145:1313-9. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Verhagen AF, Bulten J, Shirango H, et al. The clinical value of lymphatic micrometastases in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:1201-5. [Crossref] [PubMed]
- Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 2004;45:580-6. [Crossref] [PubMed]
- Rena O, Carsana L, Cristina S, et al. Lymph node isolated tumor cells and micrometastases in pathological stage I non-small cell lung cancer: prognostic significance. Eur J Cardiothorac Surg 2007;32:863-7. [Crossref] [PubMed]
- Liu H, Ye YK, Li GM, et al. Diagnosis of lymph node micrometastasis at the pN0 stage of lung adenocarcinoma using a combination of markers. Genet Mol Res 2014;13:5594-600. [Crossref] [PubMed]
- Ishiwa N, Ogawa N, Shoji A, et al. Correlation between lymph node micrometastasis and histologic classification of small lung adenocarcinomas, in considering the indication of limited surgery. Lung Cancer 2003;39:159-64. [Crossref] [PubMed]
- Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. [Crossref] [PubMed]
- Osaki T, Oyama T, Gu CD, et al. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected stage I non-small-cell lung cancer. J Clin Oncol 2002;20:2930-6. [Crossref] [PubMed]
- Gu CD, Osaki T, Oyama T, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: impact on recurrence and Survival. Ann Surg 2002;235:133-9. [Crossref] [PubMed]
- Ding N, Mao Y, Gao S, et al. Predictors of lymph node metastasis and possible selective lymph node dissection in clinical stage IA non-small cell lung cancer. J Thorac Dis 2018;10:4061-8. [Crossref] [PubMed]
- Hu S, Luo M, Li Y. Machine Learning for the Prediction of Lymph Nodes Micrometastasis in Patients with Non-Small Cell Lung Cancer: A Comparative Analysis of Two Practical Prediction Models for Gross Target Volume Delineation. Cancer Manag Res 2021;13:4811-20. [Crossref] [PubMed]
- Yu Y, Zhao Q, He XP, et al. Signal transducer and activator of transcription 3 overexpression promotes lymph node micrometastasis in early-stage non-small cell lung cancer. Thorac Cancer 2018;9:516-22. [Crossref] [PubMed]
- Song Q, Shang J, Zhang C, et al. Impact of the homogeneous and heterogeneous risk factors on the incidence and survival outcome of bone metastasis in NSCLC patients. J Cancer Res Clin Oncol 2019;145:737-46. [Crossref] [PubMed]
- Samayoa AX, Pezzi TA, Pezzi CM, et al. Rationale for a Minimum Number of Lymph Nodes Removed with Non-Small Cell Lung Cancer Resection: Correlating the Number of Nodes Removed with Survival in 98,970 Patients. Ann Surg Oncol 2016;23:1005-11. [Crossref] [PubMed]
- Zhang Z, Wang A, Zhan Z, et al. T1aN0M0 and T1bN0M0 non-small cell lung cancer: a retrospective study of the prognosis. Thorac Cardiovasc Surg 2014;62:109-16. [PubMed]
- Osarogiagbon RU, Van Schil P, Giroux DJ, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Overview of Challenges and Opportunities in Revising the Nodal Classification of Lung Cancer. J Thorac Oncol 2023;18:410-8. [Crossref] [PubMed]
- Smeltzer MP, Faris NR, Ray MA, et al. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80-7. [Crossref] [PubMed]
- Zhou D, Yue D, Zhang Z, et al. Prognostic significance of 4R lymph node dissection in patients with right primary non-small cell lung cancer. World J Surg Oncol 2022;20:222. [Crossref] [PubMed]
- Wo Y, Li H, Chen Z, et al. Lobe-Specific Lymph Node Dissection May be Feasible for Clinical N0 Solid-Predominant Part-Solid Lung Adenocarcinoma With Solid Component Diameter ≤2 cm. Clin Lung Cancer 2023;24:437-44. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]


