
Intracranial response to capmatinib after progression on crizotinib in a patient with MET exon 14 skipping non-small cell lung cancer—a case report
Highlight box
Key findings
• A non-small cell lung cancer (NSCLC) patient with isolated central nervous system (CNS) metastasis and MET exon 14 skipping mutation D1028N underwent crizotinib treatment (22+3 months), showing complete extracerebral response but later developing multiple hemorrhagic CNS metastases.
• The change in therapy to capmatinib for a duration of six months resulted in remission in one metastasis and radionecrosis in others.
• Retrospective DNA-based hybrid-capture-next generation sequencing (NGS) identified a bypass PIK3CA mutation, potentially contributing to treatment resistance.
What is known and what is new?
• Capmatinib is a highly selective and potent MET tyrosine kinase inhibitor (TKI); however, addressing intracranial disease progression is challenging due to resistance issues.
• We report a patient with a bypass PIK3CA resistance mutation detected with subsequent, comprehensive and comparative molecular analyses.
What is the implication, and what should change now?
• Thorough and subsequent NGS analyses are required to detect the complex resistance to capmatinib.
• Clinical trials investigating the capmatinib in METex14 NSCLC with CNS-only progression on another MET TKI are warranted.
Introduction
Capmatinib is a highly selective and potent MET tyrosine kinase inhibitor (TKI) that has demonstrated remarkable anti-tumor efficacy in patients with MET exon 14 skipping (METex14) non-small cell lung cancer (NSCLC). Based on these promising results, capmatinib has been approved for the treatment of METex14-positive NSCLC (1,2). However, the efficacy of capmatinib in patients with NSCLC who have progressed on other MET TKIs is not well established. To address this issue, an ongoing prospective study of capmatinib is being conducted in patients with NSCLC and MET alterations previously treated with MET TKI (3). Brain metastases frequently occur in patients with NSCLC and are associated with reduced survival rates (4,5). Patients with brain metastases have limited treatment options, which usually involve radiation therapy or stereotactic radiosurgery (SRS), with the latter being considered a less toxic alternative (5). More recently, there has been a shift towards considering TKIs that can penetrate the central nervous system (CNS) to regain control of brain disease in patients with an actionable oncogenic driver (5). Capmatinib, a selective MET inhibitor, has shown anti-tumor efficacy in METex14 NSCLC and demonstrated intracranial activity in a limited set of patients (2). There is evidence indicating that capmatinib may elicit a leptomeningeal response in a patient with METex14 NSCLC who had progressed on crizotinib (6). Other reports have suggested intercranial efficacy of capmatinib; however, more data are required (7,8). Besides the pharmacokinetic obstacles mentioned, addressing the challenges associated with targeted treatment may be necessary throughout the course of treatment. Like other TKIs (9,10), both crizotinib and capmatinib could potentially encounter resistance mechanisms, encompassing both driver-dependent (11,12) and driver-independent pathways (13,14).
In this case report, we describe a patient with advanced METex14 NSCLC who experienced CNS-relapse with multiple progressing lesions after a complete surgical resection and remission of the lung tumor with crizotinib treatment. We report on the observed intracranial activity of capmatinib on one of the crizotinib-resistant brain lesions and discuss the potential implications of this finding for the use of capmatinib in the treatment of brain metastases in patients with METex14 NSCLC who have progressed on other TKIs. We present this case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-769/rc).
Case presentation
The patient was a 77-year-old female who had never smoked and presented with shortness of breath, pain and vertigo, with unknown family history of cancer and without significant co-morbidities. She was diagnosed with metastatic Union for International Cancer Control (UICC) stage IV lung carcinoma of the left lung. The patient’s therapeutic timeline is summarized in Figure 1. At the time of diagnosis, the patient had one isolated CNS metastasis. Molecular testing using next generation sequencing (NGS) revealed that the patient had METex14 [p.D1028N, NM_001127500.3:c.3082G>A, NM_001127500.3:p.Asp1028Asn; variant allelic frequency (VAF): 48%] with concurrent amplifications of the cyclin-dependent kinase-4 (CDK4) and the mouse double minute 2 homolog (MDM2). Additionally, the patient had a programmed death-ligand 1 tumor proportion score of 2%.

Following the diagnosis of METex14 lung adenocarcinoma, the patient underwent resection of the CNS metastasis, adjuvant irradiation of the tumor bed to 40 Gy, and induction therapy with carboplatin area under curve 6 mg/d intravenous on day 1 and paclitaxel 200 mg/m2 on day 1, once every 3 weeks for 2 cycles. As a result, the patient experienced complete remission in the CNS, but there was no change observed in the thoracic lesion.
As crizotinib was the sole drug available in Germany at that time for treating patients positive for METex14, the patient was subsequently treated with crizotinib 250 mg [carboplatin (CBDCA): area under the curve (AUC) 6.0] twice daily for 39 days, which resulted in regression of the lung tumor. The tumor was subsequently resected, as described by Junker et al. (15) (Figure 2). Nevertheless, the patient exhibited elevated liver enzyme levels during the induction therapy.

After approximately 6 months of remission of the lung tumor, the patient experienced a decrease in CNS edema but subsequently relapsed in the CNS and developed multiple hemorrhagic CNS metastases, with notable involvement of the right cerebellar region and compression of the fourth ventricle. Given the location of this specific tumor, surgical intervention was likely to result in speech impairment. Consequently, the patient refused to undergo surgery, leading to the administration of SRS, which resulted in the eradication of some of the CNS metastases.
The patient resumed treatment with crizotinib approximately 22 months after the start of induction, which resulted in a complete response outside the brain. Unfortunately, despite this response, there was a progression of all CNS tumors with significant progression of the left precentral metastasis during the first 3 months of resumed treatment. Due to the lack of response to crizotinib, it was decided to discontinue the treatment. Instead, capmatinib at a dose of 400 mg (CBDCA: AUC 6.0) twice daily was started. Initially, no side effects were observed.
The patient underwent magnetic resonance imaging (MRI) scans 1 month after starting capmatinib treatment, which showed stable disease of the left parieto-occipital metastasis and improvement of the CNS edema. Continued shrinkage of the left parietal metastasis and further improvement of the CNS edema were observed after 2 months of treatment, with no progression of the remnants of Aspergillus manifestations in the lung and no suspicion of extracerebral progression (Figure 3).
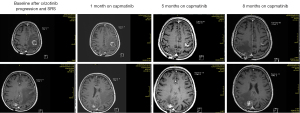
After 5 months of capmatinib treatment, MRI scans revealed remission of the left parietal metastasis and radionecrosis for the other metastases (Figure 3).
However, the patient developed grade 2 peripheral edema after 6 months of treatment, which led to a reduction in capmatinib dose to 200 mg bid. Subsequent MRI brain scans showed regression of the left parieto-occipital metastasis, but disease progression was noted for the right occipital metastasis (Figure 3).
Despite this, capmatinib therapy persisted, and following a span of 10 months under capmatinib treatment, the patient underwent surgical intervention for the right occipital metastasis. Histological examination of paraffin-embedded or cryopreserved tumor specimens was executed to ascertain the existence of viable tumor cells. In conjunction, NGS was conducted to ascertain conceivable mutations that might have arisen as resistance mechanisms against MET TKIs. Utilizing a conventional RNA-based NGS panel to confirm the functional Exon 14 skipping mutation, no discernible resistance-associated mutations were detected within the MET gene or other genetic loci (Archer FusionPlex panel, ArcherDX, Boulder, CO, USA). The nucleic acid isolation protocol followed in this study utilized the Maxwell 48 system (Promega Corp., Madison, WI, USA) according to the manufacturer’s instructions. Likewise, the manufacturer’s guidelines were observed for the RNA-based analysis; we utilized 2 ng of RNA isolated at a concentration of 12.5 ng/µL, with an average fragment length of 125 nucleotides, followed by transcription to generate 250 ng of cDNA. No quality issues were observed during nucleic acid isolation, hybrid capture, and sequencing processes.
However, a subsequent retrospective examination of the identical formalin fixed, paraffin embedded (FFPE) material employing an expanded, DNA-based hybrid capture NGS panel [Supplementary file (Appendix 1), Assay based on hybrid capture enrichment XT HS2 chemistry by Agilent Technologies, Santa Clara, CA, USA] corroborated the initial MET driver mutation (VAF 10%), detected alterations in copy numbers within the MDM2 and CDK4 genes, and unveiled an extra activating H1047R mutation in the PIK3CA gene (NM_006218.4:c.3140A>G; NM_006218.4:p.His1047Arg, VAF 5%). For the DNA hybrid capture analysis, 100 ng of fragmented DNA was employed as input, with a hybridization amount of 1,000 ng.
The patient deceased 66 months after the initial diagnosis in September 2022.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The study was approved by the Ethics Committee of University of Oldenburg (FP-Project 2014-I). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The present case report highlights the challenges in therapy of intracranial disease progression in patients with METex14 NSCLC treated with MET TKIs. In such cases, limited CNS drug penetration could be a potential cause of disease progression, which can be addressed by switching to another MET TKI with higher penetration, such as capmatinib that previously had shown intercranial response (7,8).
Our case report showed that capmatinib demonstrated intracranial activity on a lesion resistant to crizotinib and not responding to SRS. However, a new CNS lesion developed and progressed on capmatinib, suggesting the need for better understanding of the mechanism of resistance to different TKIs. Subsequent and thorough genetic testing of different CNS lesions or liquid biopsies may be informative in this regard (3).
Another challenge pertains to the identification of genetic alterations occurring during CNS progression on crizotinib therapy. Similar to the scenario in NSCLC with epidermal growth factor receptor (EGFR) mutations treated with osimertinib (9,10), a diverse range of potential resistance mechanisms to crizotinib have been reported in the scientific literature, which could arise from alterations linked to both driver-dependent (11,12) and driver-independent, bypass resistance pathways, including activation of EGFR, KIT and the MAPK pathway (13,14). Previously, co-occurring activating mutations of PIK3CA and MET-TKI triggered PI3K pathway activation have been recurrently documented as contributing factors in the development of resistance against MET-TKI treatment (16-18). Furthermore, preclinical investigations have indicated that a therapeutic opportunity may exist by combining MET-TKI agents and inhibitors targeting PIK3CA (19). Nevertheless, bypass alterations frequently lack direct and approved actionability, thus often omitting their inclusion within the fundamental routine analysis protocol. This omission is exemplified by the contrast between the results obtained from the RNA-based NGS and the detected additional PIK3CA mutation in the DNA-based panel [Supplementary file (Appendix 1)]. Our results show that meticulously devised, comprehensive DNA-based HC-NGS possesses the capacity to discern primary driver mutations (MET D1028N), foundational alterations (MDM2 and CDK4 amplifications), as well as subsequent acquired resistance modifications (PIK3CA H1047R). The PIK3CA mutation most probably emerged as a resistance mechanism to capmatinib, evident in the metastasis which progressed despite capmatinib treatment and subsequently required resection.
There is a need for clinical trials investigating the efficacy of capmatinib in METex14 NSCLC with CNS-only progression on another MET TKI. This will help establish the optimal sequencing of MET TKIs in the treatment of intracranial disease progression and identify biomarkers that can predict response to therapy (3). Furthermore, it is important to explore the combination of MET TKIs with other treatment modalities, such as next generation MET-TKIs or immunotherapy, to improve outcomes in patients with advanced METex14 NSCLC.
Conclusions
This current case report imparts significant insights regarding the complexities associated with addressing intracranial disease progression within individuals afflicted by METex14 NSCLC. Transitioning to a different MET-TKI characterized by enhanced CNS permeability, exemplified by capmatinib, emerges as a potentially viable avenue in these circumstances. Nonetheless, additional investigation is imperative to unravel the intricacies of resistance mechanisms through the utilization of sophisticated diagnostic assays such as DNA-based HC-NGS across diverse TKIs. Moreover, the pursuit of optimal therapeutic strategies for this specific patient cohort necessitates further inquiry and exploration.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-769/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-769/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-769/coif). M.F. reports receiving consulting and lecture fees from Roche, AstraZeneca, Novartis, Boehringer Ingelheim, and Pfizer. M.T. reports receiving honoraria for lectures and presentation for Novartis. F.G. has received consulting fees, payment or honoraria for lectures, presentations, speakers’ bureaus and manuscript writing from Roche, Pfizer, MSD, Takeda, BMS, Abbvie, Sanofi, AMGEN and Novartis. He has also been supported by Pierre Fabre, Roche, Abbvie, Daiichi Sankyo, AstraZeneca and Takeda for attending meetings or travelling. He is a member of data safety monitoring board and advisory board in AstraZeneca, Roche, Pfizer, Merck and Takeda. His institution received payment for expert testimony by Merck, MSD, Roche, Lilly, Janssen, CSL, Behring, BEigene, AOP, Jazz, Pfizer, Takeda, Novartis, AstraZeneca, BMS, Abbvie, AMGEN, Incyte, Sobi and GSK for expert meetings in NSCLC, Oncology and Hematology Wrap ups. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The study was approved by the Ethics Committee of University of Oldenburg (FP-Project 2014-I). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- US Food and Drug Administration. TABRECTA (capmatinib) Prescribing Information; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf, accessed at: November 3, 2023.
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Dagogo-Jack I, Moonsamy P, Gainor JF, et al. A Phase 2 Study of Capmatinib in Patients With MET-Altered Lung Cancer Previously Treated With a MET Inhibitor. J Thorac Oncol 2021;16:850-9. [Crossref] [PubMed]
- He J, Wang X, Xiao R, et al. Risk factors for brain metastases from non-small-cell lung cancer: A protocol for observational study. Medicine (Baltimore) 2021;100:e24724. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Cravero P, Vaz N, Ricciuti B, et al. Leptomeningeal Response to Capmatinib After Progression on Crizotinib in a Patient With MET Exon 14-Mutant NSCLC. JTO Clin Res Rep 2020;1:100072. [Crossref] [PubMed]
- Paik PK, Goyal RK, Cai B, et al. Real-world outcomes in non-small-cell lung cancer patients with MET Exon 14 skipping mutation and brain metastases treated with capmatinib. Future Oncol 2023;19:217-28. [Crossref] [PubMed]
- Illini O, Fabikan H, Swalduz A, et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): a retrospective analysis from an early access program. Ther Adv Med Oncol 2022;14:17588359221103206. [Crossref] [PubMed]
- Jóri B, Schatz S, Kaller L, et al. Comparison of Resistance Spectra after First and Second Line Osimertinib Treatment Detected by Liquid Biopsy. Cancers (Basel) 2021;13:2861. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Heist RS, Sequist LV, Borger D, et al. Acquired Resistance to Crizotinib in NSCLC with MET Exon 14 Skipping. J Thorac Oncol 2016;11:1242-5. [Crossref] [PubMed]
- Jin W, Shan B, Liu H, et al. Acquired Mechanism of Crizotinib Resistance in NSCLC with MET Exon 14 Skipping. J Thorac Oncol 2019;14:e137-9. [Crossref] [PubMed]
- Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol 2016;27:iii42-50. [Crossref] [PubMed]
- Lin JJ, Choudhury NJ, Yoda S, et al. Spectrum of Mechanisms of Resistance to Crizotinib and Lorlatinib in ROS1 Fusion-Positive Lung Cancer. Clin Cancer Res 2021;27:2899-909. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]
- Nisa L, Häfliger P, Poliaková M, et al. PIK3CA hotspot mutations differentially impact responses to MET targeting in MET-driven and non-driven preclinical cancer models. Mol Cancer 2017;16:93. [Crossref] [PubMed]
- Fujino T, Suda K, Mitsudomi T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges. Lung Cancer (Auckl) 2021;12:35-50. [Crossref] [PubMed]
- Rotow JK, Gui P, Wu W, et al. Co-occurring Alterations in the RAS-MAPK Pathway Limit Response to MET Inhibitor Treatment in MET Exon 14 Skipping Mutation-Positive Lung Cancer. Clin Cancer Res 2020;26:439-49. [Crossref] [PubMed]
- Jamme P, Fernandes M, Copin MC, et al. Alterations in the PI3K Pathway Drive Resistance to MET Inhibitors in NSCLC Harboring MET Exon 14 Skipping Mutations. J Thorac Oncol 2020;15:741-51. [Crossref] [PubMed]


