
Durvalumab plus platinum-etoposide chemotherapy for extensive-stage small cell lung cancer: a retrospective real-world study
Highlight box
Key findings
• This retrospective study of the efficacy and safety of durvalumab plus platinum-etoposide (PE) in extensive-stage small cell lung cancer (ES-SCLC) in real-world clinical practice showed the median progression-free survival and the median overall survival (OS) comparable to the CASPIAN study.
What is known and what is new?
• Durvalumab plus PE significantly improved OS in ES-SCLC over PE alone in strict inclusion criteria.
• This is the first study to purely evaluate the therapeutic efficacy and feasibility of durvalumab plus PE as primary treatment of ES-SCLC in real-world clinical practice.
What is the implication, and what should change now?
• Durvalumab plus PE should also be considered in patients who are ineligible for clinical trials, such as the elderly or poor performance status.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases (1). About two-thirds of patients with a diagnosis of SCLC have extensive-stage SCLC (ES-SCLC) because of its rapid progression (1). Platinum (cisplatin or carboplatin)-etoposide (PE), which has long been the standard therapy for ES-SCLC, has a favorable response rate of 60–70%, but most patients relapse within 6 months owing to drug resistance (2). An effective therapy for treating recurrences has yet to be found; thus, long-term survival in SCLC is generally thought to be difficult to achieve (3).
Immune checkpoint inhibitors (ICIs) have modified the standard of care for SCLC, which had been unchanged for about 30 years. The CASPIAN trial demonstrated that durvalumab plus PE improved overall survival (OS) in SCLC significantly more than PE alone (4). Atezolizumab plus carboplatin and etoposide was also found to have comparable treatment efficacy in the IMpower133 trial (5,6). However, the outcomes of these trials may differ from the actual, clinical efficacy observed in the real-world setting because their strict inclusion criteria exclude patients with poor performance status (PS), active brain metastases, problematic comorbidities or organ function problems. Furthermore, only 13% of the patients enrolled in the CASPIAN trial were Asian, mostly of Japanese descent, and the information pertaining to Asian patients was inadequate (4,7). The atezolizumab regimen has demonstrated some efficacy and safety in ineligible patients, but there is a dearth of real-world data (RWD) on the durvalumab regimen (8-12). The present study therefore aimed to assess the real-world outcomes of durvalumab plus PE for ES-SCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/rc).
Methods
Study design and patients
The present, monocentric, retrospective cohort study was conducted at the Tokyo Metropolitan Cancer and Infectious Diseases Center of Komagome Hospital in Japan. Between September 2020 and February 2023, patients who received durvalumab plus PE as the first-line treatment for ES-SCLC or as treatment for recurrent, limited-stage SCLC (LS-SCLC) were enrolled. Among the patients with recurrent LS-SCLC, only those receiving durvalumab plus PE as the initial treatment for a relapse after surgery or chemoradiation were included. The primary endpoint was the assessment of therapeutic efficacy [best overall response, disease control rate (DCR), progression-free survival (PFS), and OS] and safety of durvalumab plus PE for ES-SCLC. The following patient characteristics were extracted from the patients’ medical records: age, sex, smoking history, PS, clinical stage, details of any platinum regimen, presence of any brain metastasis, presence of any liver metastasis, use of polyethylene glycol conjugated granulocyte-colony stimulating factor (G-CSF), and survival. Elderly patients were defined as 71 years of age or older and PS 2–3 as poor PS. The cutoff date for enrollment was February 28, 2023.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Komagome Hospital, Tokyo, Japan ethics committee (code: 3226) and individual consent for this retrospective analysis was waived. Written informed consent was waived owing to the retrospective nature of this study.
Evaluation
The follow-up period was defined as the interval between the initiation of treatment and the cutoff date. The Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 was used to determine the overall response rate (ORR) and DCR. PFS was defined as the time from the start of treatment to disease progression or death. OS was defined as the time from the start of treatment to death. Patients with continuing therapy without progression at the last follow-up date were censored for PFS at that date. Patients who were alive at the last follow-up were censored for OS. The Common Terminology Criteria for Adverse Events (CTCAEs), version 5.0 was used to evaluate adverse events (AEs).
Statistical analysis
The 95% confidence interval (CI) for the median follow-up period was calculated using the bootstrap method. PFS and OS data were analyzed using Kaplan-Meier estimation, and the survival endpoints were compared using the log-rank test. The hazard ratio was calculated using the log-rank test and Cox regression analysis. Univariate and multivariate analyses with Cox proportional hazard analysis were used to evaluate prognostic factors. P<0.05 was considered to indicate statistical significance. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) Statistics, version 28.
Results
Patient characteristics
The present study enrolled 40 patients, consisting of 39 Japanese and one Myanmarese participant. Table 1 shows the patients’ baseline characteristics. The patients’ median age was 68 years (range, 45–89 years). Most of the patients were male [32 (80.0%)] and had a history of smoking [38 (95.0%)]. Seventeen patients (42.5%) were elderly (age >70 years), and 15 (37.5%) had poor PS (PS 2 or 3). In the total cohort, four patients (10.0%) had LS-SCLC that relapsed after chemoradiotherapy, and five patients (12.5%) had a brain metastasis, of whom four had PS 2 or 3. Among the five patients with brain metastases, two were symptomatic, and one of them underwent tumor resection prior to immunochemotherapy due to poor PS resulting from symptoms of brain metastases. None of the patients underwent radiation therapy before immunochemotherapy. Of the 25 patients who received carboplatin-etoposide treatment, seven patients received etoposide 100 mg/m2, and 18 patients received 80 mg/m2. The carboplatin dosage of all the patients fell in the area under the curve 5 mg/mL/min. Fifteen patients with cisplatin-etoposide received cisplatin 80 mg/m2 and etoposide 100 mg/m2.
Table 1
| Characteristics | Values |
|---|---|
| Age (years) | |
| Median (range) | 68 (45–89) |
| ≤70, n (%) | 23 (57.5) |
| >70, n (%) | 17 (42.5) |
| Sex, n (%) | |
| Male | 32 (80.0) |
| Female | 8 (20.0) |
| Smoking, n (%) | |
| Yes | 38 (95.0) |
| No | 2 (5.0) |
| Performance status, n (%) | |
| 0 | 6 (15.0) |
| 1 | 19 (47.5) |
| 2 | 10 (25.0) |
| 3 | 5 (12.5) |
| Stage, n (%) | |
| IIIC | 1 (2.5) |
| IVA | 14 (35.0) |
| IVB | 21 (52.5) |
| Relapse LS-SCLC | 4 (10.0) |
| Platinum regimen, n (%) | |
| CDDP | 15 (37.5) |
| CBDCA | 25 (62.5) |
| Brain metastases, n (%) | |
| Present | 5 (12.5) |
| Absent | 35 (87.5) |
| Liver metastases, n (%) | |
| Present | 10 (25.0) |
| Absent | 30 (75.0) |
| Use of G-CSF, n (%) | |
| Yes | 15 (37.5) |
| Temporary | 9 (22.5) |
| No | 16 (40.0) |
LS-SCLC, limited-stage small cell lung cancer; CDDP, cisplatin; CBDCA, carboplatin; G-CSF, granulocyte colony stimulating factor.
Efficacy
The median follow-up time was 13.0 months (95% CI: 8.0–22.2 months). The patients underwent computed tomography (CT) at generally 2–3 months intervals to evaluate treatment efficacy. Complete response (CR) was achieved in three (7.5%), a partial response (PR) in 25 (62.5%), stable disease (SD) in three (7.5%), and progressive disease (PD) in four (10.0%) patients (Table 2). Five patients did not receive a response assessment owing either to treatment discontinuation or reaching the cutoff date before treatment evaluation. The ORR was 80.0% (95% CI: 63.1–91.6%), and the DCR was 88.6% (95% CI: 73.3–96.8%) (Table 2). The median PFS was 5.9 months (95% CI: 4.9–6.9), and the median OS was 25.4 months (95% CI: 4.6–46.2) (Figure 1). Fourteen patients had no evidence of disease progression and were continuing treatment at the cutoff date. Table 3 shows the results of the univariate analysis. There was no difference in PFS or OS by age, sex, PS, stage, platinum regimen, presence of brain metastasis or presence of liver metastasis. Kaplan-Meier curves for PFS were similar for patients aged 70 years or younger and those older than 70 years; this was also true for patients with PS 0 or 1 versus those with PS 2 or 3 (Figure 2). Multivariate analysis also showed no factors affecting PFS and OS (Table 4).
Table 2
| Therapeutic efficacy | Values |
|---|---|
| Response, n (%) | |
| CR | 3 (7.5) |
| PR | 25 (62.5) |
| SD | 3 (7.5) |
| PD | 4 (10.0) |
| NE | 5 (12.5) |
| ORR (%) (95% CI) | 80.0 (63.1–91.6) |
| DCR (%) (95% CI) | 88.6 (73.3–96.8) |
DCR, disease control rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, overall response rate; CI, confidence interval.

Table 3
| Characteristics | N | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Median (months) | HR (95% CI) | P value | Median (months) | HR (95% CI) | P value | |||
| Age (years) | 1.05 (0.45–2.47) | 0.91 | 0.47 (0.15–1.49) | 0.20 | ||||
| ≤70 | 23 | 5.9 | NE | |||||
| >70 | 17 | 6.2 | 10.6 | |||||
| Sex | 2.40 (0.79–7.29) | 0.12 | 2.69 (0.35–20.99) | 0.35 | ||||
| Male | 32 | 5.7 | 13.7 | |||||
| Female | 8 | 7.3 | 10.6 | |||||
| Performance status | 0.63 (0.27–1.48) | 0.29 | 0.37 (0.11–1.22) | 0.10 | ||||
| 0–1 | 25 | 5.9 | 25.4 | |||||
| 2–3 | 15 | 6.2 | 10.6 | |||||
| Stage | 1.36 (0.31–5.88) | 0.67 | 0.68 (0.08–5.71) | 0.73 | ||||
| ES-SCLC | 36 | 5.9 | 25.4 | |||||
| Recurrent LS-SCLC | 4 | 5.7 | 8.8 | |||||
| Platinum regimen | 1.51 (0.64–3.58) | 0.35 | 0.60 (0.18–1.99) | 0.40 | ||||
| CDDP | 15 | 4.8 | NE | |||||
| CBDCA | 25 | 7.1 | 13.7 | |||||
| Brain metastasis | 0.67 (0.19–2.35) | 0.54 | 1.68 (0.21–13.23) | 0.62 | ||||
| Absent | 35 | 5.9 | 13.7 | |||||
| Present | 5 | 6.2 | NE | |||||
| Liver metastasis | 0.76 (0.27–2.13) | 0.61 | 0.53 (0.14–2.04) | 0.36 | ||||
| Absent | 30 | 5.7 | 25.4 | |||||
| Present | 10 | 6.2 | 10.1 | |||||
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; OS, overall survival; NE, not evaluable; ES-SCLC, extensive-stage small cell lung cancer; LS-SCLC, limited-stage small cell lung cancer; CDDP, cisplatin; CBDCA, carboplatin.
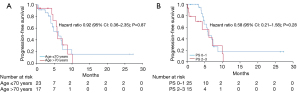
Table 4
| Characteristics | PFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≤70 years) | – | – | 0.46 (0.14–1.49) | 0.19 | |
| Sex (male) | 2.39 (0.78–7.31) | 0.13 | – | – | |
| Performance status (0–1) | 0.64 (0.27–1.51) | 0.31 | 0.37 (0.11–1.21) | 0.10 | |
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
One patient with a SD response to treatment received concurrent radiation therapy for superior vena cava (SVC) syndrome. The subsequent treatment rate for each PS was as follows: 100% (3 of 3) for PS 0, 80% (8 of 10) for PS 1, 0% (0 of 2) for PS 2, and 100% (3 of 3) for PS 3. Of the 18 patients who had disease progression, 14 (77.8%) received the second-line treatment consisting of amrubicin (AMR). The four patients with disease progression caused by oligometastatic disease during durvalumab maintenance therapy received radiation therapy for the oligometastatic site and continued the durvalumab therapy.
Safety
AEs from any cause and of any grade occurred in 39 (97.5%) of the 40 patients treated with durvalumab plus PE (Table 5). Grade 3 or higher AEs occurred in 26 (65.0%) patients, and AEs leading to treatment discontinuation occurred in three patients (7.5%). The most common grade 3 or higher AE was neutropenia. Nonhematological toxicities were generally mild. There was no difference in the incidence of AEs between patients aged ≤70 years and those aged >70 years. Two patients with PS 2 and 3 at the initiation of treatment, respectively, died (5.0%) from an AE from any cause. Immune-mediated AEs (imAEs) occurred in five patients (12.5%), of whom two with encephalitis and bullous pemphigoid, respectively, discontinued durvalumab.
Table 5
| Adverse events | Any grade | ≥ grade 3 |
|---|---|---|
| Any event, n (%) | 39 (97.5) | 26 (65.0) |
| Adverse events of any grade with an incidence of at least 10%, n (%) | ||
| Anemia | 32 (80.0) | 8 (20.0) |
| Neutropenia | 29 (72.5) | 19 (47.5) |
| White blood cell decrease | 23 (57.5) | 11 (27.5) |
| Thrombocytopenia | 20 (50.0) | 5 (12.5) |
| Appetite decrease | 16 (40.0) | 2 (5.0) |
| ALT increase | 12 (30.0) | 1 (2.5) |
| Alopecia | 12 (30.0) | 0 |
| Hyponatremia | 11 (27.5) | 4 (10.0) |
| Constipation | 11 (27.5) | 0 |
| Hyperkalemia | 10 (25.0) | 0 |
| Nausea | 10 (25.0) | 0 |
| ALP increase | 8 (20.0) | 1 (2.5) |
| AST increase | 8 (20.0) | 0 |
| Hypoalbuminemia | 8 (20.0) | 0 |
| Dermatitis/rash | 7 (17.5) | 1 (2.5) |
| Hiccups | 7 (17.5) | 0 |
| Febrile neutropenia | 5 (12.5) | 5 (12.5) |
| Creatinine increase | 5 (12.5) | 0 |
| Malaise | 4 (10.0) | 0 |
| Fever | 4 (10.0) | 0 |
| Immune-mediated adverse events, n (%) | ||
| Type 1 diabetes mellitus | 1 (2.5) | 1 (2.5) |
| Hyperthyroid event | 1 (2.5) | 0 |
| Encephalitis | 1 (2.5) | 1 (2.5) |
| Bullous pemphigoid | 1 (2.5) | 1 (2.5) |
| Asteatotic eczema | 1 (2.5) | 0 |
| Age (years), n (%) | ||
| ≤70 (n=23) | 23 (100.0) | 15 (65.2) |
| >70 (n=17) | 16 (94.1) | 11 (64.7) |
| Any event leading to discontinuation, n (%) | 3 (7.5) | 3 (7.5) |
| Any event leading to death, n (%) | 2 (5.0) | 2 (5.0) |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase.
Discussion
The present study evaluated patients receiving durvalumab plus PE as the first-line treatment for ES-SCLC or as the initial treatment for recurrent LS-SCLC. In the entire cohort of 40 patients, the ORR, median PFS, and median OS was 80.0%, 5.9 months, and 25.4 months, respectively, which were better than the ORR, median PFS, and median OS of 68.0%, 5.1 months, and 12.9 months, respectively, in the durvalumab plus PE arm of the CASPIAN trial (4,13,14). The RWD studies of durvalumab plus PE for ES-SCLC, which were conducted in China, included about 20–30% patients with concomitant radiation therapy (15-17). In our study, only one patient received palliative radiotherapy for SVC syndrome and had PFS and OS of 4.6 and 13.7 months, respectively. In Japan, the combination of chemotherapy and radiotherapy is not recommended as standard treatment for ES-SCLC. To the best of our knowledge, the present study is the first genuinely to assess the therapeutic efficacy and feasibility of durvalumab plus PE as a first-line treatment for ES-SCLC.
The number of SCLC patients older than 80 years is increasing each year (18). Patients with ES-SCLC often have poor PS and distant metastases at diagnosis owing to the rapid progression of the disease (1). The CASPIAN trial, which demonstrated that durvalumab plus PE improved OS in patients with ES-SCLC, excluded individuals with PS 2 or higher, active brain metastases, renal impairment or severe liver dysfunction; however, it included individuals with asymptomatic or previously treated brain metastases (4,19). In the durvalumab plus PE arm of the CASPIAN trial, 62% (167 patients) of the patients were aged <65 years and 13% (36 patients) were Asian, including those of Japanese descent (7%, n=18 patients) (4,7). Based on these facts, the outcomes of the CASPIAN trial were not entirely representative of patients in the real-world, clinical setting. Recent retrospective studies of chemoimmunotherapy for ES-SCLC have demonstrated the efficacy and tolerability of atezolizumab plus carboplatin and etoposide in patients who were ineligible for participation in a clinical trial (8-11). Although the efficacy of durvalumab is generally considered equivalent to that of atezolizumab, no studies have examined the differences between these treatments. Therefore, it is important to demonstrate the feasibility of durvalumab plus PE for ES-SCLC in real-world, clinical practice.
Our analysis demonstrated that the median PFS was comparable to that observed in the CASPIAN trial (5.9 vs. 5.1 months) whereas the median OS was superior in our study (25.4 vs. 12.9 months). There are several, possible reasons for this. First, in our study, 77.8% of the patients with disease progression received some form of subsequent treatment compared to 46.6% in the CASPIAN study (13). Second, all the patients with any subsequent therapy received AMR, which is only available in Japan, as second-line therapy. Although AMR is almost as effective as other drugs, there are many, other options for subsequent treatment available in Japan (20). Third, patients with a relapsed stemming from oligometastatic disease following their initial or subsequent therapy received radiation therapy at the oligometastatic site and continued the same treatment thereafter. These interventions may have prolonged OS in our study. On the other hand, it is essential to consider that the small sample sizes, the short follow-up period, and the fact that only Asians participated may have influenced the OS.
The CASPIAN trial included only patients with PS 0 or 1 while our study included 10 patients (25.0%) with PS 2 and five patients (12.5%) with PS 3. The favorable response of SCLC patients to chemotherapy underscored the benefit of PE therapy in patients with PS 3 (21). There are few data on chemoimmunotherapy for ES-SCLC patients with poor PS. Our study found no difference in the median PFS between patients with PS 0–1 and those with PS 2–3 (5.9 vs. 6.2 months), suggesting that durvalumab plus PE is useful regardless of PS. However, since most of the patients with PS 2 or 3 experienced grade 3 or higher AEs, chemotherapy dose reduction or G-CSF administration may be considered in these patients. Although there was no significant difference in OS according to PS, OS tended to be longer in cases with good PS (PS 0 or 1) (Tables 3,4) possibly because patients with poor PS at the start of treatment often also have poor PS at disease progression, making it difficult to start post-treatment. Several previous studies have also reported no significant difference in OS between an atezolizumab group and a chemotherapy group with PS 2, suggesting that OS in patients with poor PS may not benefit from ICI therapy (9,11). Because the study cited had a small sample size, larger studies of the efficacy of durvalumab plus PE in patients with poor PS are warranted. A single-arm, phase II trial is currently underway in Japan to confirm the efficacy and safety of durvalumab plus PE in patients with poor PS in ES-SCLC (22).
The present study included four patients with recurrent LS-SCLC. The combination of carboplatin and etoposide has been shown to be effective against recurrent LS-SCLC in patients who received PE more than 90 days previously (23). On the other hand, the efficacy of durvalumab plus PE in these patients is unknown; the CASPIAN trial did not enroll this patient population. Univariate analysis in the present study demonstrated that PFS was comparable in patients with recurrent LS-SCLC and ES-SCLC (5.7 vs. 5.9 months) (Table 3). However, the number of cases was very small, and a future study with a larger cohort is warranted.
The rate of all AEs, including those of grade 3 or higher, was comparable to that reported by the CASPIAN trial (7). The primary AE was hematological toxicity, but its rate was much higher than in the CASPIAN trial possibly because our study included patients with poor PS or elderly patients (24,25). Therefore, the real-world, clinical outcomes of both studies may in fact be similar. Most of the AEs, including febrile neutropenia, were manageable. Five patients (12.5%) had imAEs, a lower incidence rate compared to that reported by the CASPIAN trial (12.5% vs. 20.0%) (4). Because the present study included patients with a short follow-up period, the imAEs may not have been adequately evaluated. Owing to AEs, two patients discontinued all treatment, and one patient discontinued durvalumab alone. AEs leading to death occurred in two patients, both of whom had poor PS and multiple, distant metastases and experienced tumor lysis syndrome shortly after the initial therapy. Caution should be exercised in patients with poor PS, metastatic disease or bulky tumors (26). The overall incidence of AEs in our study was similar to that observed in the CASPIAN trial, suggesting that the AEs associated with the durvalumab plus PE combination therapy may be well tolerated by SCLC patients in real-world, clinical practice.
A retrospective analysis of the IMpower133 trial and the CASPIAN trial suggested that SCLC-I is a predictive biomarker for immunotherapy of ES-SCLC (27). We did not measure or examine any biomarkers in our study. Given the rapid relapse tendency of ES-SCLC, predicting the response to immunotherapy is of paramount importance. Further studies by SCLC subtype are warranted.
The present study has several limitations. First, it was a monocentric, retrospective study enrolling patients with a short follow-up period. Second, AEs not included in the laboratory data may have been overlooked, and the chemotherapy dosages and combined use of G-CSF varied among the patients. On the other hand, durvalumab is used in most cases at the study center in the absence of contraindications, such as active interstitial pneumonia and autoimmune disease. This may have reduced the impact of any selection bias. Given the small sample size of ineligible patients in this study, a larger, prospective trial is needed to verify the clinical utility of our findings. Second, the timing of the CT assessments was determined at the discretion of the physicians, thus possibly affecting PFS. However, most patients were evaluated at a relatively appropriate time because they were monitored regularly for tumor markers to detect early signs of disease progression. Therefore, the impact of the timing of the CT assessments was likely to be minimal. Third, since this study is not a comparative trial, it is not known whether durvalumab plus PE is more effective than PE in ESLC in real-world, clinical practice. However, many previous studies and clinical trials of atezolizumab have demonstrated that chemoimmunotherapy is more effective than chemotherapy alone. Therefore, durvalumab should be used in combination if there are no contraindications to ICIs.
Conclusions
The present study produced results comparable to those of the CASPIAN trial. The data indicated that the durvalumab plus PE combination regimen for ES-SCLC is feasible in real world, clinical practice.
Acknowledgments
We thank James R. Valera for his assistance with editing this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/coif). K.W. received payment or honoraria for lectures and presentations from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Merck Biopharma, MSD, Novartis, Ono Pharmaceutical, Riken Genesis, Sysmex Corporation and Takeda. M.S. received grants or contracts from Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Nippon Kayaku, Kyowa Hakko Kirin and payment or honoraria for lectures and presentations from AstraZeneca, MSD, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Pfizer, Novartis, Takeda, Kyowa Hakko Kirin, Nippon Kayaku, Daiichi-Sankyo Company, Merck Biopharma, Amgen. Y.H. received payment or honoraria for lectures and presentations from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Kyowa Hakko Kirin, Nihon Kayaku, Takeda, Eisai, Novartis and Pfizer. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Komagome Hospital, Tokyo, Japan ethics committee (code: 3226) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oronsky B, Reid TR, Oronsky A, et al. What's New in SCLC? A Review. Neoplasia 2017;19:842-7. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Hotta K, Nishio M, Saito H, et al. First-line durvalumab plus platinum-etoposide in extensive-stage small-cell lung cancer: CASPIAN Japan subgroup analysis. Int J Clin Oncol 2021;26:1073-82. [Crossref] [PubMed]
- Shiono A, Imai H, Wasamoto S, et al. Real-world data of atezolizumab plus carboplatin and etoposide in elderly patients with extensive-disease small-cell lung cancer. Cancer Med 2023;12:73-83. [Crossref] [PubMed]
- Falchero L, Guisier F, Darrason M, et al. Long-term effectiveness and treatment sequences in patients with extensive stage small cell lung cancer receiving atezolizumab plus chemotherapy: Results of the IFCT-1905 CLINATEZO real-world study. Lung Cancer 2023;185:107379. [Crossref] [PubMed]
- Kim SH, Jo EJ, Mok J, et al. Real-world evaluation of atezolizumab and etoposide-carboplatin as a first-line treatment for extensive-stage small cell lung cancer. Korean J Intern Med 2023;38:218-25. [Crossref] [PubMed]
- Fujimoto D, Morimoto T, Tamiya M, et al. Outcomes of Chemoimmunotherapy Among Patients With Extensive-Stage Small Cell Lung Cancer According to Potential Clinical Trial Eligibility. JAMA Netw Open 2023;6:e230698. [Crossref] [PubMed]
- Morimoto K, Yamada T, Takeda T, et al. Efficacy and Safety of Programmed Death-Ligand 1 Inhibitor Plus Platinum-Etoposide Chemotherapy in Patients With Extensive-Stage SCLC: A Prospective Observational Study. JTO Clin Res Rep 2022;3:100353. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022;7:100408. [Crossref] [PubMed]
- Qu J, Kalyani FS, Shen Q, et al. Efficacy and Safety of PD-L1 Inhibitors plus Chemotherapy versus Chemotherapy Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: A Retrospective Real-World Study. J Oncol 2022;2022:3645489. [Crossref] [PubMed]
- Zou Y, Ren X, Zhang H, et al. Efficacy and safety of durvalumab + chemotherapy vs. atezolizumab + chemotherapy in the treatment of small cell lung cancer: a retrospective comparative cohort study. J Thorac Dis 2023;15:3339-49. [Crossref] [PubMed]
- Qiu G, Wang F, Xie X, et al. A retrospective real-world experience of immunotherapy in patients with extensive stage small-cell lung cancer. Cancer Med 2023;12:14881-91. [Crossref] [PubMed]
- Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017;7:1339. [Crossref] [PubMed]
- Chen Y, Paz-Ares LG, Dvorkin M, et al. First-line durvalumab plus platinum-etoposide in extensive-stage (ES)-SCLC (CASPIAN): Impact of brain metastases on treatment patterns and outcomes. Journal of Clinical Oncology 202;38:9068.
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [Crossref] [PubMed]
- Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162-9. [Crossref] [PubMed]
- Asao T, Watanabe S, Tanaka T, et al. A phase II study of carboplatin and etoposide plus durvalumab for previously untreated extensive-stage small-cell lung cancer (ES-SCLC) patients with a poor performance status (PS): NEJ045A study protocol. BMC Cancer 2022;22:1135. [Crossref] [PubMed]
- Baize N, Monnet I, Greillier L, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2020;21:1224-33. [Crossref] [PubMed]
- Morimoto K, Yamada T, Yokoi T, et al. Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer 2021;161:26-33. [Crossref] [PubMed]
- Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol 2010;28:620-7. [Crossref] [PubMed]
- Alqurashi RM, Tamim HH, Alsubhi ZD, et al. Tumor Lysis Syndrome in Patients With Solid Tumors: A Systematic Review of Reported Cases. Cureus 2022;14:e30652. [Crossref] [PubMed]
- Zhang S, Cheng Y. Immunotherapy for extensive-stage small-cell lung cancer: current landscape and future perspectives. Front Oncol 2023;13:1142081. [Crossref] [PubMed]


