
Real-world efficacy of low dose osimertinib as second-line treatment in patients with epidermal growth factor receptor-mutated advanced non-small cell lung cancer
Highlight box
Key findings
• Treatment with low dose osimertinib in epidermal growth factor receptor (EGFR)-mutated advanced non-small-cell lung cancer (NSCLC) post-first- or second-generation EGFR tyrosine kinase inhibitor (TKI) demonstrated an overall response rate of 77.3%, median progression-free survival of 10.0 months [95% confidence interval (CI): 8.6–11.4] and median overall survival of 13.0 months (95% CI: 9.4–16.6).
What is known and what is new?
• Lower doses of osimertinib [20 mg once daily (OD) and 40 mg OD] resulted in similar response rates as the recommended dose (80 mg OD).
• In real-world clinical practice, low dose osimertinib treatment resulted in good survival outcomes in EGFR-mutated advanced NSCLC patients who had failed first-line treatment with first- or second-generation EGFR TKIs due to acquired T790M mutation.
What is the implication, and what should change now?
• A randomized controlled trial is needed to establish whether treatment with lower doses of osimertinib is as effective as the recommended dose in terms of response rates and survival outcomes.
Introduction
Epidermal growth factor receptor (EGFR) mutations, which are common in patients with non-small cell lung cancer (NSCLC), can be effectively targeted with EGFR tyrosine kinase inhibitor (TKI) (1). The first-generation TKIs (e.g., gefitinib, erlotinib, lapatinib, and icotinib) are effective for common EGFR mutations (exon 19 deletion and L858R mutation) (2), with the former mutation having the best progression-free survival (PFS) (3). However, patients may develop resistance within 10–14 months of treatment (4). In one of these trials, the median time to progression for patients with lung adenocarcinomas and acquired resistance to erlotinib or gefitinib was 13 months (5). The second-generation TKIs (e.g., afatinib, neratinib, and dacomitinib) are more efficacious against these common EGFR mutations (2). For example, patients on afatinib had significantly longer PFS and time to treatment failure than gefitinib (1). However, second-generation EGFR TKIs cause more side effects due to its irreversible EGFR inhibition (2).
Regardless of whether patients were treated with first- or second-generation TKIs, they eventually develop disease progression due largely to the emergence of EGFR exon 20 T790M mutation which happens in 50–60% of the patients (2,6). In the AURA3 trial, a phase III, open label, randomized study, treatment with osimertinib in a group of patients with EGFR exon 20 T790M mutation resulted in significantly longer PFS and better response rate compared to chemotherapy (7).
The first- and second-generation EGFR TKIs are also less effective for patients with brain metastases due to the relatively low drug penetration of the blood-brain barrier (8). In contrast, osimertinib showed significantly better central nervous system effectiveness with higher overall response rate (ORR), duration of response (DoR), and PFS than first-generation EGFR TKIs and chemotherapy in the AURA3 trial and FLAURA study, respectively (8,9).
In Malaysia, first-and second-generation EGFR TKIs are the first-line treatment of choice for patients with EGFR-mutant advanced NSCLC in the public healthcare system (10) because of their lower costs. When patients progress on these early generation TKIs, chemotherapy or other alternative treatments are usually offered (10). Although osimertinib is approved for use as first and second-line treatment in the country, access to the drug is limited due to its high cost (10), with patients having to pay for the treatment themselves (approximate cost of USD 3,000 per month). Therefore, some patients are taking half of the recommended dose of 80 mg once daily (OD), 80 mg every other day (EOD), or 40 mg EOD. The cost of the 40 and 80 mg tablets are the same in Malaysia. Therefore, less wealthy patients prefer to split the 80 mg tablet in half rather than buy the 40 mg tablet. The lack of access to novel, targeted therapies have a detrimental effect on survival outcomes (11).
The AURA1 study is a phase I/II clinical trial on the dose, safety, and efficacy of the recommended osimertinib dose of 80 mg OD in patients with EGFR TKI-pretreated EGFR mutated T790M-positive advanced NSCLC (12). The study also showed that lower doses of osimertinib (20 and 40 mg OD) are associated with ORRs similar to 80 mg OD (13). However, as there is a lack of evidence on the effect of lower doses of osimertinib on PFS and OS, the objectives of this retrospective study were to assess the efficacy and safety of lower osimertinib doses for patients with EGFR-mutated advanced NSCLC whose disease had progressed on earlier generation EGFR TKIs in a real-world clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-243/rc).
Methods
This was a retrospective multicenter study using data from case notes of patients from four hospitals (i.e., Hospital Tengku Ampuan Afzan, Universiti Malaya Medical Centre, Beacon Hospital, and Hospital Sultanah Bahiyah) between 1st October 2018 and 30th September 2021.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Universiti Malaya Medical Centre Medical Research Ethics Committee (No. MECID 20201115–9217), and all participating hospitals/institutions were informed and agreed to the study. The Universiti Malaya Medical Centre Medical Research Ethics Committee waived the need for informed consent as patient confidentiality was preserved using identification code numbers.
Study population
Adult patients (18 years or older) of both genders, with EGFR-mutated advanced NSCLC who failed first- or second-generation EGFR TKI treatment due to acquired T790M mutation and subsequently received low doses of osimertinib (i.e., 40 mg OD, 40 mg EOD or 80 mg EOD) as second-line treatment were included in the study. The recommended dose of osimertinib in Malaysia is 80 mg OD, but some physicians and patients opt for lower doses of osimertinib due to cost constraints (i.e., patients not covered by insurance and having to pay for treatment out-of-pocket). There were no changes in the dosage of osimertinib for NSCLC patients who received a low dose of osimertinib throughout the course of their treatment. Dose reductions at the start of osimertinib were not due to toxicity, neither were there any increases in dosage due to achieved tolerability in our study as the patients were given low dose osimertinib from the start due to these cost constraints. Patients who missed treatment or were lost to follow-up were excluded from the analysis.
Outcome variables
Demographic and clinical characteristics such as age, gender, ethnicity, smoking status, EGFR mutation subtype, Eastern Cooperative Oncology Group (ECOG) performance status, presence or absence of brain metastases, treatment history, treatment response history, and osimertinib dose were recorded. Documented adverse events (AEs), ORR, PFS, and OS were analyzed. Patients were assigned as good performance status if they had scores 0, 1, or 2 [as the use of chemotherapy is justified (13)] and as poor performance status if they had scores 3 or 4. The ORR was defined as proportion of patients with partial response or complete response to therapy, PFS as time from initiation of osimertinib until progression or death, and OS as the time from initiation of osimertinib until death from any cause. The intensity of each AE was determined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical analysis
Data was retrieved from the case notes of patients from four hospitals (i.e., Hospital Tengku Ampuan Afzan, Universiti Malaya Medical Centre, Beacon Hospital, and Hospital Sultanah Bahiyah) between 1st October 2018 and 30th September 2021. These were screened for missing values. Any missing data were cross-examined with the site investigator. Data analysis was performed using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA). Descriptive data were presented as percentages. Continuous data were presented as median. The duration of time was described in months. All P values reported were two sided and considered significant at the 0.05 threshold. The Kaplan-Meier method was used to estimate the PFS and OS and log-rank test was used to test the survival differences between groups. Any patients lost to follow up or still alive at the time of analysis were censored.
Results
Demographic and disease characteristics
Twenty-three patients were recruited to the study. However, one patient who was prescribed low-dose osimertinib dropped out. The patient was uncontactable. This study included 22 patients (8 males and 14 females) with EGFR-mutated advanced NSCLC between the ages of 44 and 82 years (Table 1). Five patients were on 40 mg EOD, ten on 40 mg OD, and seven on 80 mg EOD (Table 1). Seven patients had good ECOG performance status (ECOG 1 or 2) while 15 had poor ECOG performance status of 3 or 4. There were no patients with ECOG 0. The EGFR mutations consisted of exon 19 deletion (68.2%) and exon 21 L858R mutation (31.8%). Four patients had brain metastases at first progression while on first- or second-generation EGFR TKI treatment and all of them were on 40 mg OD (Table 1).
Table 1
| Characteristics | Dose | |||
|---|---|---|---|---|
| 40 mg EOD (n=5) | 40 mg OD (n=10) | 80 mg EOD (n=7) | Total (N=22) | |
| Age, years | 63 [44, 81] | 64 [57, 78] | 66 [55, 82] | 64 [44, 82] |
| Gender | ||||
| Male | 4 (80.0) | 2 (20.0) | 2 (28.6) | 8 (36.4) |
| Female | 1 (20.0) | 8 (80.0) | 5 (71.4) | 14 (63.6) |
| Ethnicity | ||||
| Malay | 3 (60.0) | 4 (40.0) | 4 (57.1) | 11 (50.0) |
| Chinese | 2 (40.0) | 6 (60.0) | 3 (42.9) | 11 (50.0) |
| Smoking status | ||||
| Never smoker | 2 (40.0) | 8 (80.0) | 6 (85.7) | 16 (72.7) |
| Former smoker | 3 (60.0) | 1 (10.0) | 1 (14.3) | 5 (22.7) |
| Current smoker | 0 | 1 (10.0) | 0 | 1 (4.6) |
| EGFR mutation subtype | ||||
| Exon 19 deletion | 3 (60.0) | 6 (60.0) | 6 (85.7) | 15 (68.2) |
| Exon 21 L858R | 2 (40.0) | 4 (40.0) | 1 (14.3) | 7 (31.8) |
| ECOG performance status | ||||
| 0 | 0 | 0 | 0 | 0 |
| 1 or 2 | 0 | 4 (40.0) | 3 (42.9) | 7 (31.8) |
| 3 or 4 | 5 (100.0) | 6 (60.0) | 4 (57.1) | 15 (68.2) |
| Presence of brain metastasis | 0 | 4 (40.0) | 0 | 4 (18.2) |
| First- or second-generation EGFR TKI | ||||
| Afatinib | 2 (40.0) | 8 (80.0) | 5 (71.4) | 15 (68.2) |
| Gefitinib | 3 (60.0) | 2 (20.0) | 1 (14.3) | 6 (27.3) |
| Erlotinib | 0 | 0 | 1 (14.3) | 1 (4.5) |
| Osimertinib dose | ||||
| 40 mg OD | 10 (45.5) | |||
| 80 mg EOD | 7 (31.8) | |||
| 40 mg EOD | 5 (22.7) | |||
Data are presented as median [range] or n (%). EOD, every other day; OD, once daily; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; TKI, tyrosine kinase inhibitor.
EGFR TKI treatments
All patients in the study were previously on first- or second-generation EGFR TKIs (erlotinib, gefitinib, or afatinib; Table 1). Upon disease progression, all patients underwent liquid biopsy for EGFR exon 20 T790M mutation, and if found negative, a repeat tissue biopsy was performed. Majority of patients (86%) were detected to have the acquired T790M mutation on liquid biopsy. Patients with acquired T790M mutation were then offered osimertinib as second-line treatment. Of the 22 patients who received osimertinib as second-line treatment, 45.5% of them received 40 mg OD, 31.8% were treated with 80 mg EOD, and 22.7% of them received 40 mg EOD (Table 1).
Safety profile
Treatment-related AEs were documented in eight patients (Table 2). All AEs were Grade 1, except paronychia which was Grade 2 in one patient and Grade 1 in two other patients. There were no Grade 3 or 4 AEs.
Table 2
| Adverse events | Number of events, N (%) |
|---|---|
| Any adverse event | 8 (36.4) |
| Paronychia* | 3 (13.6) |
| Diarrhea | 1 (4.5) |
| Facial swelling | 1 (4.5) |
| Fatigue | 1 (4.5) |
| Skin itch | 1 (4.5) |
| Acneiform rash | 1 (4.5) |
| Pneumonitis | 0 |
| Stomatitis | 0 |
*, one patient had Grade 2 paronychia, all other adverse events were recorded as Grade 1.
Treatment response
Most of the patients in the study achieved good and durable responses with low doses of osimertinib in the second-line setting, with an ORR of 77.3% (i.e., 17 patients with partial response). Two patients (9.1%) had stable disease. The remaining three (13.6%) patients died before the follow-up evaluation.
PFS and overall survival (OS)
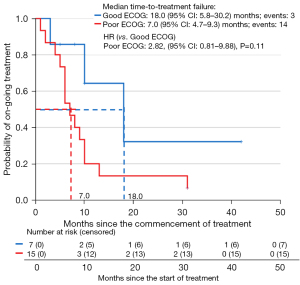
In all the 22 patients, the median PFS was 10.0 months [95% confidence interval (CI): 8.6–11.4] and median OS was 13.0 months (95% CI: 9.4–16.6). In patients with ECOG 1 or 2, the median PFS was 18.0 months (95% CI: 5.8–30.2) (Figure 1) and median OS was not reached (Figure 2). Reasonable survival outcomes were also seen in patients with poor performance status [median PFS: 7.0 months (95% CI: 4.7–9.3) and median OS: 10.0 months (95% CI: 7.5–12.5)].

Patients on 80 mg EOD had a longer median PFS (10 months) compared to 40 mg EOD (8 months) and 40 mg OD (6 months) (Figure 3). The 80 mg EOD group also had a longer median OS (14 months) than the other two doses (10 months for 40 mg EOD and 13 months for 40 mg OD) (Figure 4).


Figure 5 shows the swimmer plot for PFS according to osimertinib dose.

Discussion
This real-world study showed that most patients with EGFR-mutated advanced NSCLC whose disease progressed on afatinib, erlotinib or gefitinib responded to low dose osimertinib (ORR of 77.3%). This finding was comparable to the results in the AURA3 trial, which showed that patients on the recommended osimertinib dose of 80 mg daily had an ORR of 71% (14).
In our study, the median PFS for patients on low-dose osimertinib after progressing on earlier EGFR TKI was 10 months, which is consistent with the findings of the AURA3 study (14), despite the fact our study also included patients with poor ECOG performance status. This shows that patients with poor ECOG performance status should be treated with osimertinib to prolong their survival. Moreover, it is common to see improvement in ECOG performance status after targeted therapy. Our study revealed a median OS of 12 months, which is shorter than that of the AURA3 study (7,14) because we included patients with poor ECOG performance status. The median OS for patients with ECOG 1 and 2 was not reached at the time of analysis. In the AURA3 study, which only included patients with ECOG 0 and 1 (14), the median OS was 26.8 months with osimertinib, but this was not significantly better than the median OS of 22.5 months with chemotherapy because a high proportion of patients on chemotherapy (73%) crossed over to receive osimertinib (7). The anaplastic lymphoma kinase (ALK) TKI trial (J-ALEX) also did not show superiority of OS in Japanese patients treated upfront with lower doses of alectinib over crizotinib in ALK-positive advanced NSCLC patients, which again, was due to a high crossover rate (15). In patients who were treated with alectinib, the 5-year OS rate was 60.9% (15). The international ALEX trial showed a higher 5-year OS rate with alectinib, but the OS data remain immature (16). Moreover, the PROFILE 1014 trial showed that ALK TKI-treated patients who received a subsequent ALK TKI had a similar 5-year OS rate, consistent with the J-ALEX trial (17). The findings of our study provide support that patients should be given effective subsequent targeted therapy regardless of ECOG performance status. Lower doses of osimertinib can be used to prolong the PFS and possibly OS.
As far as we know, there is little data on the clinical outcomes of lower doses of osimertinib other than another small study which found 40 mg OD to be efficacious in patients with T790M-positive advanced NSCLC patients (18). This was also reflected in our study whereby patients who were on 80 mg EOD, 40 mg EOD and 40 mg OD had a median PFS of 10, 8 and 6 months respectively. These patients also had a remarkable median OS of 14, 10 and 13 months respectively.
Our patients with poor performance status (ECOG 3 & 4) which are contraindications for chemotherapy had a median PFS of 7 months with low dose osimertinib. Patients with poor ECOG scores [3–4] on osimertinib 80 mg OD in another study had a median PFS of 5.5 months (19). The efficacy of osimertinib in patients with poor performance has also been demonstrated elsewhere (20,21). The inclusion of patients with brain metastases also did not affect the results of our study, which was expected as osimertinib’s efficacy in this group of patients was demonstrated in the AURA3 study (8). Furthermore, as brain magnetic resonance imaging is not routinely performed in our clinical practice, the incidence of brain metastasis of 18% in our patients could have been higher.
The standard dose of osimertinib at 80 mg OD has been associated with 23% of Grade 3 or higher toxicity and 13% of permanent discontinuation due to AEs (22). In our small study, the tolerable safety profile with no Grade 3 AEs with lower doses of osimertinib is encouraging despite 68% of our patients having ECOG 3 and 4 performance status. Therefore, patients with poor ECOG performance status (especially those who are bedridden) should be considered for lower doses of osimertinib to avoid AEs such as diarrhea and rashes which may be difficult to handle in such patients. We, however, acknowledge that in a real-world study, AEs or serious AEs (SAEs) may be less commonly documented compared to randomized controlled studies. Manageable tolerability profiles have also been reported with low doses of afatinib. A global, real-world study (RealGiDo) found reduction in the severity of adverse drug reactions with afatinib doses 30 mg and lower (23). Malaysian studies have also found that afatinib at 40 or 30 mg OD were effective maintenance doses and not commonly associated with severe side effects in actual clinical practice (24,25).
Our study was limited by its small sample size and its retrospective design, in addition to a lack of a comparator arm of osimertinib 80 mg OD (due to an equally small sample size), making this comparison insufficiently powered to detect any difference in efficacy between those given full dose versus low dose osimertinib. We recommend larger scale, muti-centered, prospective studies to confirm our findings. However, the broad inclusive nature of our study including patients with poor performance status and those with untreated brain metastases who are under-represented in randomized controlled trials is more reflective of real-world clinical practice.
Conclusions
Treatment with lower doses of osimertinib resulted in comparable PFS and ORR compared to 80 mg OD in patients with EGFR-mutated advanced NSCLC whose disease had progressed on first- or second-generation EGFR TKI due to T790M mutation in real-world clinical practice. However, a randomized controlled trial comparing low dose vs. 80 mg OD osimertinib is needed to establish whether lower doses of osimertinib treatment is as effective as the recommended dose in terms of ORR, PFS and OS. Lower dose is potentially cost saving and can be associated with lower incidence of AEs and SAEs.
Acknowledgments
The authors thank the study coordinators at each of the participating centers for data collection and management, and Anne John Michael (MedPhrase) for help in preparing the manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-243/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-243/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-243/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-243/coif). M.E.P. received honoraria and fees for lectures from AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer and Roche. S.B. received honoraria for a talk from Menarini. C.K.L. received research grants from AstraZeneca and Boehringer Ingelheim; received honoraria and fees for lectures and advisory board meetings from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Merck, MSD, Novartis, Pfizer, Roche and Zuellig Pharma. J.L.T. received honoraria for lecture/presentation from Boehringer Ingelheim, Eli Lilly, MSD, and Pfizer; received support to attend the World Conference on Lung Cancer 2023 from MSD; and participated in the Advisory Board for Neoadjuvant Immunotherapy for Bristol Myers Squibb. L.M.T. is the President of the Lung Cancer Network Malaysia; received honoraria from AstraZeneca, Eisai, Ipsen, Janssen, MSD, Novartis, Pfizer, and Roche; and received support for attending meetings from AstraZeneca, MSD, and Roche. S.H.H. received clinical trial grants to his institution from AstraZeneca, Boehringer Ingelheim, Janssen, MSD, Novartis, and Roche; received payment for attending Advisory Board meetings from AstraZeneca, Janssen, MSD, and Roche; received payment for publication of clinical trial results from AstraZeneca and Janssen; and received support to attend the World Conference on Lung Cancer 2023 from MSD. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Universiti Malaya Medical Centre Medical Research Ethics Committee (No. MECID 20201115–9217), and all participating hospitals/institutions were informed and agreed to the study. The Universiti Malaya Medical Centre Medical Research Ethics Committee waived the need for informed consent as patient confidentiality was preserved using identification code numbers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takeda M, Nakagawa K. First- and Second-Generation EGFR-TKIs Are All Replaced to Osimertinib in Chemo-Naive EGFR Mutation-Positive Non-Small Cell Lung Cancer? Int J Mol Sci 2019;20:146. [Crossref] [PubMed]
- Guardiola S, Varese M, Sánchez-Navarro M, et al. A Third Shot at EGFR: New Opportunities in Cancer Therapy. Trends Pharmacol Sci 2019;40:941-55. [Crossref] [PubMed]
- Hong W, Wu Q, Zhang J, et al. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol Lett 2019;18:3887-95. [Crossref] [PubMed]
- Wu L, Ke L, Zhang Z, et al. Development of EGFR TKIs and Options to Manage Resistance of Third-Generation EGFR TKI Osimertinib: Conventional Ways and Immune Checkpoint Inhibitors. Front Oncol 2020;10:602762. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020;31:1536-44. [Crossref] [PubMed]
- Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref]
- How SH, Liam CK, Zainal Abidin MA, et al. Outcomes of Patients with EGFR-Mutant Advanced NSCLC in a Developing Country in Southeast Asia. Cancer Manag Res 2022;14:1995-2005. [Crossref] [PubMed]
- Tamura K, Nukiwa T, Gemma A, et al. Real-world treatment of over 1600 Japanese patients with EGFR mutation-positive non-small cell lung cancer with daily afatinib. Int J Clin Oncol 2019;24:917-26. [Crossref] [PubMed]
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol 2017;35:1288-96. [Crossref] [PubMed]
- Ito K, Hataji O. Osimertinib therapy as first-line treatment before acquiring T790M mutation: from AURA1 trial. J Thorac Dis 2018;10:S3071-7. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Hotta K, Hida T, Nokihara H, et al. Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 2022;7:100527. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Sonobe S, Taniguchi Y, Saijo N, et al. The efficacy of a reduced dose (40mg) of osimertinib with T790M-positive advanced non-small-cell lung cancer. Ann Oncol 2017;28:X130.
- Kato Y, Hosomi Y, Watanabe K, et al. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis 2019;11:2350-60. [Crossref] [PubMed]
- Igawa S, Fukui T, Kasajima M, et al. First-line osimertinib for poor performance status patients with EGFR mutation-positive non-small cell lung cancer: A prospective observational study. Invest New Drugs 2022;40:430-7. [Crossref] [PubMed]
- Tsubata Y, Watanabe K, Saito R, et al. Osimertinib in poor performance status patients with T790M-positive advanced non-small-cell lung cancer after progression of first- and second-generation EGFR-TKI treatments (NEJ032B). Int J Clin Oncol 2022;27:112-20. [Crossref] [PubMed]
- Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. [Crossref] [PubMed]
- Halmos B, Tan EH, Soo RA, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: Results from a global real-world study (RealGiDo). Lung Cancer 2019;127:103-11. [Crossref] [PubMed]
- Ho GF, Chai CS, Alip A, et al. Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer 2019;19:896. [Crossref] [PubMed]
- Poh ME, Chai CS, Liam CK, et al. Does dose reduction of afatinib affect treatment outcomes of patients with EGFR-mutant metastatic non-small cell lung cancer in real-world clinical practice? Transl Lung Cancer Res 2024;13:307-20. [Crossref] [PubMed]


