
Lymph nodes rather than pleural metabolic activity in 18F-FDG PET/CT correlates with malignant pleural effusion recurrence in advanced non-small cell lung cancer
Highlight box
Key findings
• Lymph nodes (LN)’ highest metabolic activity is associated with malignant pleural effusion (MPE) recurrence for treatment-naïve non-small cell lung cancer (NSCLC) patients. The change of systemic treatment was more crucial for the first MPE recurrence while the previous intrapleural perfusion presented favorable effect on second MPE recurrence.
What is known and what is new?
• Frequently recurrent MPE significantly hampers the life quality of advanced NSCLC patients.
• LN rather than pleural metabolic activity or uptake patterns could predict MPE recurrence for advanced NSCLC patients with or without targeted therapy. The change of systemic treatment and intrapleural perfusion treatment were respectively effective for the first and the second MPE recurrence.
What is the implication, and what should change now?
• For Asian patients, MPE patients with LN SUVmax >4.50 g/mL, male gender, bone metastases, and non-targeted therapy could collectively indicate MPE recurrence with an optimal 300-day area under the curve of 0.83, which is representative of higher recurrence risks.
• We should re-consider the application of intrapleural perfusion treatment for first-onset MPE and prompt it more at the moment of recurrent MPE.
Introduction
Malignant pleural effusion (MPE) refers to the accumulation of fluid in the pleural cavity originating from pleura or secondary to ectopic malignancies. It is commonly observed in lung cancer and breast cancer (1) and leads to poor prognoses. In non-small cell lung cancer (NSCLC), 10–15% of patients are diagnosed with concurrent MPE at the onset, with a suffered median survival of 7.7 months (2) and a tendency to harbor actionable mutation (3). It was reported that almost 50% of MPEs tend to reaccumulate within 90 days for Caucasians (4) and within 300 days for Asians (5). Although some remain asymptomatic, the majority of patients present dyspnea, coughing, and chest pain caused by recurrent MPE, which drastically decreases life quality. Several studies have developed predictive models for survival in patients with MPE, including LENT [pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group (ECOG) performance score (PS), neutrophil-to-lymphocyte ratio and tumour type] (6) and PROMISE (haemoglobin, C-reactive protein, white blood cell count, ECOG PS, cancer type, pleural fluid tissue inhibitor of metalloproteinases 1 and previous chemo- or radiotherapy) score (7). However, these two widely accepted scoring systems were derived from unspecific malignancies; therefore, our group established and validated a novel predictive system “RECLS” for NSCLC patients (8). Initially, GROSU et al. explored the potential risk factors for MPE recurrence regardless of tumor type. They found that the amount and size of effusion on chest X-rays were reliable indicators (9). Subsequently, Schwalk et al. narrowed the participants into NSCLC with actionable mutation and proposed the correlation between pleural fluid lactate dehydrogenase (LDH) and positive cytologic examination results (10) and recurrence, which was renovated by our group’s preliminary study with a longer follow-up period for Asian NSCLC patients with various treatment lines (5). Nevertheless, these studies were mostly designed based on Caucasian patients with prior treatment history and did not elaborate on discriminative performances of the predictive models, which in turn brought in bias.
It has been pointed out that individual qualitative or semi-quantitative parameters of fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) might not be equipped with adequate predictive value in the differential diagnosis of MPE (11). The establishment of an integrated PET/CT scoring system (12), as well as pleural uptake patterns (13), would improve the dilemma with an improved area under the curve (AUC) of 0.949. Nevertheless, the existing studies have been mostly launched on pan-cancer cohorts and no studies have applied PET/CT to predict MPE recurrence in lung cancer.
The standard and prioritized management of symptomatic MPE requiring intervention is thoracentesis and indwelling pleural catheter drainage (13). Intrapleural perfusion, pleurodesis or video-assisted thoracic surgery (VATS) have shown promising potential as additional MPE control measures which improved overall survival (OS) and prolonged the time to MPE recurrence (14-17). Several studies have explored the effect of intrapleural perfusion on the MPE control (18,19), nevertheless, no specific analysis was performed based on a first-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) treatment NSCLC cohort.
In this study, we aimed to apply the non-invasive PET/CT to identify the NSCLC patients with first-onset MPE who have a higher recurrence risk with or without actionable mutation. We also explore the effects of local intervention as well as the progression patterns of MPE patients receiving first-line EGFR-TKI treatment. These might assist in the identification of treatment-naïve patients with higher MPE recurrence risks. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-291/rc).
Methods
Study design
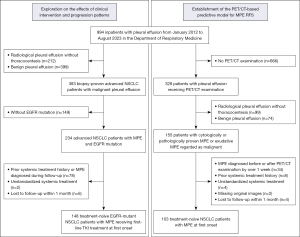
From January 2012 to August 2023, a total of 994 inpatients with pleural effusion in the Department of Respiratory and Critical Care Medicine in Jinling Hospital were retrospectively screened. To establish the PET/CT-based predictive model for MPE recurrence, treatment-naïve NSCLC patients who were diagnosed with MPE at the onset and received PET/CT scanning were recruited. Eligible patients were required to meet the following criteria: (I) MPE cytologically proven by thoracentesis or histologically proven by pleural biopsy, or exudative pleural effusion highly suggestive of malignancy excluding other non-cancer reasons (20,21); (II) receiving PET/CT examination within 1 week of the diagnosis of MPE; (III) pathologically proven NSCLC; (IV) MPE occurring concurrently at the diagnosis of NSCLC. Patients were excluded for the following reasons: (I) MPE diagnosed before or after PET/CT examination by over 1 week; (II) prior systemic treatment history; (III) receiving unstandardized systemic treatment (such as receiving chemotherapy with actionable mutations, or targeted therapy without actionable mutations); (IV) missing original images; (V) lost to follow up within 1 month. To elaborate on the effects of clinical intervention and progression patterns, a cohort of treatment-naïve EGFR-mutant NSCLC patients with MPE receiving first-line TKI treatment were enrolled. Eligible patients were required to satisfy the following criteria, including MPE cytologically proven by thoracentesis or histologically proven by pleural biopsy, or exudative pleural effusion highly suggestive of malignancy excluding other non-cancer reasons; pathologically proven NSCLC; MPE occurring concurrently at the diagnosis of NSCLC; with EGFR mutation and first-line EGFR-TKI treatment. Patients with prior systemic treatment history, MPE occurring during follow-up and lost to follow-up within 1 month were ruled out. The flowchart of the study is illustrated in Figure 1. The last follow-up time was in February 2024. Actionable mutations included common anaplastic lymphoma kinase (ALK) rearrangement and EGFR mutations (EGFR Del19, L858R, T790M), as well as b-Raf proto-oncogene (BRAF), c-Mesenchymal-epithelial transition factor (c-MET), proto-oncogene (RET), Kirsten rat sarcoma viral oncogene (KRAS), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) and human epidermal growth factor receptor 2 (HER2) alterations. Detailed targeted therapy regimens contained first-generation EGFR-TKI (gefitinib, erlotinib and icotinib), second-generation EGFR-TKI (afatinib and dacomitinib), third-generation EGFR-TKI (osimertinib, almonertinib and furmonertinib), ALK/ROS1-TKI (crizotinib and lorlatinib), HER2-TKI (dacomitinib), c-MET-TKI (savolitinib), RET-TKI (pralsetinib) and trametinib for BRAF V600E mutation. The study was conducted following the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Jinling Hospital (registration ID: 2022NZKY-006-07). Informed consent from individuals was waived due to the retrospective nature of this study.
PET/CT imaging protocol
Three different PET/CT scanners were used: Scanner A (n=68), Discovery 710 PET/CT scanner (General Electric Medical Systems, Chicago, USA); Scanner B (n=26), Biograph LSO Sensation 16 PET/CT scanner (Siemens Medical Solutions, Malvern, USA); Scanner C (n=9), PoleStar m660 PET/CT scanner (SinoUnion, Beijing, China). Briefly, patients were injected with FDG according to current guidelines (range, 3.0–4.0 MBq/kg), and images were acquired 60 min later (median 60 min). The PET scan was obtained from the skull base to the proximal thighs after an initial low-dose CT. PET images were reconstructed using three iterative algorithms respectively (Scanner A: 3D-Ordered Subset Expectation Maximization (OSEM) algorithm with time-of-flight (TOF) and point spread function (PSF) correction, 128×128 matrix, voxel size 3.91×3.91×3.27 mm3; Scanner B: OSEM algorithm with TOF, 4 iterations, 8 subsets, 128×128 matrix, voxel size 5×5×5.3 mm3; Scanner C: OSEM algorithm with TOF, 2 iterations, 10 subsets, 192×192 matrix, 3.15×3.15×1.87 mm3).
Clinical and PET/CT parameters extraction
Baseline information including age, gender, stage, treatment regimen, ECOG PS, bone metastases, liver metastases, contralateral lung metastases, adrenal gland metastases, brain metastases and mutation status, as well as pleural fluid parameters such as lactate dehydrogenase (p-LDH), glucose (p-Glucose), and carcinoembryonic antigen (p-CEA) were collected from electronic records. MPE control measures included indwelling pleural catheter and intrapleural perfusion therapy. Progression patterns represented the first sign of recurrence/progression during follow-up, including progressive disease (PD) confirmed by Response Evaluation Criteria in Solid Tumors 1.1 criteria (RECIST 1.1) (22), MPE recurrence, both (PD and MPE recurrence) and no progression/recurrence. Patients were divided into with and without systemic treatment change subgroups depending on whether the first MPE recurrence was regarded as a failure of systemic treatment.
We further analyzed the FDG-avid hypermetabolic tumor lesions on PET images throughout the body via LIFEx 7.4.0 software (www.lifexsoft.org) (23). Primary, pleural, and intrathoracic lymph nodes (LN)’ maximum standardized uptake value (SUVmax) were measured by drawing regions of interest (ROIs) in 3D semiautomatically with a threshold of 42% of SUVmax (24). Each ROI on PET and CT images was respectively resampled into 3×3×3 mm3 and 1×1×1 mm3. Furthermore, to correct for multi-scanner and multi-protocol effects, we processed PET-related parameters using a previously validated post-reconstruction harmonization approach “ComBat” in PET studies (25). Further, all ROIs were reviewed by two nuclear physicians (T Liu and J.W.) with 6 and 20 years’ experience who were blinded to the endpoints. LN exhibiting elevated uptake (higher than adjacent normal soft tissue) with no calcification or attenuation of less than 70 Hounsfield units (HU) are classified as positive. The mediastinal background SUVmean was calculated using 2D ROIs in three successive slices in the right pulmonary artery (below the carina) (26). The pleural activity was defined as positive if the was higher than the mediastinal background activity. If the LN or pleura was deemed PET negative, mediastinal background SUVmean was utilized alternatively (27). LN SUVmax in each region including N1, N2, and N3 (28) was respectively calculated. Pleural FDG intake patterns were divided into four categories, namely negative, nodular, linear, and encasement. Encasement meant the pleural lesions with abnormal uptake occupied the majority of pleura on the CT transects. Noteworthily, since different uptake patterns might occur simultaneously at different layers, a patient might be featured with two or three patterns.
Endpoint events
MPE recurrence was defined as re-accumulation of cytologically proven ipsilateral MPE requiring intervention, including thoracentesis and/or chest tube drainage. Specifically, recurrence-free survival 1 (RFS1) was defined as the time from the MPE onset to the first recurrence. Recurrence-free survival 2 (RFS2) was defined as the time from the first to second MPE recurrence. The primary endpoint event was MPE recurrence during the whole follow-up period. Moreover, 100-day MPE recurrence and 300-day MPE recurrence were also evaluated according to the high recurrence rate for MPE within 1 month (10,29) and 300 days by published studies (5). Progression-free survival (PFS) was defined as the time from onset to PD according to RECIST 1.1 (22).
Statistical analyses
Continuous variables with normal distribution were described as mean (standard deviation) and otherwise as median [interquartile range (IQR)]. Categorical variables were presented as counts (percentage). The cut-off values of pleural fluid as well as PET/CT parameters were recognized using the “survminer” R package with recurrence as the endpoint. Kaplan-Meier (K-M) survival curves with log-rank test and univariate Cox regression analysis were used to explore the association between parameters and RFS. To choose predictive features, variables with P<0.05 by univariate Cox regression were further incorporated into the multivariable Cox regression model with a backward step-wise selection method and the Akaike information criterion as the stopping criteria. Cox regression model (Cox) and random survival forest (RSF) model were used to establish predictive models for RFS1. RSF model was applied using the “randomForestSRC” R package with the number of trees, max number of levels in each decision tree and random seed set to 1,000, 20 and 123. Time-dependent receiver operating characteristic (ROC) curve analysis and concordance-index (C-index) were used to evaluate the predictive efficiency. The DeLong test was applied for model comparison at different time points. The bootstrap C-index for the model with 1,000 resampling groups was calculated for internal validation. Variables with two-sided P<0.05 were considered significant. All statistical analyses were conducted via R 4.3.3 and GraphPad Prsim 9 software.
Results
Baseline characteristics
In the cohort of EGFR-mutant treatment-naïve NSCLC patients with first-onset MPE regardless of the implementation of PET/CT examination, a total of 148 patients were eventually recruited during the median follow-up period of 683 (IQR, 406–1,147) days (Figure 1). Among them, 76 (51.4%) patients harbored exon 19 deletion and 63 (42.6%) harbored exon 21 mutation. The majority of patients received a first-generation EGFR-TKI [97 (65.5%)] regimen, followed by 38 (25.7%) and 10 (6.8%) with third- and second-generation EGFR-TKI. The MPE control measures within 30 days included intrapleural perfusion and indwelling pleural catheter treatment, which was implemented on 91 (61.5%) and 134 (90.5%) patients respectively. A sum of 69 (46.6%) patients witnessed the first MPE recurrence with a median RFS1 of 783 [IQR, 587–not reached (NR)] days, while 35 (23.6%) recurred more than once (Table 1).
Table 1
| Parameters | Total (n=148) |
|---|---|
| Age (years) | 61 [51–69] |
| Gender | |
| Male | 90 (60.8) |
| Female | 58 (39.2) |
| EGFR-activating mutations | |
| Exon 19 deletion | 76 (51.4) |
| Exon 21 mutation | 63 (42.6) |
| Unknown | 9 (6.1) |
| ECOG PS | |
| 0–1 | 140 (94.6) |
| ≥2 | 8 (5.4) |
| Contralateral lung metastases (yes/no) | 25 (16.9)/123 (83.1) |
| Liver metastases (yes/no) | 15 (10.1)/133 (89.9) |
| Bone metastases (yes/no) | 55 (37.2)/93 (62.8) |
| Adrenal gland metastases (yes/no) | 8 (5.4)/140 (94.6) |
| Systemic targeted treatment within 30 days | |
| First-generation TKI | 97 (65.5) |
| Second-generation TKI | 10 (6.8) |
| Third-generation TKI | 38 (25.7) |
| Unknown | 3 (2.0) |
| MPE control measures within 30 days | |
| Intrapleural perfusion | 91 (61.5) |
| Indwelling pleural catheter | 134 (90.5) |
| First sign of recurrence/progression during follow-up | |
| Progressive disease† | 65 (43.9) |
| MPE recurrence | 29 (19.6) |
| Both | 20 (13.5) |
| No progression/recurrence | 34 (23.0) |
| First MPE recurrence requiring drainage | |
| Recurrence within 100 days | 25 (16.9) |
| Recurrence within 300 days | 59 (39.9) |
| Recurrence | 69 (46.6) |
| Second MPE recurrence requiring drainage | |
| Recurrence within 100 days | 27 (18.2) |
| Recurrence | 35 (23.6) |
Data are presented as median [interquartile range] or n (%). †, according to the Response Evaluation Criteria in Solid Tumors 1.1 criteria. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; MPE, malignant pleural effusion; ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor.
During the median follow-up period of 376 (IQR, 138–728) days, a total of 103 treatment-naïve NSCLC patients with MPE at the onset receiving PET/CT examination were recruited (Figure 1). Median RFS1 reached 424 (IQR, 251–NR) days in the total cohort, 1,183 (IQR, 511–NR) days for patients with actionable mutation, and 89 (IQR, 65–590) days for those without actionable mutation. A total of 30 (29.1%) and 40 (38.8%) patients experienced recurrence within 100 and 300 days separately. Most patients were adenocarcinomas [94 (91.3%)] and harbored actionable mutations with standardized first-line TKI therapy [58 (56.3%)]. It was worth mentioning that the majority [75 (80.6%)] of patients received indwelling pleural catheter drainage and 62 (60.2%) received additional intrapleural perfusion therapy via a chest tube, including the injection of cytotoxic drugs, antiangiogenic drugs, and traditional Chinese medicine (Table 2). In terms of PET/CT parameters, few patients [38 (37.9%)] had lesions closely adjoining to the pleura, but most of them presented pleural [77 (74.8%)] and intrathoracic LN metastases [80 (77.7%)] with abnormal FDG uptake. An investigation of the FDG uptake patterns in pleural metastases revealed that linear [42 (40.8%)] and nodular [61 (59.2%)] lesions occurred frequently, while the abnormal uptake encasing the pleura vastly was less [8 (7.8%)] observed (Figure 2). Detailed information of the PET-based and pleural fluid parameters is listed in Table 3.
Table 2
| Parameters | Actionable mutation (+) (n=58) | Actionable mutation (−) (n=45) | Total (n=103) |
|---|---|---|---|
| Age (years) | 60 [51–68] | 68 [58–75] | 65 [23–89] |
| Gender | |||
| Male | 26 (44.8) | 33 (73.3) | 59 (57.3) |
| Female | 32 (55.2) | 12 (26.7) | 44 (42.7) |
| ECOG PS | |||
| 0–1 | 56 (96.6) | 38 (84.4) | 94 (91.3) |
| ≥2 | 2 (3.4) | 7 (15.6) | 9 (8.7) |
| Histological type | |||
| Adenocarcinoma | 58 (100.0) | 36 (80.0) | 94 (91.3) |
| Non-adenocarcinoma | 0 (0.0) | 9 (20.0) | 9 (8.7) |
| Contralateral lung metastases | |||
| No | 50 (86.2) | 42 (93.3) | 92 (89.3) |
| Yes | 8 (13.8) | 3 (6.7) | 11 (10.7) |
| Bone metastases | |||
| No | 35 (60.3) | 31 (68.9) | 66 (64.1) |
| Yes | 23 (39.7) | 14 (31.1) | 37 (35.9) |
| Liver metastases | |||
| No | 53 (91.4) | 40 (88.9) | 93 (90.3) |
| Yes | 5 (8.6) | 5 (11.1) | 10 (9.7) |
| Brain metastases | |||
| No | 51 (87.9) | 41 (91.1) | 92 (89.3) |
| Yes | 7 (12.1) | 4 (8.9) | 11 (10.7) |
| Adrenal gland metastases | |||
| No | 53 (91.4) | 40 (88.9) | 93 (90.3) |
| Yes | 5 (8.6) | 5 (11.1) | 10 (9.7) |
| Stage | |||
| IVA | 25 (43.1) | 24 (53.3) | 49 (47.6) |
| IVB | 33 (56.9) | 21 (46.7) | 54 (52.4) |
| Systemic treatment within 30 days | |||
| Mut (−)—chemotherapy | 0 (0.0) | 30 (66.7) | 30 (29.1) |
| Mut (+)—targeted therapy | 58 (100.0) | 0 (0.0) | 58 (56.3) |
| Mut (−)—immunotherapy | 0 (0.0) | 15 (33.3) | 15 (14.6) |
| MPE control measures within 30 days | |||
| Intrapleural perfusion | 39 (67.9) | 23 (51.1) | 62 (60.2) |
| Indwelling pleural catheter | 44 (84.6) | 31 (68.9) | 75 (80.6) |
| First MPE recurrence requiring drainage | |||
| Recurrence within 100 days | 8 (13.8) | 22 (48.9) | 30 (29.1) |
| Recurrence within 300 days | 15 (25.9) | 25 (55.6) | 40 (38.8) |
| Recurrence | 27 (46.6) | 32 (71.1) | 59 (57.3) |
Data are presented as median [interquartile range] or n (%). NSCLC, non-small cell lung cancer; MPE, malignant pleural effusion; PET/CT, positron emission tomography/computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; mut, actionable mutation.
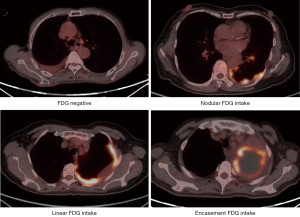
Table 3
| Parameters | Actionable mutation (+) (n=58) | Actionable mutation (−) (n=45) | Total (n=103) |
|---|---|---|---|
| Qualitative parameters | |||
| Primary lesion adjacent to pleura (yes/no) | 24 (41.4)/34 (58.6) | 14 (31.1)/31 (68.9) | 38 (36.9)/65 (63.1) |
| Pleural FDG uptake (positive/negative) | 44 (75.9)/14 (24.1) | 33 (73.3)/12 (26.7) | 77 (74.8)/26 (25.2) |
| Intrathoracic lymph nodes FDG uptake (positive/negative) | 45 (77.6)/13 (22.4) | 35 (77.8)/10 (22.2) | 80 (77.7)/23 (22.3) |
| Pleural FDG uptake pattern | |||
| Pleural FDG uptake (−) | 14 (24.1) | 12 (26.7) | 26 (25.2) |
| Encasement | 4 (6.9) | 4 (8.9) | 8 (7.8) |
| Nodular | 37 (63.8) | 24 (53.3) | 61 (59.2) |
| Linear | 23 (39.7) | 19 (42.2) | 42 (40.8) |
| Quantitative parameters | |||
| LN SUVmax (g/mL) | 5.74 [3.72–9.41] | 6.38 [3.80–8.82] | 6.38 [3.80–8.82] |
| N1 SUVmax (g/mL) | 4.44 [2.81–7.07] | 4.29 [2.58–7.31] | 4.29 [2.58–7.31] |
| N2 SUVmax (g/mL) | 4.87 [3.05–8.69] | 4.79 [3.50–7.46] | 4.79 [3.50–7.46] |
| N3 SUVmax (g/mL) | 3.27 [1.78–5.00] | 2.84 [1.92–5.54] | 2.84 [1.92–5.54] |
| Pleural SUVmax (g/mL) | 5.14 [3.13–8.61] | 4.97 [3.20–7.91] | 4.97 [3.20–7.91] |
| Pleural MTV (mL) | 6.76 [0.00–27.60] | 13.52 [0.00–38.19] | 7.89 [0.00–30.41] |
| Pleural TLG (g) | 21.20 [0.00–79.86] | 30.32 [0.00–112.78] | 21.20 [0.00–91.99] |
| Primary SUVmax (g/mL) | 10.93 [6.88–13.72] | 9.53 [6.71–11.34] | 9.53 [6.71–11.34] |
| Pleural fluid parameters | |||
| p-CEA (μg/L) | 139.80 [22.59–709.98] | 11.69 [3.57–236.96] | 93.70 [4.22–528.92] |
| p-LDH (U/L) | 441.00 [258.50–723.80] | 287.00 [196.20–522.50] | 355.00 [225.00–673.80] |
| p-Glucose (mmol/L) | 6.20 [5.18–7.65] | 6.30 [4.90–7.90] | 6.20 [5.00–7.90] |
Data are presented as median [interquartile range] or n (%). PET/CT, positron emission tomography/computed tomography; NSCLC, non-small cell lung cancer; MPE, malignant pleural effusion; FDG, 18F-fluorodeoxyglucose; LN, lymph nodes; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; p-CEA, pleural fluid carcinoma embryonic antigen; p-LDH, pleural fluid lactate dehydrogenase.
Progression patterns and the predictors of MPE recurrence for patients with MPE and first-line EGFR-TKI treatment
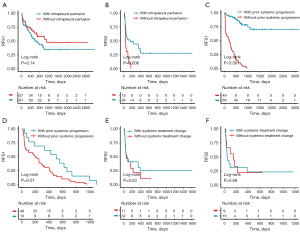
To explore the progression patterns of the patients with MPE, we enrolled a relatively homogeneous treatment-naïve cohort with MPE and first-line EGFR-TKI treatment. During the follow-up period, the majority of patients [65 (43.9%)] witnessed systemic PD as the first sign of recurrence/progression, while merely 29 (19.6%) patients underwent MPE recurrence alone as the first sign. Moreover, 20 (13.5%) patients had MPE recurrence accompanied by systemic PD simultaneously (Table 1). Further survival analysis indicated that neither the indwelling pleural catheter nor intrapleural perfusion therapy at baseline was associated with the first MPE recurrence (P=0.71; P=0.14) (Table S1, Figure 3A), while the log-rank test indicated that intrapleural perfusion therapy at first recurrence seemed to be a protective indicator for second MPE recurrence (P=0.006) (Figure 3B). Furthermore, we explore whether the prior history of systemic progression of other sites exerted a certain effect on MPE recurrence. It was indicated that prior systemic progression was a protective factor for RFS1, either for all the patients (P<0.001) or the subgroup with at least one MPE recurrence during follow-up (P<0.001; P=0.01) (Figure 3C,3D). Moreover, we discussed whether the first MPE recurrence regarded as the failure of systemic treatment, which in turn led to the change of systemic treatment, could influence the time from the first to second MPE recurrence. It was indicated that the change of systemic treatment was not linked with RFS2 for patients with MPE recurrence as the first sign of progression/recurrence, regardless of the presence of simultaneous progression of other sites (P=0.53; P=0.69) (Figure 3E,3F). For 148 EGFR-mutant treatment-naïve NSCLC patients with MPE at diagnosis receiving EGFR-TKI treatment, multivariate analyses indicated that only combined chemotherapy was significantly associated with 300-day MPE recurrence [hazard ratio (HR), 0.21; 95% confidence interval (CI): 0.07–0.62; P=0.005], while the generation of TKI (second- vs. first-generation, P=0.83; third- vs. first-generation, P=0.86) and intrapleural perfusion treatment (P=0.91) were not (Table S1).
Establishment, evaluation and validation of PET/CT-based predictive model for the first MPE RFS
The Log-rank test illustrated that patients with LN SUVmax >4.50 g/mL tended to witness shorter 300-day RFS1 (P=0.001) and unfavorable PFS (P=0.02) (Figure 4A,4B). Subgroup analysis suggested that LN SUVmax >4.50 g/mL remained as an independent risk factor for RFS1 and PFS for patients with actionable mutation (P=0.01; P=0.02) (Figure 4C,4D).
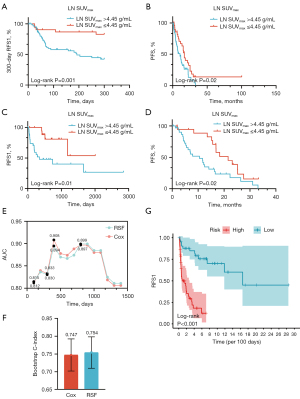
Furthermore, to establish a robust predictive model for the first MPE recurrence, four predictors were eventually reserved via multivariate step-wise Cox regression and constituted the Cox model for RFS1, including female gender (HR, 0.40; 95% CI: 0.20–0.81; P=0.01), bone metastases (HR, 3.16; 95% CI: 1.59–6.28; P=0.001), systemic treatment within 30 days (targeted therapy vs. chemotherapy, HR: 0.32, 95% CI: 0.15–0.66; P=0.002; immunotherapy vs. chemotherapy, HR: 0.99, 95% CI: 0.38–2.58; P=0.97) and LN SUVmax >4.50 g/mL (HR, 2.54; 95% CI: 1.16–5.56; P=0.01) (Table 4). RSF model was simultaneously established based on the identical selected parameters, however, no significant differences were observed between RSF and Cox models at diverse time points by Delong tests (Table S2). Therefore, we took the simplified Cox model as the appropriate predictive model for RFS1, with an AUC of ROC of 0.81 (95% CI: 0.72–0.91) at 100 days and 0.83 (95% CI: 0.74–0.93) at 300 days (Figure 4E, Table S2). The bootstrap C-index of the Cox model was 0.747 (standard error of mean, 0.045) (Figure 4F). The K-M survival curve illustrated that patients in the high-risk group according to the Cox model had distinctly shorter RFS1 compared to those in the low-risk group (P<0.001) (Figure 4G).
Table 4
| Parameters | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Clinical parameters | |||||
| Age | 1.01 (0.99–1.03) | 0.35 | – | – | |
| Gender (female) | 0.47 (0.26–0.86) | 0.01 | 0.40 (0.20–0.81) | 0.01 | |
| Histology (adenocarcinoma) | 0.74 (0.26–2.09) | 0.57 | – | – | |
| ECOG PS ≥2 | 1.06 (0.38–2.96) | 0.91 | – | – | |
| Stage IVB | 1.77 (0.98–3.18) | 0.057 | – | – | |
| Liver metastases | 0.80 (0.25–2.59) | 0.71 | – | – | |
| Bone metastases | 1.87 (1.05–3.33) | 0.03 | 3.16 (1.59–6.28) | 0.001 | |
| Contralateral lung metastases | 0.53 (0.16–1.70) | 0.28 | – | – | |
| Brain metastases | 1.34 (0.53–3.39) | 0.54 | – | – | |
| Adrenal gland metastases | 1.08 (0.42–2.75) | 0.87 | – | – | |
| Systemic treatment within 30 days | |||||
| Mut (−)—chemotherapy | Ref. | Ref. | |||
| Mut (+)—targeted therapy | 0.26 (0.14–0.51) | <0.001 | 0.32 (0.15–0.66) | 0.002 | |
| Mut (−)—immunotherapy | 0.58 (0.24–1.38) | 0.20 | 0.99 (0.38–2.58) | 0.97 | |
| MPE control measurements within 30 days | |||||
| Indwelling pleural catheter | 1.18 (0.53–2.64) | 0.68 | – | – | |
| Intrapleural perfusion | 1.34 (0.73–2.45) | 0.34 | – | – | |
| Qualitative parameters | |||||
| Primary lesion adjacent to pleura | 0.61 (0.32–1.13) | 0.11 | – | – | |
| Pleural FDG uptake (+) | 1.01 (0.56–1.82) | 0.96 | – | – | |
| Lymph nodes FDG uptake (+) | 2.27 (1.11–4.63) | 0.02 | – | – | |
| Pleural FDG uptake pattern | |||||
| Encasement | 1.64 (0.65–4.17) | 0.29 | – | – | |
| Nodular | 1.10 (0.66–1.86) | 0.71 | – | – | |
| Linear | 0.95 (0.56–1.59) | 0.83 | – | – | |
| Quantitative parameters | |||||
| LN SUVmax >4.50 g/mL | 2.80 (1.38–5.69) | 0.004 | 2.54 (1.16–5.56) | 0.01 | |
| N1 SUVmax >3.76 g/mL | 2.97 (1.53–5.79) | 0.001 | – | – | |
| N2 SUVmax >5.04 g/mL | 1.73 (0.96–3.11) | 0.06 | – | – | |
| N3 SUVmax >6.46 g/mL | 2.76 (1.38–5.51) | 0.004 | – | – | |
| Pleural SUVmax >3.66 g/mL | 0.80 (0.44–1.46) | 0.46 | – | – | |
| Pleural MTV >18.00 mL | 0.81 (0.44–1.50) | 0.50 | – | – | |
| Pleural TLG >30.32 g | 0.73 (0.41–1.31) | 0.29 | – | – | |
| Primary SUVmax >10.23 g/mL | 0.66 (0.36–1.22) | 0.18 | – | – | |
| Pleural fluid parameters | |||||
| p-CEA >15.6 μg/L | 0.59 (0.32–1.09) | 0.08 | – | – | |
| p-LDH >786 U/L | 1.58 (0.81–3.09) | 0.17 | – | – | |
| p-Glucose >6.9 mmol/L | 1.49 (0.82–2.71) | 0.19 | – | – | |
MPE, malignant pleural effusion; ECOG PS, Eastern Cooperative Oncology Group performance status; FDG, 18F-fluorodeoxyglucose; LN, lymph nodes; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; p-CEA, pleural fluid carcinoma embryonic antigen; p-LDH, pleural fluid lactate dehydrogenase; HR, hazard ratio; CI, confidence interval; Ref., reference.
Moreover, we ulteriorly performed multivariate Cox regression analysis for RFS1 in patients with actionable mutation. It was shown that LN SUVmax >4.50 g/mL (HR, 4.26; 95% CI: 1.24–14.65; P=0.02) and stage IVB (HR, 8.46; 95% CI: 2.35–30.41; P=0.001) were reserved via step-wise Cox regression as the optimal prediction model for RFS1 (Table S3).
Discussion
As far as we know, this is the first study using PET/CT to develop a model predicting MPE recurrence for advanced NSCLC patients with MPE at the onset. In this study, for MPE patients with first-line EGFR-TKI treatment, the implementation of intrapleural perfusion therapy exerts no definitive effects on the time to the first MPE recurrence, but could potentially prolong the time from first to second MPE recurrence. Conversely, the prior change of systemic treatment could improve the time to the first MPE recurrence. Moreover, the higher level of intrathoracic LN SUVmax rather than primary or pleural SUVmax was an independent risk factor for MPE recurrence in NSCLC patients with or without actionable mutation. Specifically, LN SUVmax, gender, bone metastases, and systemic treatment could collectively suggest MPE recurrence with an optimal 300-day AUC of 0.83. For patients with actionable mutation, LN SUVmax >4.50 g/mL and stage ⅣB could forecast MPE recurrence independently. However, pleural SUVmax, MTV, TLG, as well as uptake patterns, had no connection to MPE recurrence.
In clinical scenarios, whether the first MPE recurrence without other sites’ progression was regarded as a failure of systemic treatment was a comprehensive judgment according to the serum CEA level and rate of progression/recurrence established by a panel of physicians-in-charge. According to our research, it is worth mentioning whether the MPE recurrence being seen as the failure of systemic treatment, which in turn resulted in the change of systemic therapy, was not associated with RFS2 in our cohort. Conversely, the intrapleural perfusion treatment at the first recurrence significantly prolonged the time from the first to second MPE recurrence, which further emphasized the necessity of local rather than systemic treatment on recurrent MPE (e.g., at the second recurrence). Intriguingly, the prior systemic progression was a protective factor for the first MPE recurrence compared to those without prior systemic progression. It would be explained by the fact that the prior systemic progression would lead to the change of systemic therapy beforehand. These preliminary findings should be validated in larger prospective cohorts.
The currently widely applied MPE control measures contained the prioritized thoracentesis and indwelling pleural catheter drainage (14). Additionally, intrapleural perfusion, pleurodesis and VATS have been proven effective in improving OS and time to MPE recurrence (15-17). In a 30-patient NSCLC cohort with MPE receiving first-line EGFR-TKI treatment, pleurodesis using sterile talc or hypotonic cisplatin was a proven protective factor for time to MPE recurrence (17). Another retrospective study (30) enrolled 195 non-squamous NSCLC patients with MPE who received chest tube drainage plus chemotherapy perfusion or VAST plus chemotherapy perfusion respectively, and the median OS of patients in the VATS plus chemotherapy group was higher than that of the drainage plus chemotherapy perfusion group (25 vs. 11 months, P<0.05). Unfortunately, although pleurodesis is recommended as the first-line definitive pleural intervention for the management of dyspnea according to American guidelines (4), it was not routinely carried out in our center. According to our analysis, the non-hyperthermic intrapleural perfusion treatment could not hinder the time to the first MPE recurrence under the background of EGFR-TKI treatment. However, it was a protective factor for time from first to second MPE recurrence. It implies that we should re-consider the application of intrapleural perfusion treatment for first-onset MPE and prompt it more at the moment of recurrent MPE. Contrary to our findings, previous studies inferred that intrapleural perfusion could improve RFS (19,31). However, these studies enrolled patients with diverse systemic treatment regimens or previous treatment history, which would inevitably introduce bias. Moreover, due to the relatively limited sample size, we could not subdivide the specific intrapleural perfusion agents, which should be tackled in the future.
When characteristics linked to MPE recurrence are identified at the time of diagnosis, the initial local intervention can be tailored to the patient, thereby reducing the negative effects on quality of life. For example, we could prompt pleurodesis or VATS in patients with higher recurrence risks. Previous studies have established diverse predictive models for MPE recurrence mainly for Caucasians (9,10). In light of this, our team (5) established another model for MPE recurrence in Chinese, with patients presenting diverse treatment lines. This might hamper the models’ application since patients at more advanced stages might present frequently recurrent MPE (32). Moreover, the discriminative performances of the aforementioned models were unreported and mentioned merely once with an unpleasing AUC of 0.55 (9), while our model was superior with an AUC of 0.83 at 300 days and 0.81 at 100 days. Therefore, we regard it compulsory to introduce these more persuasive parameters with a detailed presentation of model performances.
PET/CT is a noninvasive imaging tool embedded with tumor metabolic information which has been proven to be a convincible survival predictor. Pioneeringly, Duysinx et al. (33) first applied PET/CT in a 25-patient lung cancer cohort with MPE and impli1ffed that the degree of metabolic activity of pleural extension originating from the lung rather than the primary lesion is a stronger survival indicator. Conventionally, MPE is featured by pleural thickening ≥1 cm on CT (34) and scattered or diffused pleural FDG uptake in the parietal pleura on PET images. In summary, 75.6–84.5% of patients with MPE have pleural thickening coupled with increased FDG uptake, and 54.8–66.3% present positive mediastinal LN (12,35). Similarly, in our cohort, nearly a quarter of patients presented pleural thickening without FDG avid lesions. Considering the subjectivity of visual assessments, we employed more unified semiquantitative parameters like SUVmax to explore its effect on MPE recurrence. Sadly, neither the dichotomous positive pleural uptake nor the pleural SUVmax was associated with MPE recurrence. Considering a sole SUV which is usually calculated over a small area of the pleura might not be suitable for the often-extensive pleural involvement in MPE, we followed and renovated the pleural uptake classification by Cohen et al. and hypothesized that the severity of the pleural involvement might in turn affect recurrence (36). Unfortunately, both volume parameters including pleural MTV and TLG and pleural FDG intake patterns including negative, nodular, linear, and encasement failed radically in presenting disparate recurrence hazards.
Mediastinal LN metastases have displayed reliable predictive capacity for survival and brain metastases (37) in NSCLC. It was shown that the higher clinical N stage distinctly suggested worse survival for patients with MPE (38). Moreover, for early-stage NSCLC patients, LN metastasis was found to be positively correlated with postoperative molecular residual disease, which indicated poor prognosis (39). Concurrently, PET/CT is a splendid tool in the staging and diagnostics of mediastinal LN (40-42). For NSCLC patients receiving neoadjuvant chemoimmunotherapy or chemotherapy, studies (43,44) have found that pathologic N2/N1 suggested inferior disease-free survival and PET/CT N2 (+) could ulteriorly stratify survival. However, to our knowledge, no studies have probed into LN’ role in MPE recurrence. Lymphatic has been proven essential in the generation of MPE (45,46), which is caused by the direct mechanism of extravasation from hyper-permeable parietal or visceral pleura, or/and indirect ways via tumor arteries or obstructed mediastinal lymphatic outflows. Post-mortem studies demonstrated that the effusion occurrence is inferred by LN metabolic activity detected by PET/CT, which might not only indicate the production of pleural fluid but profoundly influence MPE recurrence. Inspiringly, we similarly found that a higher level of LN was a reliable recurrence indicator. Underlyingly, higher LN SUVmax might indicate severer tumor metastases in the subordinate drainage LN, which blocks the outflow of pleural fluid thus leading to quick recurrence.
For Asian NSCLC patients with MPE, targeted therapy has prolonged the time to recurrence, with a median RFS of 21.7 months compared to 2.5 months in the chemotherapy group without pleurodesis (47). Another study suggested that compared to chemotherapy, targeted therapy observed an improved recurrence period from 88 to 182 days (17). Consistent with these studies, in our analysis, only a small proportion of patients experienced recurrence (at 100 and 300 days) under the supervision of EGFR-TKI. The favorable outcomes brought by TKIs emphasized the necessity of reaffirming the predictive parameters in the targeted therapy setting. Pleasingly, LN SUVmax maintained its predictive capacity in patients with actionable mutation. More studies are warranted to validate our findings.
Compared to the previous research, our study is superior as the first study applying the non-invasive imaging tool to develop a predictive model for MPE recurrence in advanced NSCLC and is well-equipped with an improved discriminative performance. Also, we explored the progression/recurrence patterns of MPE patients and compared the contribution of systemic and intrapleural perfusion treatment for first and second MPE recurrence. Despite the trivial findings, this study harbored distinct limitations. First and foremost, the sample size was relatively small and the retrospective design might hamper the selection of a representative cohort. Moreover, it has to be admitted that we did not probe into the repeatedly mentioned clinical parameters such as the amount and size of the effusion, and specific intrapleural perfusion agents consequently. In truth, we aimed merely at exploring the PET/CT parameters but not these clinical variables. Finally, most participants received PET/CT merely at baseline, making it impossible to evaluate prompt therapy response and the decreased FDG uptake after initiation of systemic therapy. Therefore, prospective studies with larger cohorts and reassessment are warranted to validate our findings and encompass more clinical parameters.
Conclusions
In conclusion, we comprehensively profiled the characteristics of pleural lesions in advanced treatment-naïve NSCLC patients with MPE. LN rather than pleural metabolic activity could predict MPE recurrence for patients with or without targeted therapy. Moreover, we should re-consider the application of intrapleural perfusion treatment for first-onset MPE and prompt it more at the moment of recurrent MPE. Promisingly, we could probably apply the non-invasive tool to identify the candidate risk factors for MPE recurrence.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-291/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-291/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-291/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-291/coif). Y.S. serves as an Editor-in-Chief of Translational Lung Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted following the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Jinling Hospital (registration ID: 2022NZKY-006-07). Informed consent from individuals was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mongardon N, Pinton-Gonnet C, Szekely B, et al. Assessment of chronic pain after thoracotomy: a 1-year prevalence study. Clin J Pain 2011;27:677-81. [Crossref] [PubMed]
- Ryu JS, Ryu HJ, Lee SN, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol 2014;32:960-7. [Crossref] [PubMed]
- Zou J, Bella AE, Chen Z, et al. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: Implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res 2014;42:1110-7. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Xu K, Wu X, Chen L, et al. Risk factors for symptomatic malignant pleural effusion recurrence in patients with actionable mutations in advanced lung adenocarcinoma. Transl Lung Cancer Res 2023;12:1887-95. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol 2018;19:930-9. [Crossref] [PubMed]
- Zhang T, Chen X, Wan B, et al. Development of RECLS score to predict survival in lung cancer patients with malignant pleural effusion. Transl Lung Cancer Res 2021;10:1318-26. [Crossref] [PubMed]
- Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology 2019;24:76-82. [Crossref] [PubMed]
- Schwalk AJ, Ost DE, Saltijeral SN, et al. Risk Factors for and Time to Recurrence of Symptomatic Malignant Pleural Effusion in Patients With Metastatic Non-Small Cell Lung Cancer with EGFR or ALK Mutations. Chest 2021;159:1256-64. [Crossref] [PubMed]
- Fjaellegaard K, Koefod Petersen J, Reuter S, et al. Positron emission tomography-computed tomography (PET-CT) in suspected malignant pleural effusion. An updated systematic review and meta-analysis. Lung Cancer 2021;162:106-18. [Crossref] [PubMed]
- Yang MF, Tong ZH, Wang Z, et al. Development and validation of the PET-CT score for diagnosis of malignant pleural effusion. Eur J Nucl Med Mol Imaging 2019;46:1457-67. [Crossref] [PubMed]
- Thomas R, Azzopardi M, Muruganandan S, et al. Protocol of the PLeural Effusion And Symptom Evaluation (PLEASE) study on the pathophysiology of breathlessness in patients with symptomatic pleural effusions. BMJ Open 2016;6:e013213. [Crossref] [PubMed]
- Mierzejewski M, Korczynski P, Krenke R, et al. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respir Res 2019;20:247. [Crossref] [PubMed]
- Cao Y, Zhang Q, Huang Z, et al. Better effect of intrapleural perfusion with hyperthermic chemotherapy by video-assisted thoracoscopic surgery for malignant pleural effusion treatment compared to normothermic chemoperfusion of the pleural cavity. Cancer Med 2022;11:348-57. [Crossref] [PubMed]
- Wang CQ, Xu J, Jiang H, et al. The evidence framework of traditional Chinese medicine injection (Aidi injection) in controlling malignant pleural effusion: A clustered systematic review and meta-analysis. Phytomedicine 2023;115:154847. [Crossref] [PubMed]
- Chiang KY, Ho JC, Chong P, et al. Role of early definitive management for newly diagnosed malignant pleural effusion related to lung cancer. Respirology 2020;25:1167-73. [Crossref] [PubMed]
- Zhao WY, Chen DY, Chen JH, et al. Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion and ascites. Cell Biochem Biophys 2014;70:623-8. [Crossref] [PubMed]
- Du N, Li X, Li F, et al. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer‑mediated malignant pleural effusion. Oncol Rep 2013;29:2332-40. [Crossref] [PubMed]
- Ost DE, Jimenez CA, Lei X, et al. Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters. Chest 2014;145:1347-56. [Crossref] [PubMed]
- Thomas R, Fysh ETH, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Nioche C, Orlhac F, Boughdad S, et al. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res 2018;78:4786-9. [Crossref] [PubMed]
- Meignan M, Sasanelli M, Casasnovas RO, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging 2014;41:1113-22. [Crossref] [PubMed]
- Orlhac F, Boughdad S, Philippe C, et al. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J Nucl Med 2018;59:1321-8. [Crossref] [PubMed]
- Toney LK, Vesselle HJ. Neural networks for nodal staging of non-small cell lung cancer with FDG PET and CT: importance of combining uptake values and sizes of nodes and primary tumor. Radiology 2014;270:91-8. [Crossref] [PubMed]
- Rogasch JMM, Michaels L, Baumgärtner GL, et al. A machine learning tool to improve prediction of mediastinal lymph node metastases in non-small cell lung cancer using routinely obtainable [18F]FDG-PET/CT parameters. Eur J Nucl Med Mol Imaging 2023;50:2140-51.
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Ost DE, Niu J, Zhao H, et al. Quality Gaps and Comparative Effectiveness of Management Strategies for Recurrent Malignant Pleural Effusions. Chest 2018;153:438-52. [Crossref] [PubMed]
- Li X, Li M, Lv J, et al. Survival Benefits for Pulmonary Adenocarcinoma With Malignant Pleural Effusion After Thoracoscopic Surgical Treatment: A Real-World Study. Front Oncol 2022;12:843220. [Crossref] [PubMed]
- Tao H, Meng Q, Li M, et al. Outcomes of bevacizumab combined with chemotherapy in lung adenocarcinoma-induced malignant pleural effusion. Thorac Cancer 2018;9:298-304. [Crossref] [PubMed]
- Abrão FC, de Abreu IRLB, de Oliveira MC, et al. Prognostic factors of recurrence of malignant pleural effusion: what is the role of neoplasia progression? J Thorac Dis 2020;12:813-22. [Crossref] [PubMed]
- Duysinx B, Corhay JL, Larock MP, et al. Prognostic value of metabolic imaging in non-small cell lung cancers with neoplasic pleural effusion. Nucl Med Commun 2008;29:982-6. [Crossref] [PubMed]
- Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Li Y, Mu W, Li Y, et al. Predicting the nature of pleural effusion in patients with lung adenocarcinoma based on (18)F-FDG PET/CT. EJNMMI Res 2021;11:108. [Crossref] [PubMed]
- Cohen SE, Betancourt J, Soo Hoo GW. Pleural Uptake Patterns in (F18)Fluorodeoxyglucose-Positron Emission Tomography (FDG-PET) Scans Improve the Identification of Malignant Pleural Effusions. J Clin Med 2023;12:6977. [Crossref] [PubMed]
- Dong X, Wang K, Yang H, et al. Choice of radiotherapy modality for the combined treatment of non-small cell lung cancer with brain metastases: whole-brain radiation therapy with simultaneous integrated boost or stereotactic radiosurgery. Front Oncol 2023;13:1220047. [Crossref] [PubMed]
- Dai C, Ren Y, Xie D, et al. Does Lymph Node Metastasis Have a Negative Prognostic Impact in Patients with NSCLC and M1a Disease? J Thorac Oncol 2016;11:1745-54. [Crossref] [PubMed]
- Dong D, Zhang S, Jiang B, et al. Correlation analysis of MRD positivity in patients with completely resected stage I-IIIA non-small cell lung cancer: a cohort study. Front Oncol 2023;13:1222716. [Crossref] [PubMed]
- Hua J, Li L, Liu L, et al. The diagnostic value of metabolic, morphological and heterogeneous parameters of 18F-FDG PET/CT in mediastinal lymph node metastasis of non-small cell lung cancer. Nucl Med Commun 2021;42:1247-53. [Crossref] [PubMed]
- Togo M, Yokobori T, Shimizu K, et al. Diagnostic value of (18)F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8(+)tumour-infiltrating lymphocytes in oral squamous cell carcinoma. Br J Cancer 2020;122:1686-94. [Crossref] [PubMed]
- Lv YL, Yuan DM, Wang K, et al. Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 2011;6:1350-8. [Crossref] [PubMed]
- Deng H, Xiong S, Zhong R, et al. "Major pathologic response" in lymph nodes: a modified nodal classification for non-small cell lung cancer patients treated with neoadjuvant immunochemotherapy. Exp Hematol Oncol 2023;12:40. [Crossref] [PubMed]
- Zhang L, E H, Huang J, et al. Clinical utility of [18F]FDG PET/CT in the assessment of mediastinal lymph node disease after neoadjuvant chemoimmunotherapy for non-small cell lung cancer. Eur Radiol 2023;33:8564-72.
- Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012;186:487-92. [Crossref] [PubMed]
- Ryu JS, Lim JH, Lee JM, et al. Minimal Pleural Effusion in Small Cell Lung Cancer: Proportion, Mechanisms, and Prognostic Effect. Radiology 2016;278:593-600. [Crossref] [PubMed]
- Kashiwabara K, Fuji S, Tsumura S, et al. Prognosis of EGFR-mutant Lung Adenocarcinoma Patients With Malignant Pleural Effusion Receiving First-line EGFR-TKI Therapy Without Pleurodesis: A Single-institute Retrospective Study. Anticancer Res 2020;40:1117-21. [Crossref] [PubMed]


