
Effect of laterality on the postoperative survival of non-small cell lung cancer patients undergoing pneumonectomy
Highlight box
Key findings
• Patients who undergo right-sided pneumonectomy for non-small cell lung cancer (NSCLC) have a higher risk of mortality during the initial 6 months post-surgery, with the impact of laterality on long-term prognosis leveling off after this critical period.
What is known and what is new?
• It is widely acknowledged that patients with NSCLC who undergo right-sided pneumonectomy often experience a poorer prognosis. However, the precise impact of laterality remains unclear.
• This study reveals that the high-risk period for patients following right-sided pneumonectomy is primarily within the initial 6 months post-operation. The influence of laterality on prognosis diminishes beyond this high-risk phase.
What is the implication, and what should change now?
• Patients with NSCLC who undergo right-sided pneumonectomy often have a higher risk of mortality during the initial 6 months post-surgery. Therefore, those patients planning to undergo this procedure should undergo a thorough preoperative evaluation. Additionally, patients undergoing right-sided pneumonectomy should receive extended perioperative follow-up, including no less than 6 months of postoperative monitoring.
Introduction
As one of the most common cancers and the leading cause of cancer-related death worldwide for several decades, lung cancer severely affects human life and health (1). Graham performed the first successful one-stage left-sided pneumonectomy to treat lung cancer in 1933 (2). For a long time, pneumonectomy remained the standard surgical procedure for treating lung cancer. However, technological advances and improved medical concepts have led to the widespread acceptance of lobectomy and sleeve resection in the treatment of non-small cell lung cancer (NSCLC) (3). As a result, in recent decades, much fewer patients with resectable lung cancer have undergone pneumonectomy (4). Nevertheless, current guidelines recommend that patients with locally advanced diseases, such as those with centrally located tumors or tumors with hilum invasion, undergo pneumonectomy to achieve radical resection and improve their long-term survival (5). Unilateral pneumonectomy can completely remove the lesion, but it always leads to a greater proportion of pulmonary function loss and has a significant effect on the patient’s respiratory and circulatory systems. Thus, optimal perioperative evaluation and management will facilitate accelerated patient recovery.
Studies on the survival prognosis of NSCLC patients undergoing pneumonectomy have shown that gender, age, tumor stage, laterality, and lymph node dissection are independently associated with perioperative mortality and long-term prognosis (6,7). Previous studies have suggested that laterality affects the short-term prognosis but not the long-term prognosis of patients undergoing a pneumonectomy (8). However, the exact extent of the effect of laterality on the short-term prognosis of patients after pneumonectomy has not yet been determined (9,10).
This study analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database, which has a large sample size and relatively complete follow-up data related to patients after pneumonectomy. This study aimed to explore the effect of laterality on the prognosis of NSCLC patients undergoing pneumonectomy and to quantify the approximate timing and magnitude of this effect. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-700/rc).
Methods
Data source
This retrospective observational cohort study was based on the SEER program, which was established in 1973 (11). Anonymized information is made available to medical researchers upon formal request. The requirement of informed consent was waived due to the anonymous, observational, and registry-based nature of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The investigators consecutively reviewed and obtained the Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) data set through SEER*Stat (Version 8.4.0) software (https://seer.cancer.gov/data-software/documentation/seerstat/).
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have been diagnosed between 2004 and 2015 (because of the staging standards modification); (II) have undergone pneumonectomy (surgical primary site codes: 55, 56, 65, 66, and 70); (III) have the lung as the primary site (primary site-labelled: C34.0–C34.9); (IV) have a pathological confirmation of NSCLC; and (V) have stage I–III disease (American Joint Committee on Cancer, 6th ed.) (12). Patients were excluded from the study if they met any of the following exclusion criteria: (I) had an autopsy as the reporting source; (II) had an unknown laterality side; (III) had been diagnosed with (an)other malignant tumor(s); and/or (IV) had incomplete follow-up data.
Variables
The general demographic characteristics encompassed age, sex, racial identity, year of disease diagnosis, marital status, vital condition, and duration of survival in months. Tumor characteristics such as laterality, histopathological subtypes, tumor grade, tumor dimensions, N (lymph node) stage, and treatment strategies (operation type, additional chemotherapy, or additional radiotherapy) were included in this research. The SEER variables directory specified radical pneumonectomy as pneumonectomy with mediastinal lymph node dissection, whereas extended pneumonectomy was radical pneumonectomy with additional dissection of surrounding structures, including the diaphragm, pleura, or chest wall. The primary study endpoint focused on overall survival (OS).
Statistical analysis
All the statistical analyses in this study were performed using R software 4.0.5 (The R foundation, Vienna, Austria) and SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA). Propensity score matching (PSM) was conducted using the MatchIt R package to reduce selection bias. To examine the balance between the left- and right-sided pneumonectomy groups, a 1:1 matching protocol was used without replacement with a caliper width of 0.02 standard deviations (SDs) of the logit of the propensity score. A stepwise logistic regression model analysis was employed to analyze the correlation of laterality and other potential variables with postoperative mortality at 0–3, 4–6, and 7–9 months using the rms R package.
All the research variables were included in the univariable logistic analysis and odds ratios (ORs) with 95% confidence intervals (CIs) were compared. The factors significantly associated with postoperative mortality (P<0.1) in the univariable analysis were included in the multivariable analysis to identify the independent risk factors associated with mortality. Kaplan-Meier (KM) curves were used to further assess the effect of laterality on OS.
Numbers and proportions were calculated for the categorical variables, and the groups were compared using the Chi-squared test or Fisher’s exact test. The normally distributed continuous variables were analyzed using the independent samples Student’s t-test or paired Student’s t-test, and are expressed as the mean ± SD. The non-normally distributed variables were analyzed using the Mann-Whitney U test or Wilcoxon signed-rank test, and are presented as the median with interquartile range (IQR). Two-sided P values <0.05 were considered statistically significant.
Results
A total of 4,763 patients met the enrollment criteria, including 1,988 (41.7%) and 2,775 (58.3%) patients who underwent right-sided and left-sided pneumonectomies, respectively. The screening scheme for the subjects is provided in Figure 1. The median age of the included patients was 63 [56–70] years. Squamous cell carcinoma (N=2,423; 50.9%) was a more common diagnosis than adenocarcinoma. The median tumor size was 4.9 (3.3–7.0) cm, and 62.2% (N=2,962) of the patients had lymph node metastasis. Radical or extended pneumonectomy was performed in 62.2% (N=2,962) of the patients, while 50.5% (N=2,407) and 22.3% (N=1,060) of the patients underwent chemotherapy and radiotherapy, respectively.

The overall postoperative mortality rates at 0 (intra-operation or in-hospital mortality), 1, 3, 6, and 12 months were 1.66%, 4.74%, 10.96%, 17.17%, and 28.15%, respectively. The postoperative mortality rates at 0, 1, 3, 6, and 12 months of the left-sided pneumonectomy group were 1.23%, 3.42%, 8.22%, 13.37%, and 24.36%, while those of the right-sided pneumonectomy group were 2.26%, 6.59%, 14.79%, 22.48%, and 33.45%, respectively.
After 1:1 PSM, 1,911 patients were included in each matched cohort to achieve a balance in the confounding covariates between the groups (Table 1). Postoperative deaths occurred in 2,594 (67.9%) patients, including 1,336 (51.5%) who underwent right-sided pneumonectomies, and 1,258 (48.5%) who underwent left-sided pneumonectomies with a median follow-up time of 69 months. The mortality times for each laterality group within 3 years are shown in Figure 2A. Postoperative deaths were most common during the first 6 months after surgery. During this period, deaths occurred in 32.0% (428/1,336) and 19.9% (250/1,258) of the patients in the right-sided and left-sided pneumonectomy groups, respectively. The proportion of deaths in the right-sided pneumonectomy group was initially higher, but both groups showed similar trends after the initial 6 months.
Table 1
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Right-sided (N=1,988) | Left-sided (N=2,775) | P value | Right sided (N=1,911) | Left sided (N=1,911) | P value | ||
| Gender | 0.057 | 0.34 | |||||
| Female | 643 (32.3) | 971 (35.0) | 621 (32.5) | 594 (31.1) | |||
| Male | 1,345 (67.7) | 1,804 (65.0) | 1,290 (67.5) | 1,317 (68.9) | |||
| Age (years) | 0.002 | 0.35 | |||||
| ≤60 | 843 (42.4) | 1,051 (37.9) | 790 (41.3) | 762 (39.9) | |||
| >60 | 1,145 (57.6) | 1,724 (62.1) | 1,121 (58.7) | 1,149 (60.1) | |||
| Race | 0.20 | 0.24 | |||||
| White | 1,693 (85.2) | 2,399 (86.5) | 1,634 (85.5) | 1,659 (86.8) | |||
| Other | 295 (14.8) | 376 (13.5) | 277 (14.5) | 252 (13.2) | |||
| Marriage | 0.93 | 0.23 | |||||
| Married | 1,242 (62.5) | 1,737 (62.6) | 1,198 (62.7) | 1,233 (64.5) | |||
| Other | 746 (37.5) | 1,038 (37.4) | 713 (37.3) | 678 (35.5) | |||
| Grade | 0.67 | 0.62 | |||||
| I | 97 (4.8) | 136 (4.9) | 92 (4.8) | 97 (5.1) | |||
| II | 697 (35.1) | 967 (34.8) | 668 (35.0) | 703 (36.8) | |||
| III/IV | 1,011 (50.9) | 1,443 (52.0) | 983 (51.4) | 950 (49.7) | |||
| Unknown | 183 (9.2) | 229 (8.3) | 168 (8.8) | 161 (8.4) | |||
| Histology | <0.001 | 0.15 | |||||
| ADC | 733 (36.9) | 829 (29.9) | 676 (35.4) | 690 (36.1) | |||
| SCC | 940 (47.3) | 1,483 (53.4) | 930 (48.7) | 964 (50.4) | |||
| NEC | 45 (2.3) | 82 (3.0) | 43 (2.3) | 41 (2.1) | |||
| Other | 270 (13.6) | 381 (13.7) | 262 (13.7) | 216 (11.3) | |||
| Tumor size (cm) | 0.006 | <0.001 | |||||
| T ≤3 | 431 (21.7) | 595 (21.4) | 398 (20.8) | 570 (29.8) | |||
| 3< T ≤5 | 609 (30.6) | 969 (34.9) | 598 (31.3) | 779 (40.8) | |||
| 5< T ≤7 | 442 (22.2) | 615 (22.2) | 437 (22.9) | 309 (16.2) | |||
| T >7 | 457 (23.0) | 533 (19.2) | 441 (23.1) | 204 (10.7) | |||
| Unknown | 49 (2.5) | 63 (2.3) | 37 (1.9) | 49 (2.6) | |||
| N stage | 0.001 | 0.20 | |||||
| N0 | 797 (40.1) | 1,004 (36.2) | 764 (40.0) | 815 (42.6) | |||
| N1 | 690 (34.7) | 1,112 (40.1) | 665 (34.8) | 663 (34.7) | |||
| N2 | 476 (23.9) | 636 (22.9) | 462 (24.2) | 418 (21.9) | |||
| N3 | 25 (1.3) | 23 (0.8) | 20 (1.0) | 15 (0.8) | |||
| Surgery type | 0.30 | 0.20 | |||||
| Pneumonectomy | 608 (30.6) | 833 (30.0) | 578 (30.2) | 599 (31.3) | |||
| Radical Pneu | 1,297 (65.2) | 1,848 (66.6) | 1,252 (65.5) | 1,251 (65.5) | |||
| Extended Pneu | 83 (4.2) | 94 (3.4) | 81 (4.2) | 61 (3.2) | |||
| Chemotherapy | 0.002 | 0.41 | |||||
| Yes | 951 (47.8) | 1,456 (52.5) | 925 (48.4) | 950 (49.7) | |||
| No | 1,037 (52.2) | 1,319 (47.5) | 986 (51.6) | 961 (50.3) | |||
| Radiotherapy | 0.03 | 0.45 | |||||
| Yes | 413 (20.8) | 647 (23.3) | 402 (21.0) | 421 (22.0) | |||
| No | 1,575 (79.2) | 2,128 (76.7) | 1,509 (79.0) | 1,490 (78.0) | |||
PSM, propensity score matching; ADC, adenocarcinoma; SCC, squamous cell carcinoma; NEC, neuroendocrine carcinoma; Pneu, pneumonectomy.
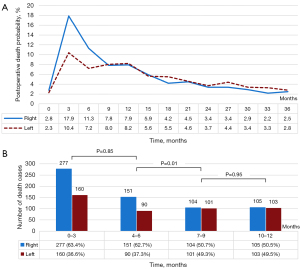
A further analysis of postoperative mortality over 12 months revealed that the patients who underwent right-sided pneumonectomies had higher proportions of deaths during the 0–3- and 4–6-month periods (63.4% and 62.7%, respectively, P=0.85; Figure 2B). In the right-sided pneumonectomy group, postoperative mortality at 7–9 months was significantly lower than that at 4–6 months (P=0.01). A similar trend was also observed in the left-sided pneumonectomy group. There was no significant difference in postoperative mortality between the two groups during the 7–9- and 10–12-month periods (P=0.95).
In the univariate analysis, age, laterality, pathological type, chemotherapy, and radiotherapy were found to be related to postoperative mortality at both 3 months and 4–6 months. While, gender (Table 2) and N stage (Table 3) were found to be correlated with postoperative mortality at 0–3 and 4–6 months, respectively.
Table 2
| Variables | Univariable logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Gender (male vs. female) | 1.655 (1.309–2.093) | <0.001* | 1.455 (1.119–1.893) | 0.005 | |
| Age (>60 vs. ≤60 years) | 2.297 (1.828–2.887) | <0.001* | 1.748 (1.354–2.258) | <0.001 | |
| Race (other vs. White) | 0.905 (0.672–1.218) | 0.50 | |||
| Marriage (other vs. married) | 1.045 (0.850–1.284) | 0.67 | |||
| Laterality (right vs. left) | 1.362 (1.229–1.510) | <0.001* | 1.421 (1.269–1.592) | <0.001 | |
| Grade | 0.08* | 0.004 | |||
| II vs. I | 1.425 (0.819–2.480) | 0.21 | 1.582 (0.884–2.833) | 0.12 | |
| III/IV vs. I | 1.684 (0.977–2.901) | 0.06 | 2.142 (1.206–3.804) | 0.009 | |
| Histology | 0.02* | 0.37 | |||
| SCC vs. ADC | 1.311 (1.048–1.639) | 0.01 | 1.096 (0.850–1.414) | 0.47 | |
| NEC vs. ADC | 0.456 (0.164–1.264) | 0.13 | 0.391 (0.117–1.306) | 0.12 | |
| Other vs. ADC | 1.309 (0.947–1.809) | 0.10 | 1.034 (0.703–1.521) | 0.86 | |
| Tumor size (cm) | 0.23 | ||||
| 3< T ≤5 vs. T ≤3 | 0.982 (0.754–1.280) | 0.89 | |||
| 5< T ≤7 vs. T ≤3 | 1.156 (0.858–1.557) | 0.33 | |||
| T >7 vs. T ≤3 | 1.281 (0.946–1.736) | 0.10 | |||
| N stage | 0.13 | ||||
| N1 vs. N0 | 1.066 (0.851–1.336) | 0.57 | |||
| N2 vs. N0 | 0.831 (0.634–1.090) | 0.18 | |||
| N3 vs. N0 | 1.919 (0.826–4.456) | 0.12 | |||
| Surgery type | 0.07* | 0.19 | |||
| Radical Pneu vs. Pneu | 0.787 (0.636–0.972) | 0.02 | 0.878 (0.695–1.111) | 0.27 | |
| Extended Pneu vs. Pneu | 0.964 (0.572–1.626) | 0.89 | 1.420 (0.770–2.618) | 0.26 | |
| Chemotherapy (no vs. yes) | 16.254 (11.150–23.694) | <0.001* | 15.850 (10.278–24.444) | <0.001 | |
| Radiotherapy (no vs. yes) | 4.669 (3.138–6.947) | <0.001* | 1.251 (0.774–2.022) | 0.36 | |
*, these factors with P value less than 0.1 were defined as parameters significantly associated with postoperative mortality in univariable analysis. OR, odds ratio; CI, confidence interval; SCC, squamous cell carcinoma; ADC, adenocarcinoma; NEC, neuroendocrine carcinoma; Pneu, pneumonectomy.
Table 3
| Variables | Univariable logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Gender (male vs. female) | 1.310 (0.976–1.758) | 0.07* | 1.330 (0.973–1.818) | 0.07 | |
| Age (>60 vs. ≤60 years) | 1.492 (1.129–1.971) | 0.005* | 1.441 (1.074–1.934) | 0.01 | |
| Race (other vs. White) | 1.024 (0.704–1.490) | 0.90 | |||
| Marriage (other vs. married) | 0.968 (0.737–1.270) | 0.81 | |||
| Laterality (right vs. left) | 1.318 (1.152–1.507) | <0.001* | 1.292 (1.122–1.488) | <0.001 | |
| Grade | 0.10 | ||||
| II vs. I | 1.236 (0.585–2.609) | 0.57 | |||
| III/IV vs. I | 1.631 (0.786–3.386) | 0.18 | |||
| Histology | 0.01* | 0.07 | |||
| SCC vs. ADC | 0.825 (0.616–1.106) | 0.19 | 0.848 (0.620–1.158) | 0.29 | |
| NEC vs. ADC | 0.717 (0.257–2.003) | 0.52 | 0.778 (0.274–2.209) | 0.63 | |
| Other vs. ADC | 1.491 (1.025–2.169) | 0.03 | 1.396 (0.941–2.071) | 0.09 | |
| Tumor size (cm) | 0.06* | 0.52 | |||
| 3< T ≤ 5 vs. T ≤3 | 1.030 (0.721–1.471) | 0.87 | 1.029 (0.714–1.481) | 0.87 | |
| 5< T ≤7 vs. T ≤3 | 1.061 (0.704–1.599) | 0.77 | 0.979 (0.642–1.492) | 0.92 | |
| T >7 vs. T ≤3 | 1.578 (1.069–2.329) | 0.02 | 1.290 (0.858–1.939) | 0.22 | |
| N stage | 0.009* | 0.01 | |||
| N1 vs. N0 | 0.899 (0.653–1.238) | 0.51 | 1.038 (0.746–1.444) | 0.82 | |
| N2 vs. N0 | 1.506 (1.097–2.067) | 0.01 | 1.698 (1.192–2.418) | 0.003 | |
| N3 vs. N0 | 2.086 (0.721–6.034) | 0.17 | 2.290 (0.756–6.932) | 0.14 | |
| Surgery type | 0.07* | 0.10 | |||
| Radical Pneu vs. Pneu | 0.885 (0.667–1.176) | 0.40 | 0.934 (0.694–1.257) | 0.65 | |
| Extended Pneu vs. Pneu | 1.664 (0.930–2.979) | 0.08 | 1.734 (0.948–3.172) | 0.07 | |
| Chemotherapy (no vs. yes) | 1.336 (1.027–1.740) | 0.03* | 1.766 (1.273–2.449) | 0.001 | |
| Radiotherapy (no vs. yes) | 0.711 (0.530–0.954) | 0.02* | 0.599 (0.417–0.862) | 0.006 | |
*, these factors with P value less than 0.1 were defined as parameters significantly associated with postoperative mortality in univariable analysis. OR, odds ratio; CI, confidence interval; SCC, squamous cell carcinoma; ADC, adenocarcinoma; NEC, neuroendocrine carcinoma; Pneu, pneumonectomy.
The multivariable logistic regression analysis showed that right-sided pneumonectomy was an independent risk factor for postoperative mortality at 0–3 months (right vs. left: OR: 1.421, 95% CI: 1.269–1.592, P<0.001; Table 2) and 4–6 months (right vs. left: OR: 1.292, 95% CI: 1.122–1.488, P<0.001; Table 3). However, laterality was not a prognostic factor associated with postoperative death at 7–9 months (right vs. left: OR: 1.016, 95% CI: 0.882–1.169, P=0.82; Table 4).
Table 4
| Variables | Univariable logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Gender (male vs. female) | 1.162 (0.852–1.585) | 0.34 | |||
| Age (>60 vs. ≤60 years) | 1.170 (0.875–1.566) | 0.29 | |||
| Race (other vs. White) | 0.941 (0.621–1.426) | 0.77 | |||
| Marriage (other vs. married) | 1.150 (0.862–1.534) | 0.34 | |||
| Laterality (right vs. left) | 1.016 (0.882–1.169) | 0.82 | |||
| Grade | 0.40 | ||||
| II vs. I | 1.740 (0.747–4.056) | 0.19 | |||
| III/IV vs. I | 1.770 (0.767–4.084) | 0.18 | |||
| Histology | 0.94 | ||||
| SCC vs. ADC | 0.980 (0.721–1.331) | 0.89 | |||
| NEC vs. ADC | 1.089 (0.428–2.770) | 0.85 | |||
| Other vs. ADC | 0.870 (0.539–1.405) | 0.56 | |||
| Tumor size (cm) | <0.001* | <0.001 | |||
| 3< T ≤ 5 vs. T ≤3 | 0.934 (0.629–1.388) | 0.73 | 0.921 (0.619–1.369) | 0.68 | |
| 5< T ≤7 vs. T ≤3 | 0.979 (0.621–1.545) | 0.92 | 0.943 (0.597–1.491) | 0.80 | |
| T >7 vs. T ≤3 | 2.142 (1.438–3.192) | <0.001 | 2.038 (1.362–3.052) | 0.001 | |
| N stage | 0.50 | ||||
| N1 vs. N0 | 0.577 (0.173–1.923) | 0.37 | |||
| N2 vs. N0 | 0.675 (0.202–2.251) | 0.52 | |||
| N3 vs. N0 | 0.535 (0.157–1.817) | 0.31 | |||
| Surgery type | 0.054* | 0.11 | |||
| Radical Pneu vs. Pneu | 0.810 (0.599–1.096) | 0.17 | 0.787 (0.577–1.072) | 0.12 | |
| Extended Pneu vs. Pneu | 1.594 (0.858–2.961) | 0.14 | 1.330 (0.705–2.507) | 0.37 | |
| Chemotherapy (no vs. yes) | 0.894 (0.675–1.185) | 0.43 | |||
| Radiotherapy (no vs. yes) | 0.630 (0.462–0.859) | 0.003* | 0.665 (0.483–0.916) | 0.01 | |
*, these factors with P value less than 0.1 were defined as parameters significantly associated with postoperative mortality in univariable analysis. OR, odds ratio; CI, confidence interval; SCC, squamous cell carcinoma; ADC, adenocarcinoma; NEC, neuroendocrine carcinoma; Pneu, pneumonectomy.
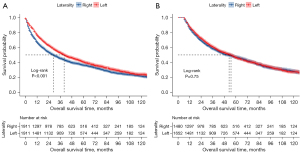
The median follow-up time for the entire patient group was 65.0 months. The median OS of patients undergoing right- and left-sided pneumonectomy was 29.0 and 40.0 months, respectively. This survival difference between the two groups demonstrated high statistical significance (log-rank P<0.001; Figure 3A). The subgroup survival analysis of the patients with follow-up times >6 months demonstrated that laterality was not significantly correlated with OS (log-rank P=0.75; Figure 3B).
Discussion
Pneumonectomy is associated with a greater operative risk compared to limited pulmonary resections, and its effect on long-term survival is still a subject of contention (13). Nevertheless, anatomical or technical constraints may necessitate this operation (14). In the present study, a comprehensive analysis was conducted to examine the effects of laterality on the short- and long-term survival of pneumonectomy patients. The results showed that the effect of laterality was limited to the initial 6 months following pneumonectomy. The subgroup analysis of patients with follow-up times >6 months revealed no significant difference in the long-term prognosis between the left- and right-sided pneumonectomy groups.
The study included a total of 4,763 patients who underwent surgery between 2004 and 2015. In the entire cohort, the 1-month postoperative mortality rate was 4.74%, with rates of 3.43% and 6.59% for the left- and right-sided pneumonectomy groups, respectively. Seder et al. analyzed data from the STS (the Society of Thoracic Surgeons) and ESTS (the European Society of Thoracic Surgeons) databases and reported that the in-hospital mortality rates for pneumonectomy patients were 4.3% and 4.9%, while the 30-day mortality rates were 6.7% and 7.3%, respectively (15). A meta-analysis reported an overall 30-day mortality rate of 7% for pneumonectomy patients, with rates of 5% and 11% for left- and right-sided pneumonectomies, respectively. The corresponding 90-day mortality rates were 12% for the entire cohort, 9% for left-sided pneumonectomies, and 20% for right-sided pneumonectomies (16). Pricopi et al. found that among NSCLC patients who underwent right-sided pneumonectomies, the 30- and 90-day mortality rates were 9.4% and 11.2%, respectively. The corresponding rates for left-sided pneumonectomies were significantly lower at 3.1% and 4.7%, respectively (9). These trends were similar to those observed in the present study.
The National Surgical Quality Improvement Program (NSQIP) database categorizes any death occurring within 30 days of non-cardiothoracic surgery, regardless of the cause, as a surgery-related death. The assessment of 30-day mortality encompasses not only the surgical risk index but also the projected survival benefit and unanticipated postoperative death (17). A 30-day window is typically adequate for documenting all procedure-related deaths following conventional thoracic surgery, including sub-lobar resections and lobectomies. However, recent investigations of the pneumonectomy procedure have demonstrated that the 90-day mortality (3.0–21.0%) can be twice as high as the 30-day mortality (1.5–12.0%) (18). Thus, the surgical risk related to pneumonectomy persists beyond the initial 30-day period or the time of discharge. As a result, pneumonectomy patients require intensive care, comprehensive treatment, and prolonged follow-up to minimize operative mortality and postoperative complications management (19).
Patients undergoing right-sided pneumonectomies were found to be at a greater risk of perioperative mortality compared to those undergoing left-sided pneumonectomies. This difference was primarily a result of postoperative complications rather than the procedure itself and may be related to several potential factors (20). First, the right lung contributes to a greater share of overall lung ventilation; therefore, a right-sided pneumonectomy can disproportionately affect lung function. The asymmetrical mediastinal shift, with the heart shifted to the left side, also provides relief on the left side after pneumonectomy. The absence of counteracting resistance on the right side can trigger a mediastinal shift, leading to detrimental structural alterations in the hilum that affect pulmonary ventilation and cardiac hemodynamic stability, posing a potentially life-threatening risk. Moreover, right-sided surgery is associated with a greater risk of serious complications, such as acute respiratory distress syndrome (ARDS) and bronchopleural fistula (BPF), resulting in greater morbidity and mortality. The greater loss of pulmonary function following right total lung surgery, with a considerably reduced pulmonary vascular bed area, leads to higher pulmonary artery pressure and increased right ventricular afterload. This, in turn, increases the risk of general complications, including postoperative ARDS and arrhythmias (21).
Following pneumonectomy, the right bronchus becomes wider than the left bronchus, which increases the tension and leads to a greater risk of BPF. The left stump is covered by the aortic arch, while the right bronchial stump lacks protection from the surrounding mediastinal tissue, making it more susceptible to inflammation and rupture. The risk of perioperative mortality is higher in the presence of respiratory complications, particularly ARDS, because of the significant reduction in lung function following pneumonectomy. A retrospective study has reported a significant association between ARDS and pulmonary edema caused by increased pulmonary blood flow after unilateral total lung resection (22). The incidence of ARDS among pneumonectomy patients ranges from approximately 0.9% to 46.0%, with perioperative morbidity and mortality rates ranging from 33.3% to 59.8% (23). Blanc et al. investigated 543 cases of total lung resection and conducted a systematic analysis to assess the occurrence, management, and prognosis of perioperative ARDS after pneumonectomy. Their multifactorial analysis revealed that right-sided pneumonectomy and a high Charlson Comorbidity Index (CCI) were independent risk factors for ARDS. This may be because of the greater perfusion and ventilation of the right lung, along with a higher gradient of elevated pulmonary artery pressure after right-sided pneumonectomy (24).
Previous studies have reported that pneumonia occurs in 2–10% of patients after pneumonectomy, mostly because of poor postoperative pain control, limited mobility, or prolonged mechanical ventilation. Pneumonectomy patients who develop pneumonia have a poorer survival prognosis than those without pneumonia (25). It has been reported that supraventricular arrhythmia, such as atrial fibrillation (AF), is the most common type of arrhythmia after pneumonectomy. Postoperative AF occurs in approximately 4–25% of pneumonectomy patients (22). The mechanism underlying postoperative AF is complex and not yet fully understood. Right-sided pneumonectomy is a known risk factor for AF. Pulmonary hypertension and right-sided cardiac insufficiency after pneumonectomy are caused by increased pulmonary blood flow on the healthy side. Severe pulmonary hypertension increases the afterload on the right ventricle, resulting in right-sided cardiac insufficiency as the heart fails to compensate (26).
The prevailing consensus in the medical community is that right-sided pneumonectomy is associated with higher perioperative mortality rates than left-sided pneumonectomy (6). Research conducted by Fernandez et al. indicates that there is a significant difference in the mortality rates at 30 and 90 days, with rates of 8% and 16% for right-sided pneumonectomies, and 4% and 9% for left-sided pneumonectomies, respectively (10). Yang et al. identified right-sided pneumonectomy as an independent risk factor for 90-day perioperative mortality (hazard ratio: 2.23, P<0.01) (27). The incidence of at least one complication in pneumonectomy patients during the perioperative period varied significantly, ranging from 21.4% to 56.7% (28). Pneumonectomy patients also had significantly higher rates of major complications than lobectomy patients.
The laterality of pneumonectomy may be related to short-term morbidity; however, the laterality of pneumonectomy did not affect the long-term survival outcomes of patients. Pneumonectomy patients undergo a sequence of anatomical alterations postoperatively. Fluid gradually accumulates in the empty chest cavity, forming a radiologically visible hydropneumothorax sign. In addition, there may be an upward shift in the diaphragm on the side of the surgery, thorax deformation, and gradual mediastinal displacement (29). The perioperative mortality risk was significantly higher in patients who underwent right-sided pneumonectomies, and this elevated risk persisted for approximately 6 months. However, beyond this period, there was no significant prognostic difference in the OS of the pneumonectomy patients regardless of laterality (30).
Riquet investigated post-pneumonectomy patients who achieved long-term survival, and found no evidence to suggest that laterality had any effect on the prognosis of pneumonectomy patients (6). This accords with the results of the present study, which found that right-sided pneumonectomy patients had a higher risk of early postoperative mortality, but that laterality had no significant effect on survival after 6 months. An analysis of the survival curves for OS in pneumonectomy patients by Fernandez et al. revealed a widening survival gap between the two patient groups during the early stages, but a convergent trend after 2 years (10). Consistent with the findings of previous studies, the present study demonstrated that right-sided pneumonectomy patients had an increased risk of early postoperative death, but laterality did not play a significant role in survival after the initial period of 6 months.
Limitations
The authors conducted a comprehensive analysis of the differences in short- and long-term survival between patients who underwent left- and right-sided pneumonectomies. The study also investigated the timeframe for the effect of laterality on post-pneumonectomy outcomes. However, this study was not without certain limitations. First, given its retrospective nature, this study faced inherent natural deviations. The research data were derived from the national cancer registration database. Due to data constraints, some of the variables, such as the specific causes of death, perioperative complications, (neo-)adjuvant treatment strategies, detailed pulmonary function, and surgical approaches (open or minimally invasive) could not be comprehensively analyzed. Additionally, surgical techniques/intensive care unit management would have changed over the study period. Even if PSM was used to balance the variables of two sets, there was still a certain degree of difference in the characteristics between the two groups of patients. Further, the SEER database includes survival data in months rather than days, which precluded a more precise assessment of short-term prognosis in this study. However, several previous studies have also analyzed perioperative survival in months. Despite these limitations, this study had a large sample size and yielded compelling evidence regarding both early and late mortality in pneumonectomy patients, specifying the exact period for the effect of laterality.
Conclusions
In conclusion, the risk of early mortality was greater after right-sided pneumonectomy, and this risk persisted for the initial 6 months. However, after this period, there was no significant difference in the survival of the pneumonectomy patients based on laterality. Thus, the long-term survival of patients does not appear to be affected by the laterality of the procedure.
Acknowledgments
This article was presented at the 30th Annual Meeting of the European Society of Thoracic Surgeons, Hague, Netherlands, 19–21 June 2022. The authors would like to thank Dr. Jiani Wang (Institute for Social Medicine, Epidemiology and Health Economics, Charité-Universitätsmedizin zu Berlin) for her statistical assistance.
Funding: This project has been supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-700/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-700/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-700/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. CA Cancer J Clin 1974;24:238-42. [Crossref] [PubMed]
- Hutchings H, Schwarze E, Was J, et al. Trauma pneumonectomy followed by extracorporeal membrane oxygenation cannulation: a case report. AME Case Rep 2024;8:10. [Crossref] [PubMed]
- Prisciandaro E, Bertolaccini L, Fieuws S, et al. Multicentre retrospective analysis on pulmonary metastasectomy: an European perspective. Eur J Cardiothorac Surg 2024;65:ezae141. [Crossref] [PubMed]
- Nitsche LJ, Jordan S, Demmy T, et al. Analyzing the impact of minimally invasive surgical approaches on post-operative outcomes of pneumonectomy and sleeve lobectomy patients. J Thorac Dis 2023;15:2497-504. [Crossref] [PubMed]
- Gu C, Wang R, Pan X, et al. Comprehensive study of prognostic risk factors of patients underwent pneumonectomy. J Cancer 2017;8:2097-103. [Crossref] [PubMed]
- Motas N, Gonzalez-Rivas D, Bosinceanu ML, et al. Uniportal robotic-assisted thoracic surgery pneumonectomy. Ann Cardiothorac Surg 2023;12:67-9. [Crossref] [PubMed]
- Stolz AJ, Harustiak T, Simonek J, et al. Pneumonectomy for non-small cell lung cancer: predictors of early mortality and morbidity. Acta Chir Belg 2014;114:25-30.
- Pricopi C, Mordant P, Rivera C, et al. Postoperative morbidity and mortality after pneumonectomy: a 30-year experience of 2064 consecutive patients. Interact Cardiovasc Thorac Surg 2015;20:316-21. [Crossref] [PubMed]
- Fernandez FG, Force SD, Pickens A, et al. Impact of laterality on early and late survival after pneumonectomy. Ann Thorac Surg 2011;92:244-9. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Nakamura A, Kuribayashi K. Do we still need to debate the merits of pleurectomy/decortication vs. extrapleural pneumonectomy for malignant pleural mesothelioma? Transl Lung Cancer Res 2023;12:193-6. [Crossref] [PubMed]
- Alexiou C, Beggs D, Onyeaka P, et al. Pneumonectomy for stage I (T1N0 and T2N0) nonsmall cell lung cancer has potent, adverse impact on survival. Ann Thorac Surg 2003;76:1023-8. [Crossref] [PubMed]
- Chen Q, Liu Y, Liu Y, et al. Effect of perioperative aspirin continuation on bleeding after pneumonectomy. Thorac Cancer 2023;14:1071-6. [Crossref] [PubMed]
- Seder CW, Salati M, Kozower BD, et al. Variation in Pulmonary Resection Practices Between The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. [Crossref] [PubMed]
- Kim AW, Boffa DJ, Wang Z, et al. An analysis, systematic review, and meta-analysis of the perioperative mortality after neoadjuvant therapy and pneumonectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:55-63. [Crossref] [PubMed]
- Rivera C, Dahan M, Bernard A, et al. Surgical treatment of lung cancer in the octogenarians: results of a nationwide audit. Eur J Cardiothorac Surg 2011;39:981-6. [Crossref] [PubMed]
- Tsukioka T, Izumi N, Komatsu H, et al. Large Tumor Size and High Neutrophil-to-lymphocyte Ratio Predicts Poor Prognosis After Pneumonectomy or Sleeve Lobectomy in Patients With Non-small-cell Lung Cancer. Anticancer Res 2022;42:3029-34. [Crossref] [PubMed]
- Jones GD, Tan KS, Caso R, et al. Time-varying analysis of readmission and mortality during the first year after pneumonectomy. J Thorac Cardiovasc Surg 2020;160:247-255.e5. [Crossref] [PubMed]
- Wang ZM, Swierzy M, Balke D, et al. Dynamic nomogram for long-term survival in patients with non-small cell lung cancer after pneumonectomy. J Thorac Dis 2021;13:2276-87. [Crossref] [PubMed]
- Thomas PA, Berbis J, Baste JM, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015;149:73-82. [Crossref] [PubMed]
- Matthews CR, Goswami D, Ramchandani NK, et al. The Influence of Airway Closure Technique for Right Pneumonectomy on Wall Tension During Positive Pressure Ventilation: An Experimental Study. Semin Thorac Cardiovasc Surg 2020;32:1076-84. [Crossref] [PubMed]
- Riquet M, Mordant P, Pricopi C, et al. A review of 250 ten-year survivors after pneumonectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:876-81. [Crossref] [PubMed]
- Blanc K, Zaimi R, Dechartres A, et al. Early acute respiratory distress syndrome after pneumonectomy: Presentation, management, and short- and long-term outcomes. J Thorac Cardiovasc Surg 2018;156:1706-1714.e5. [Crossref] [PubMed]
- Khaitan PG. Incidence of bronchopleural fistula after pneumonectomy in an era of revolution. J Thorac Dis 2023;15:226-8. [Crossref] [PubMed]
- Kopec SE, Irwin RS, Umali-Torres CB, et al. The postpneumonectomy state. Chest 1998;114:1158-84. [Crossref] [PubMed]
- Yang CJ, Shah SA, Lin BK, et al. Right-Sided Versus Left-Sided Pneumonectomy After Induction Therapy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1074-81. [Crossref] [PubMed]
- Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis 2014;6:S217-23. [Crossref] [PubMed]
- Yun J, Choi YS, Hong TH, et al. Nononcologic Mortality after Pneumonectomy Compared to Lobectomy. Semin Thorac Cardiovasc Surg 2022;34:1122-31. [Crossref] [PubMed]
- Baboudjian M, Gondran-Tellier B, Tadrist A, et al. Predictors of Postoperative Urinary Retention Following Pulmonary Resection. Semin Thorac Cardiovasc Surg 2021;33:1137-43. [Crossref] [PubMed]


