
Detailed characterization of combination treatment with MET inhibitor plus EGFR inhibitor in EGFR-mutant and MET-amplified non-small cell lung cancer
Highlight box
Key findings
• A combination of MET-tyrosine kinase inhibitor (TKI) and EGFR-TKI showed a 74.4% of response rate and 5.3 months of progression-free survival (PFS) in advanced EGFR-mutant and MET-amplified non-small cell lung cancer (NSCLC).
• Median clearance time of MET amplification in plasma circulating tumor DNA (ctDNA) was measured as 63 days.
• Patients who stopped MET-TKI within 63 days of initiation showed poorer PFS.
What is known and what is new?
• A combination of MET-TKI and EGFR-TKI showed a promising anti-tumor effect in advanced EGFR-mutant and MET-amplified NSCLC.
• Early discontinuation of MET-TKI negatively affected the survival outcomes.
What is the implication, and what should change now?
• A combination of MET-TKI and EGFR-TKI treatment needs to be maintained at least until the MET-amplified clones is cleared up.
• Plasma ctDNA might have to be monitored in conjunction with this combination treatment to verify the disappearance of the MET-amplified clone.
Introduction
Mesenchymal-epithelial transition factor (MET) gene amplification is a common mechanism of therapeutic resistance in EGFR-mutant non-small cell lung cancer (NSCLC) patients treated with EGFR inhibitors (EGFRi) (1,2). MET amplification was reported in 7–15% of these patients after progression with first-line osimertinib therapy and in 5–50% of patients after the failure of second-line osimertinib therapy (3). Thus, combination treatment with a MET inhibitor (METi) and an EGFRi has been proposed as a promising strategy to overcome MET-amplification-related resistance to EGFRi in EGFR-mutant NSCLC (4-6). The TATOON phase Ib study evaluated the efficacy and toxicity of a combination of savolitinib and osimertinib in 186 patients with advanced EGFR-mutant and MET-amplified NSCLC who developed progression on EGFRi treatment (4). In the TATOON study, the two-drug combination treatment showed promising efficacy with a tolerable toxicity profile. Interestingly, this phase I study showed a difference in the efficacy and toxicity outcomes depending on the dose of METi. In part B of that study, osimertinib 80 mg and savolitinib 600 mg daily resulted in a 66% response rate and 57% incidence of ≥ grade 3 adverse effects, while in part D of the study, osimertinib 80 mg and savolitinib 300 mg daily resulted in a 23% response rate and 38% incidence of ≥ grade 3 adverse effects. Based on these results, the subsequent SAVANNAH phase 2 study has been evaluating 300 or 600 mg once-daily or 300 mg twice-daily dose of savolitinib in combination with osimertinib 80 mg. In addition, the currently ongoing SAFFRON phase 3 study aims to compare a 300 mg twice-daily dose of savolitinib plus osimertinib 80 mg with pemetrexed and carboplatin in the same patient subset. In addition, the INSIGHT phase 2 study tested tepotinib plus gefitinib in 122 patients with EGFR-mutant NSCLC who had acquired resistance to 1st line osimertinib due to MET amplification (7). Most recent final results of this study were that the combination regimen had high effectiveness with 43.9% of response rate and 5.4 months of progression-free survival (PFS) for this population.
In recent years, this dual EGFRi plus METi treatment has been making its way into clinical practice for patients with advanced EGFR-mutant and MET-amplified NSCLC. However, there is a lack of detailed clinical data to inform personalized treatment strategies for this distinct subset. For example, the intracranial response, true toxicity profile, drug discontinuation rate and its clinical impact, and post-treatment data including mechanisms of acquired resistance and response to corresponding subsequent therapy are not well characterized. Thus, in this observational study, we report longitudinal data for patients with EGFR-mutant and MET-amplified NSCLC who were treated with a combination of EGFRi plus METi. Our findings may provide valuable insights for treatment decision-making for these patients. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-273/rc).
Methods
Patients and data collection
From the pharmacy disposition records, we identified 54 patients with NSCLC who were treated with any type of EGFRi plus METi at the National Cancer Center Hospital (Goyang, Republic of Korea) between May 2015 and April 2023. The inclusion criteria for this study were: (I) histologically or cytologically confirmed metastatic NSCLC; (II) drug-sensitive EGFR mutations; (III) previous use of EGFRi and (IV) MET amplification detected by MET-fluorescence in situ hybridization (FISH) [MET/CEP7 ≥2 or MET gene copy number (GCN) ≥15 in >10% of tumor cells] (8) or next-generation sequencing (NGS) with plasma circulating tumor DNA (ctDNA) (FoundationOne Liquid CDx). Subsequently, the medical records and radiographic images of patients were reviewed to collect clinicopathologic data, genetic characteristics, adverse effects, details of dose modification, tumor response, and survival outcomes using a predesigned data format. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of the National Cancer Center (No. NCC 2016-0208). Informed consent was waived because of the retrospective nature of the study.
Treatment and tumor assessment
The drug type and the prescribed doses of EGFRi and METi were at the discretion of the treating physician. A 4-week treatment cycle was continued until the development of disease progression, intolerable toxicity, or patient refusal. Tumor response was assessed every 8 or 12 weeks by imaging studies. The imaging modalities including chest computed tomography (CT), abdominal CT, brain magnetic resonance imaging (MRI), bone scan, and positron emission tomography (PET) were performed according to the clinical needs. Thirty-three patients who were enrolled in prospective clinical studies (TATOON, SAVANNAH, and phase Ib/II capmatinib plus gefitinib study) received the treatment and tumor evaluation according to the protocol of the clinical study.
Tumor response was assessed in accordance with the guidelines established by the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) committee (9).
Droplet digital polymerase chain reaction (ddPCR) for ctDNA
Whole-blood samples were obtained from patients who provided written informed consent for translational research before the combination treatment with METi and EGFRi and at each response assessment. A QX200 Droplet Digital PCR system (BioRad, Hercules, CA, USA) was used for ddPCR as per the manufacturer’s protocol. The TaqMan Probe mix was used to measure the MET GCN (MET copy number variation: FAM, #10031240; HEX, #10031243). After completion of the PCR, a droplet reader was used to read TaqMan Probe fluorescence in individual droplets using the QuantaSoft software.
Statistical analysis
An analysis of drug efficacy and adverse effects was performed for all patients who received at least one dose of EGFRi plus METi combination. Response rate was the percentage of patients whose best tumor response was complete response (CR) or partial response (PR). Pearson’s Chi-squared test or Fisher’s exact test was used to determine the relationship between categorical variables, where appropriate. PFS was assessed from the first day of treatment until the first documentation of disease progression or death, with censoring of patients who are lost to follow-up. Overall survival (OS) was measured from the first day of treatment until death or the most recent follow-up. Survival time was estimated using the Kaplan-Meier method and between-group differences in survival were assessed using the log-rank test. The standard Cox proportional hazards regression model was used to perform multivariate survival analysis. Two-tailed P values less than 0.05 were considered indicative of statistical significance.
Results
Patient characteristics
A total of 44 patients (mean age: 59 years; males: 56.8%) were included in this study (Table 1, Figure S1). The proportion of never smoker and adenocarcinoma histology were 38.6% and 93.2%, respectively. At the baseline, 22 (50.0%) patients had brain metastasis (BM) while 10 (22.7%) patients had pulmonary lymphangitic metastasis. MET amplification was diagnosed using FISH in 38 (86.4%) patients and by plasma ctDNA NGS in 6 (13.6%) patients. The median number of previous chemotherapy lines was 2 (range, 1–6). The EGFRi plus METi treatment was started after failure of 1st or 2nd generation EGFRi in 31 (70.5%) patients, and after failure of 3rd generation EGFRi in 13 (29.5%) patients. The following combination regimens were used in our cohort: osimertinib plus savolitinib (n=31); erlotinib plus crizotinib (n=5); osimertinib plus crizotinib (n=4); gefitinib plus capmatinib (n=2); gefitinib plus crizotinib (n=1); and afatinib plus crizotinib (n=1).
Table 1
| Characteristics | Subgroups | N (%) |
|---|---|---|
| Age | <65 years | 35 (79.5) |
| ≥65 years | 9 (20.5) | |
| Sex | Male | 25 (56.8) |
| Female | 19 (43.2) | |
| Smoking | Never | 17 (38.6) |
| Ever | 27 (61.4) | |
| ECOG PS | 0 or 1 | 36 (81.8) |
| 2 | 8 (18.2) | |
| Histology | Adenocarcinoma | 41 (93.2) |
| Non-adenocarcinoma | 3 (6.8) | |
| EGFR mutation | Exon 19 deletion | 25 (56.8) |
| Exon 21 L858R | 18 (40.9) | |
| Exon 18 G719X | 1 (2.3) | |
| Brain metastasis | Yes | 22 (50.0) |
| No | 22 (50.0) | |
| Lymphangitic metastasis | Yes | 10 (22.7) |
| No | 34 (77.3) | |
| Diagnostics for MET amplification | FISH test | 38 (86.4) |
| Plasma ctDNA NGS | 6 (13.6) | |
| Prior EGFR-TKI | 1st or 2nd generation | 31 (70.5) |
| 3rd generation | 13 (29.5) | |
| EGFR-TKI | 1st or 2nd generation | 9 (20.5) |
| 3rd generation | 35 (79.5) | |
| MET-TKI | Savolitinib | 31 (70.5) |
| Crizotinib | 11 (25.0) | |
| Capmatinib | 2 (4.5) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; FISH, fluorescence in situ hybridization; ctDNA, circulating tumor DNA; NGS, next-generation sequencing; TKI, tyrosine kinase inhibitor.
Adverse effects and drug discontinuation
The median number of treatment cycles administered in our cohort was 3 (range, 1–61). No drug-related death events occurred. Sixteen (36.4%) patients experienced severe grade 3 or 4 adverse effects, including hypersensitivity (n=7), pneumonitis (n=2), hepatitis (n=2), cellulitis (n=2), nausea (n=1), generalized pain (n=1), and deep vein thrombosis (n=1). The number of patients who permanently discontinued any drug due to adverse effects was 23 (52.3%): both drugs (n=13, 29.5%); METi only (n=10, 22.7%), and EGFRi only (n=0, 0.0%) (Table 2). The median time from starting treatment to discontinuing both drugs or METi only was 84 days (range, 10–952 days) or 16 days (range, 8–117 days), respectively. The reasons for discontinuation of both drugs were pneumonitis (69.2%), hypersensitivity (15.4%), mediastinitis (7.7%), and recurrent cellulitis (7.7%). The reasons for discontinuation of only METi were hypersensitivity (50.0%), generalized pain (20.0%), hepatitis (20.0%), and deep vein thrombosis (10.0%). There was no significant difference in the discontinuation rate between 1st or 2nd generation EGFRi and 3rd generation EGFRi (44.4% vs. 54.3%). Among the three METis, the discontinuation rate of savolitinib was relatively higher than other METis (61.3% vs. 30.8%).
Table 2
| Characteristics | Subgroups | N (%) |
|---|---|---|
| Dose reduction | EGFR-TKI | 0/44 (0.0) |
| MET-TKI | 6/44 (13.6) | |
| Drug discontinuation | Both drugs | 13/44 (29.5) |
| MET-TKI only | 10/44 (22.7) | |
| Cause of discontinuation of both drugs | Pneumonitis | 9/13 (69.2) |
| Hypersensitivity | 2/13 (15.4) | |
| Mediastinitis | 1/13 (7.7) | |
| Recurrent cellulitis | 1/13 (7.7) | |
| Cause of discontinuation of MET-TKI | Hypersensitivity | 5/10 (50.0) |
| Generalized pain | 2/10 (20.0) | |
| Hepatitis | 2/10 (20.0) | |
| Deep vein thrombosis | 1/10 (10.0) | |
| Discontinuation rate per drug |
EGFR-TKI | |
| • 1st or 2nd generation | 4/9 (44.4) | |
| • 3rd generation | 19/35 (54.3) | |
| MET-TKI | ||
| • Savolitinib | 19/31 (61.3) | |
| • Crizotinib | 3/11 (27.3) | |
| • Capmatinib | 1/2 (50.0) |
TKI, tyrosine kinase inhibitor.
Among total patients, the incidence of pneumonitis was 25.0% (11/44): grade 2 (9/11, 81.8%), grade 3 (1/11, 9.1%), and grade 4 (1/11, 9.1%). The incidence and severity of pneumonitis according to the combination regimen of METi plus EGFRi were shown in Table S1. Crizotinib plus 1st or 2nd generation EGFRi had relatively higher incidence of pneumonitis compared to other regimens.
Intracranial tumor response
As of the data cutoff date (June 1, 2023), 36 (81.8%) disease progression and 38 (86.4%) death events occurred over a median follow-up of 65.2 months [95% confidence interval (CI): 0.0–139.8]. One patient was still alive receiving the EGFRi plus METi combination.
The response evaluation was available for 43 patients. The overall response rate was 74.4% [CR (n=1, 2.3%), PR (n=31, 72.1%), stable disease (SD) (n=10, 23.3%), and disease progression (n=1, 2.3%)] (Figure S2). Among 22 patients with BM, intracranial tumor response rate was 63.6%; SD (n=8) and PR (n=14). Figure 1 illustrates two patients in whom multiple BM showed prompt and dramatic response to the EGFRi plus METi combination. The first patient was a 67-year-old man with stage IV lung adenocarcinoma with EGFR 19 deletion mutation (Figure 1A). Secondary MET amplification was identified in his lung tumor tissue after progression to second-line osimertinib treatment. At the time of progression, he had dysdiadochokinesia and speech fluency disorder; brain MRI showed multiple lobulated necrotic lesions in both cerebral hemispheres, cerebellum, and left thalamus. Thus, he was prescribed erlotinib 150 mg plus crizotinib 250 mg daily. After 8 weeks, almost all brain lesions had completely resolved and his neurologic symptoms were much improved. Moreover, there was significant regression of his lung and bone metastases. His tumor response was maintained for 5.3 months. The second patient was a 59-year-old woman with stage IV lung adenocarcinoma who developed acquired resistance to second-line osimertinib treatment (Figure 1B). She had multifocal nodular or necrotic lesions in both cerebral hemispheres and cerebellum with localized leptomeningeal enhancement in the right Sylvian fissure as well as multiple bone, liver, and right adrenal gland metastases. At the time of progression, EGFR 19 deletion and T790M mutation and MET amplification were detected in her plasma ctDNA NGS test (Guardant360®). Thus, she was prescribed afatinib 20 mg and crizotinib 250 mg daily. After two cycles, multiple BM and leptomeningeal carcinomatosis were considerably attenuated along with remarkable improvement in dysarthria. Extracranial metastatic lesions were also much improved. Her tumor response was maintained for 3.9 months. Further analysis of clinicopathologic factors related to BM response showed the previous history of local treatment for BM and EGFRi type was not significantly associated with the BM response (Table S2). However, METi type was associated with the BM response (P=0.052). Crizotinib was numerically inferior to other specific MET inhibitors in terms of treating BM: the response rate to BM, savolitinib (11/14, 78.6%), crizotinib (2/7, 28.6%), and capmatinib (1/1, 100%) (P=0.052).

Predictors of PFS
The median PFS in our cohort was 5.3 months (95% CI: 3.3–7.3) and the median OS was 13.4 months (95% CI: 10.2–16.6) (Figure S3A,S3B). We further analyzed the clinicopathologic factors related to survival outcomes after treatment with EGFRi plus METi combination. In the univariate analysis of PFS, only lymphangitic metastasis was associated with PFS [hazard ratio (HR), 2.46; 95% CI: 0.14–5.34; P=0.02] (Table S3). The potential factors related to METi efficacy such as MET/CEP7 ratio on FISH test, METi type, and METi discontinuation were not significant predictors of PFS. In addition, the potential factors related to EGFRi efficacy such as EGFR mutation type, history of EGFRi therapy and its response, and EGFRi type were not related to the risk of disease progression. Among 23 patients who were treated with the combination treatment of 3rd generation EGFRi plus METi after progression to 1st or 2nd generation EGFRi, EGFR T790M mutation was simultaneously detected in 2 patients, not detected in 15 patients, and not evaluated in 6 patients. In this patient population, there was no significant difference in PFS between the tumors with or without EGFR T790M mutation (HR, 1.90; 95% CI: 0.38–9.46; P=0.43). The presence of lymphangitic metastasis remained significant even in the multivariate PFS analysis accounting for MET/CEP7 ratio, prior EGFRi therapy, and current METi type. Patients with lymphangitic metastasis showed a significantly shorter median PFS compared to those without lymphangitic metastasis (3.8 vs. 6.1 months, P=0.03) (Figure S3C,S3D).
In the univariate analysis of OS, prior EGFRi treatment type and current METi treatment type were associated with the risk of death from the combination treatment (Table S4). However, no independent predictors of OS were identified in multivariate analysis.
Timing of METi discontinuation
Serial monitoring of MET amplification in ctDNA isolated from plasma was available for nine patients who continued the combination treatment without interruption of METi. At the baseline, the median value of MET to reference gene ratio was 1.85 (range, 1.00–3.30). The median time to the normalization of ctDNA MET ratio (1.00) from the initiation of the combination treatment was estimated using Kaplan-Meier curve (Figure 2A). The median time elapsed from the initiation of combination treatment to ctDNA MET gene normalization was 63 days (95% CI: 39–not available). We divided the 23 patients in whom METi treatment was withheld due to adverse effects into two subgroups based on the median ctDNA MET normalization time. Patients who stopped METi before 63 days from the start of combination treatment (n=14) had a significantly inferior outcome compared with those who discontinued METi after ≥63 days (n=9) [PFS: HR, 2.78, 95% CI: 1.00–7.75, P=0.050; OS: HR, 2.71, 95% CI: 1.02–7.16, P=0.045] (Figure 2B,2C).

On the other hand, continuation of EGFRi even after withholding METi was not associated with survival outcomes (with vs. without EGFRi maintenance; PFS: HR, 2.06; 95% CI: 0.73–5.80, P=0.17; OS: HR, 0.95, 95% CI: 0.39–2.33, P=0.92).
Acquired resistance and subsequent treatment
Table 3 lists the details of 14 (31.8%) patients for whom the mechanism of resistance to EGFRi and METi combination was detected via genomic and histologic comparison between pretreatment and posttreatment samples. Secondary MET mutation (MET D1246H) was detected in 1 (7.1%) patient who received osimertinib and savolitinib for 11.8 months. This patient was administered a trial of a multi-receptor-tyrosine-kinase-targeting MET inhibitor (sitravatinib) and showed a PR with 40% tumor shrinkage. On the other hand, secondary EGFR mutation was detected in seven patients (50%): C797S (n=2) and T790M (n=5). In one patient who acquired EGFR C797S mutation after osimertinib and savolitinib treatment for 4 months, amivantamab, an EGFR-MET bispecific antibody, was used as subsequent therapy; this patient showed a PR with 38% tumor shrinkage. EGFR T790M mutation developed in four patients who received 1st- or 2nd-generation EGFRi plus METi and one patient who stopped osimertinib plus savolitinib due to pneumonitis before the disease progression. All five patients with EGFR T790M-mutant tumors received osimertinib with or without METi after the failure of initial EGFRi plus METi treatment. Three patients showed clinical benefit from osimertinib: PR (n=2); SD (n=1). The two non-responders to osimertinib included one patient whose tumor showed histologic transformation to squamous cell carcinoma and one patient whose tumor still had high MET amplification along with EGFR T790M mutation. After osimertinib failure, the patient with both EGFR T790M mutation and histologic transformation to squamous cell carcinoma as acquired resistance was treated with atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) and showed a PR with 52% tumor shrinkage. Secondary bypass resistance mechanisms included KRAS amplification, MAP2K1 mutation and FGFR3 amplification, CDK4 and FGFR1 amplification, CCND1 amplification, and RB1 mutation.
Table 3
| ID | Age (years)/sex | Prior EGFR-TKI |
Current regimen | Drug discontinuation due to adverse effect (time) | Response (PFS) | Progression site | Mechanism of acquired resistance | MET amplification after progression (test) | Subsequent treatment (response) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | Olmutinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | None | PR (11.8 m) | Lung, bone | MET D1246H | – | Sitravatinib (PR) |
| 2 | 55/M | Afatinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | None | PR (4.2 m) | Brain | EGFR C797S | Negative (FISH) | Amivantamab (PR) |
| 3 | 59/F | Osimertinib | Osimertinib 80 mg QD + crizotinib 250 mg QD | None | PR (12.0 m) | Lung, liver, bone | EGFR C797S | – | – |
| 4 | 53/M | Erlotinib | Gefitinib 250 mg QD + capmatinib 400 mg BID | None | PR (20.3 m) | Lung | EGFR T790M | – | Osimertinib (PR) |
| 5 | 58/M | Dacomitinib | Erlotinib 100 mg QD + crizotinib 250 mg BID | None | PR (8.2 m) | Brain, mediastinal lymph node | EGFR T790M | Positive (FISH) | Osimertinib (PD) |
| 6 | 58/F | Osimertinib | Afatinib 20 mg QD + crizotinib 250 mg QD | None | PR (3.9 m) | Lung, liver, brain | EGFR T790M | – | Osimertinib + crizotinib (SD) |
| 7 | 60/F | Erlotinib | Osimertinib 80 mg QD + savolitinib 300 mg QD | Both drugs (8 cycles) |
PR (7.9 m) | Lung | EGFR T790M | Negative (FISH) | Osimertinib (PR) |
| 8 | 61/M | Afatinib | Erlotinib 100 mg QD + crizotinib 250 mg BID | None | PR (2.6 m) | Bone | EGFR T790M and SQCC transformation | Positive (FISH) | Osimertinib + crizotinib (PD) |
| 9 | 55/M | Erlotinib | Osimertinib 80 mg QD + savolitinib 300 mg QD | Savolitinib (1 cycle) |
PR (3.8 m) | Lung | MAP2K1 E203K and FGFR3 amplification | – | PCb (PD) |
| 10 | 57/F | Gefitinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | None | SD (18.6 m) | Lung, brain | KRAS amplification | Negative (FISH) | Afatinib (PD) |
| 11 | 41/F | Gefitinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | Savolitinib (1 cycle) |
PR (2.8 m) | Pericardial effusion | EGFR wild type | Negative (FISH) | GCb (PD) |
| 12 | 60/M | Osimertinib | Osimertinib 80 mg QD + crizotinib 250 mg QD | None | SD (1.8 m) | Lung | CDK4 amplification and FGFR1 amplification | Negative (FISH) | Trametinib (PD) |
| 13 | 69/F | Erlotinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | None | PR (4.1 m) | Soft tissue | CCND1 amplification | Positive (FISH) | IP (PD) |
| 14 | 58/M | Erlotinib | Osimertinib 80 mg QD + savolitinib 600 mg QD | Savolitinib (1 cycle) |
SD (2.7 m) | Pleura | RB1 R320* | Positive (NGS) | Amivantamab (PR) |
TKI, tyrosine kinase inhibitor; M, male; F, female; QD, once daily; BID, twice daily; PFS, progression-free survival; PR, partial response; SD, stable disease; m, months; SQCC, squamous cell carcinoma; FISH, fluorescence in situ hybridization; NGS, next-generation sequencing; PD, progressive disease; PCb, paclitaxel plus carboplatin; GCb, gemcitabine plus carboplatin; IP, irinotecan plus cisplatin.
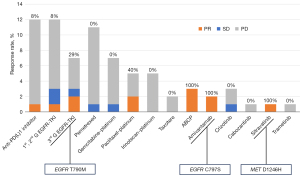
After progression while on the combination treatment, 31 (70.5%) patients underwent subsequent chemotherapy. The median number of subsequent chemotherapy regimens was 2 (range, 1–6). Figure 3 displays all types of chemotherapy regimens administered to all patients after showing progression while on EGFRi plus METi combination. The most frequently used regimen was anti-PD(L)1 inhibitor (n=12) and 1st- or 2nd-generation EGFRi (n=12). Among these regimens, the regimens that included paclitaxel and platinum, such as ABCP, showed a trend of higher response rates compared to other regimens. It seems that immune checkpoint inhibitor, retreatment with 1st- or 2nd-generation EGFRi, and pemetrexed had little effect on the tumors resistant to EGFRi plus METi combination.
Discussion
To the best of our knowledge, this is the first study to present longitudinal data about patients with EGFR-mutant and MET amplified lung cancer treated with EGFRi plus METi combination, including the efficacy, safety profile, acquired resistance mechanism, and subsequent treatment. This study suggests that the strategy of combining EGFRi and METi was highly effective for secondary MET-amplified EGFR-mutant lung cancer patients because it resulted in a prompt and dramatic tumor response with rapid symptom relief. This combination treatment provided a survival gain of approximately 1 year to previously-treated lung cancer patients with poor prognosis.
Interestingly, this study demonstrated substantial tumor response was observed in intracranial lesion as well as extracranial lesions. 1st or 2nd generation EGFRi is generally considered to be inferior to 3rd generation EGFRi in terms of the central nervous system (CNS) penetration activity (10). Crizotinib also is known to have poor penetration ability into the blood-brain barrier (11). However, two cases of this study having bulky brain metastases with or without leptomeningeal disease showed the remarkable response to the combination treatment with a 1st- or 2nd-generation EGFRi and half dose of crizotinib. Although the specific METi was numerically better in the response to BM than the nonspecific METi, these cases raise the possibility that two-drug combination strategy may have a synergistic effect against intracranial lesions. Most recently, the FLAURA2 clinical study supported this finding that the addition of chemotherapy to osimertinib improved survival outcomes compared to osimertinib monotherapy in patients with the CNS metastasis (12,13).
Nevertheless, in this study, 52.3% of patients had to discontinue both EGFRi and METi or METi alone due to drug-related adverse effects. The discontinuation rate in our study seems to be higher than that in a previous clinical trial (14). The primary cause of drug discontinuation due to adverse effects was pneumonitis for both drugs or hypersensitivity for METi alone, which can lead to a fatal and life-threatening event. Concurrent administration of two drugs may be the ideal way to overcome acquiring bypass resistance mechanisms such as MET amplification in patients treated with targeted therapy. However, in case of overlapping adverse effects of the two drugs, it is challenging to assess the effectiveness of this concurrent treatment. Thus, we further evaluated the survival impact of discontinuing METi due to adverse effects. Unexpectedly, this study found no significant difference in the survival outcomes between patients who discontinued METi due to adverse effects and those who did not. Several case series have presented the effect of METi monotherapy in the same patient subsets with EGFR-mutant and secondary MET-amplified lung cancer (15-20). Among these reports, van Veggel et al. demonstrated that MET amplification in tumors disappeared even after short treatment with METi and suggested that MET-amplified cells might be minor clones among the whole EGFRi-resistant cells (17). These previous findings might explain why the patients who had to receive short-term METi treatment owing to toxicities did not have worse survival compared to those who received long-term METi treatment in this study.
If transient use of METi could successfully eradicate METi-sensitive subclones, it might reduce the duration of concurrent administration of EGFRi and METi. Thus, we sought to determine the time required to clear METi-sensitive cells, utilizing serial plasma ctDNA data. Recent studies have demonstrated that the blood ctDNA test is a useful tool to identify minimal residual disease, which has been proposed as a surrogate biomarker to predict postoperative recurrence after curative surgery in early-staged solid cancers (21-23). In this study, the estimated time required for the disappearance of MET amplification in plasma ctDNA was 63 days from starting the combination treatment. This plasma MET amplification clearance time is shorter than the time required for the development of severe adverse effects leading to the discontinuation of both drugs (84 days). Additionally, patients who discontinued METi after 63 days of treatment showed significantly longer survival outcomes than those who discontinued METi before 63 days. Thus, it is recommended that plasma ctDNA should be monitored in conjunction with this treatment to monitor treatment response and potentially guide therapeutic decisions in this patient population.
In this study, on-target secondary mutations were identified as the most common mechanism of acquired resistance to the EGFRi plus METi combination treatment. Firstly, EGFR T790M or C797S mutation are well-known mechanisms of acquired resistance to EGFRi (3,24,25). Currently, novel 4th-generation EGFRis, such as BDTX-1535 and BLU-945, are being actively developed to treat EGFR C797S-mutant tumors (26). In our cohort, the patient with EGFR C797S mutation showed a good response to amivantamab, which has been evaluated in combination with lazertinib in the osimertinib-failed patients with EGFR-mutant NSCLC (CHRISALI-2 study) (27). This new drug may be effective in targeting both EGFR and MET signaling pathways. Another patient whose tumor had MET amplification and RB1 mutation without EGFR C797S mutation after progression on osimertinib plus savolitinib demonstrated a good response to amivantamab. On the other hand, previous case studies have reported MET D1246H mutation as a mechanism of acquired resistance to EGFRi plus METi combination treatment (28,29). In this study, the patient who acquired MET D1246H mutation after progression on osimertinib plus savolitinib was treated with sitravatinib alone and showed substantial tumor shrinkage. Recent studies have demonstrated the effectiveness of type II MET inhibitors against tumors acquiring resistance to type I MET inhibitors by secondary MET mutations (30,31). These studies suggest that tumors that acquire resistance to EGFRi and METi due to on-target secondary mutations may potentially be treated with another targeted therapy. Thus, attempts to identify the resistance mechanisms, including re-biopsy and comprehensive genetic analysis, should be continued even in patients who show progression after EGFRi plus METi combination treatment.
Among subsequent treatments including nontargeted therapy, pemetrexed and immune checkpoint inhibitors were the most frequently used but showed limited benefit after EGFRi plus METi combination treatment. The most effective cytotoxic therapy seems to be the regimen including paclitaxel and platinum [PR: 5 of 8 (62.5%)]. Especially, the patients treated with the ABCP regimen showed a promising response [PR: 3 of 3 (100%)] compared to the other chemotherapy regimens. In the IMPOWER 150 study, the ABCP regimen conferred a significant survival benefit in the subgroup of patients with EGFR mutation who are unlikely to benefit from immune checkpoint inhibitor therapy (32). Although the mechanism of the therapeutic effect of the ABCP regimen in EGFR-mutant tumors remains unknown, this study showed that this regimen can be effective even in heavily-treated patients with EGFR-mutant tumors.
The main limitations of this study should be considered while interpreting the results. First, there was significant heterogeneity in terms of patient characteristics, chemotherapy regimen, dose, and administration schedule. In particular, including both clinical trial patients and real-world patients could introduce potential bias into the evaluation of efficacy and safety data because the two groups’ baseline characteristics and management may be different. Actually, two clinical studies about METi and EGFRi combination treatment into which some patients of this study were originally enrolled, showed the difference in the following (4,5). In the TATTON study with EGFR mutation-positive, MET-amplified, and EGFR-TKI-failed NSCLC, the patient treated by savolitinib 600 mg and osimertinib 80 mg showed 57% of grade ≥3 adverse event and 28% of savolitinib treatment discontinuation, but they had a 48% response rate and a PFS of 7.6 months (4). In contrast, the phase Ib/II study of capmatinib plus gefitinib in patients with EGFR-mutated, MET-amplified or overexpressing NSCLC after progression to EGFRi, demonstrated 29.0% of grade ≥3 adverse event, 13.0% of treatment discontinuation due to adverse effects, 29% response rate and 5.5 months PFS (5). Despite these limitations, this retrospective study was conducted to provide physician treating this rare and poor patient population with more detailed and longitudinal data beyond the efficacy and safety of clinical trials. Second, the results of statistical analyses should be interpreted with caution due to the small sample size. For example, based on previous clinical trials, a subgroup of patients in whom EGFRi type may have significant impact on the overall efficacy with combination treatment, are those who received non-3rd-generation EGFRi plus METi for T790M-positive tumor after progression to non-3rd-generation EGFRi (4,33). However, in this study, the number of those patients (n=2) was too small to affect the clinical outcomes of combination treatment and thus, the EGFRi type was not significantly associated with median PFS. In addition, the plasma MET amplification clearance time was determined using data from only nine patients because this analysis was performed only for patients who continued both EGFRi and METi until disease progression. In addition, the number of patients was two small to compare the tumor response according to subsequent therapy regimens. Although secondary MET-amplified and EGFR-mutant lung cancer population is rather rare, a prospective study with a larger sample size is required to reassess these important findings.
Conclusions
The EGFRi plus METi combination therapy showed a remarkable response leading to rapid tumor-associated symptom relief in the EGFRi-treated patients with advanced EGFR-mutant and MET-amplified NSCLC. However, the occurrence of severe toxicity requiring drug continuation, such as pneumonitis, was relatively common with this combination regimen. Early discontinuation of METi negatively affected the survival outcomes. A variety of mechanisms of acquired resistance to the two drugs were detected after disease progression while receiving the combination treatment. Active surveillance to identify resistance mechanisms may help improve survival after the failure of the EGFRi plus METi combination.
Acknowledgments
We acknowledge the support from the Research Core Center Biostatistics Collaboration Team at National Cancer Center.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-273/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-273/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-273/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-273/coif). Y.L. received consulting fees from Roche, Merck, Yuhan, and Bayer. J.Y.H. received research grants from Roche, ONO, Pfizer and Takeda; consulting fee from Astra Zeneca, BMS, Eli Lilly, Merck, Novartis, Pfizer, Abion, Jints Bio; and honoraria for lecture from Astra Zeneca, BMS, Merck, Takeda and Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of the National Cancer Center (No. NCC 2016-0208). Informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roper N, Brown AL, Wei JS, et al. Clonal Evolution and Heterogeneity of Osimertinib Acquired Resistance Mechanisms in EGFR Mutant Lung Cancer. Cell Rep Med 2020;1:100007. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol 2020;21:373-86. [Crossref] [PubMed]
- Wu YL, Zhang L, Kim DW, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:3101-9. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020;8:1132-43. [Crossref] [PubMed]
- Tan DSW, Kim TM, Guarneri V, et al. Tepotinib + osimertinib for EGFR mutant (EGFRm) NSCLC with MET amplification (METamp) after first-line (1L) osimertinib. J Clin Oncol 2023;41:9021.
- Go H, Jeon YK, Park HJ, et al. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:305-13. [Crossref] [PubMed]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43-6.
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Jänne PA, Planchard D, Kobayashi K, et al. CNS Efficacy of Osimertinib With or Without Chemotherapy in Epidermal Growth Factor Receptor-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2024;42:808-20. [Crossref] [PubMed]
- Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 2023;389:1935-48. [Crossref] [PubMed]
- Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 2020;31:507-16. [Crossref] [PubMed]
- Ou SI, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 2016;98:59-61. [Crossref] [PubMed]
- Wang W, Wang H, Lu P, et al. Crizotinib with or without an EGFR-TKI in treating EGFR-mutant NSCLC patients with acquired MET amplification after failure of EGFR-TKI therapy: a multicenter retrospective study. J Transl Med 2019;17:52. [Crossref] [PubMed]
- van Veggel B, de Langen AJ, Hashemi S, et al. Crizotinib treatment for patients with EGFR mutation positive NSCLC that acquire cMET amplification after EGFR TKI therapy results in short-lived and heterogeneous responses. Lung Cancer 2018;124:130-4. [Crossref] [PubMed]
- Yoshimura K, Inui N, Karayama M, et al. Successful crizotinib monotherapy in EGFR-mutant lung adenocarcinoma with acquired MET amplification after erlotinib therapy. Respir Med Case Rep 2017;20:160-3. [Crossref] [PubMed]
- Baldacci S, Mazieres J, Tomasini P, et al. Outcome of EGFR-mutated NSCLC patients with MET-driven resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2017;8:105103-14. [Crossref] [PubMed]
- Choi YR, Kang EH, Kim S, et al. Single targeting of MET in EGFR-mutated and MET-amplified non-small cell lung cancer. Br J Cancer 2023;128:2186-96. [Crossref] [PubMed]
- Tie J, Cohen JD, Wang Y, et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol 2019;5:1710-7. [Crossref] [PubMed]
- Tarazona N, Gimeno-Valiente F, Gambardella V, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol 2019;30:1804-12. [Crossref] [PubMed]
- Jung HA, Ku BM, Kim YJ, et al. Longitudinal Monitoring of Circulating Tumor DNA From Plasma in Patients With Curative Resected Stages I to IIIA EGFR-Mutant Non-Small Cell Lung Cancer. J Thorac Oncol 2023;18:1199-208. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Li Y, Mao T, Wang J, et al. Toward the next generation EGFR inhibitors: an overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer. Cell Commun Signal 2023;21:71. [Crossref] [PubMed]
- Shu CA, Goto K, Ohe Y, et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J Clin Oncol 2022;40:9006.
- Lim SM, Yang SD, Lim S, et al. Brief Report: Heterogeneity of Acquired Resistance Mechanisms to Osimertinib and Savolitinib. JTO Clin Res Rep 2021;2:100180. [Crossref] [PubMed]
- Wang K, Du R, Roy-Chowdhuri S, et al. Brief Report: Clinical Response, Toxicity, and Resistance Mechanisms to Osimertinib Plus MET Inhibitors in Patients With EGFR-Mutant MET-Amplified NSCLC. JTO Clin Res Rep 2023;4:100533. [Crossref] [PubMed]
- Bahcall M, Sim T, Paweletz CP, et al. Acquired METD1228V Mutation and Resistance to MET Inhibition in Lung Cancer. Cancer Discov 2016;6:1334-41. [Crossref] [PubMed]
- Riedel R, Fassunke J, Tumbrink HL, et al. Resistance to MET inhibition in MET-dependent NSCLC and therapeutic activity after switching from type I to type II MET inhibitors. Eur J Cancer 2023;179:124-35. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Yang JJ, Fang J, Shu YQ, et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Invest New Drugs 2021;39:477-87. [Crossref] [PubMed]


