
TQB2450 with or without anlotinib as maintenance treatment in subjects with locally advanced/unresectable non-small cell lung cancer that have not progressed after prior concurrent/sequential chemoradiotherapy (R-ALPS): study protocol for a randomized, double-blind, placebo-controlled, multicenter phase III trial
Introduction
Approximately one-third of patients with non-small cell lung cancer (NSCLC) have locally advanced stage III disease at diagnosis (1), which is complex to manage and the management strategy is a subject of debate. Prior to the advent of immune checkpoint inhibitors (ICIs), the standard treatment for unresectable stage III NSCLC with a good performance status was platinum-based definitive chemoradiotherapy (dCRT) (2). However, the prognosis of these patients is poor, with a 5-year survival of only 15% (1,3).
ICIs targeting programmed cell death protein 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1) have revolutionized the treatment of stage III NSCLC and new treatment combinations are constantly being evaluated (4,5). A growing body of evidence suggests that anti-angiogenic therapy can transform the immune-inhibitory tumor microenvironment into a more immune-active state (6,7). Anti-angiogenic therapy can rapidly normalize the blood vessels, which in turn stabilizes endothelial cells, reduces vascular permeability, increases drug and oxygen delivery, and promotes the infiltration of effector T cells into tumors (8,9). The combination of atezolizumab and bevacizumab has shown synergistic antitumor activity in advanced non-squamous NSCLC, regardless of PD-L1 expression (10). Similarly, the IMpower150 trial reported enhanced efficacy of anti-angiogenic and immunological therapy in combination with chemotherapy in patients with metastatic NSCLC (11).
Anlotinib, an oral multitarget tyrosine kinase inhibitor (TKI), has shown efficacy in relapsed and advanced NSCLC by suppressing the activation of proangiogenic signals such as vascular endothelial growth factor receptor 1–3 (VEGFR1–3), platelet-derived growth factor receptor-beta (PDGFR-β), fibroblast growth factor receptor 1 (FGFR1), and stem cell factor receptor (c-Kit) (12-17), which led to its approval by National Medical Products Administration (NMPA) of China (18,19). Based on preclinical data, first-line sintilimab combined with anlotinib in patients with advanced NSCLC attained a disease control rate (DCR) of 100% and an objective response rate (ORR) of 73%, with a median progression-free survival (PFS) of 15 months (20).
TQB2450 (benmelstobart) is a novel humanized immunoglobulin G1 (IgG1) monoclonal antibody against PD-L1 developed by Chia Tai Tianqing Pharmaceutical Group Co. Ltd. Ongoing early phase clinical studies, including NSCLC and other solid tumors, demonstrate that TQB2450 in combination with anlotinib has promising antitumor activity and acceptable safety (7,21-26). A phase Ib study randomized pretreated stage IIIB or IV NSCLC to receive TQB2450 with or without anlotinib, and the median PFS was 8.7 months for TQB2450 plus anlotinib compared with 2.8 months for TQB2450 plus placebo. Further subgroup analysis revealed that in patients with PD-L1 ≥1% the ORR was 50.0% in the TQB2450 plus anlotinib group, and 5.3% in the TQB2450 plus placebo group (21). ETER701 was a double-blind, randomized, placebo-controlled phase III trial that investigated the efficacy of TQB2450 with anlotinib and standard chemotherapy for treating extensive-stage small-cell lung cancer. It demonstrated that the median overall survival (OS) was prolonged with TQB2450 and anlotinib plus etoposide/carboplatin compared with chemotherapy alone (19.3 vs. 11.9 months; P<0.001) (26).
The Radiotherapy and Anlotinib Let PD-L1 Superb (R-ALPS) study will explore the efficacy and safety of this treatment combination in participants with locally advanced/unresectable (stage III) NSCLC. The objectives of the R-ALPS study are to: (I) enhance the efficacy of treatment after dCRT in patients with NSCLC by adding TQB2450 with or without anlotinib; (II) evaluate the safety and tolerability of the combination of immunological and anti-angiogenic therapy after dCRT as the maintenance treatment for NSCLC; and (III) collect tumor tissue and blood samples for translational research. The protocol is presented in accordance with the SPIRIT reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-362/rc).
Methods
Study design and characteristics of participants
This randomized, double-blind, placebo-controlled, multicenter phase III trial will examine the treatment efficacy and tolerability of TQB2450, with or without anlotinib, in the maintenance treatment of patients with stage III NSCLC after dCRT. Because durvalumab had not yet been approved by the NMPA and was unavailable during the application of this trial to the Center for Drug Evaluation of China (September 2019), and observation after dCRT was still the standard treatment for locally advanced/unresectable NSCLC in China at the time, a placebo control arm was set up in this study. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013), and supervised by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. [a contract research organization (CRO)].
Given that durvalumab has been approved by the NMPA for maintenance treatment of patients with stage III NSCLC after concurrent chemoradiotherapy (December 2019), our study encourages patients to receive standard immunotherapy. Patients who decline maintenance therapy with durvalumab and are eligible for study inclusion will be approached and informed about the study when they arrive at the medical site. At the time of inclusion in the study, all participants must have been diagnosed with stage III NSCLC without progression after prior dCRT, independent of the study, as part of the standard treatment (Figure 1). Prior to study enrollment, all archives of documents and their imaging will be reviewed by a board-certified radiation oncologist and radiotherapist to estimate whether lung tumor formation after dCRT can safely be treated with immunological and anti-angiogenic therapy. Patients who have previously received other PD-1/PD-L1 targeting therapies or anlotinib will be excluded. The detailed eligibility criteria for the study are provided in Table 1. The Institutional Review Boards (IRBs) of each center have approved the trial, including Sun Yat-sen University Cancer Center (No. A2021-080-01), Zhejiang Cancer Hospital {No. IRB-[2020]62}, Guangxi Medical University Cancer Hospital {No. CS2020[11]}, Tianjin Medical University Cancer Institute & Hospital (No. E2020090), The Second People’s Hospital of Neijiang (No. 2020-024-001), Henan Cancer Hospital (No. 2020022609), Qilu Hospital of Shandong University (No. 2020011), Xiangya Hospital Central South University (No. 202004075), Shandong Cancer Hospital & Institute (No. SDZLEC2021-041-01), and Shanxi Cancer Hospital (No. YW2020034). The complete list of the IRB names of 42 sites participating in the study and their registration numbers can be found at Table S1.
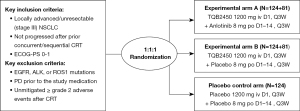
Table 1
| Inclusion criteria |
| • Fully-informed written consent with well compliance |
| • Confirmed stage III NSCLC |
| • Expected survival time ≥3 months |
| • ECOG-PS: 0–1 |
| • Completed at least two cycles of platinum-based concurrent/sequential CRT without PD |
| (I) Within 42 days between last radiotherapy and the first medication |
| (II) For sequential CRT, the interval between the end of the chemotherapy cycle and the start of radiotherapy should not exceed 6 weeks |
| (III) Consolidation chemotherapy after radiotherapy was not allowed, however, chemotherapy before concurrent chemoradiotherapy was permitted |
| (IV) The total dose of radiotherapy was 60 Gy±10% (54–66 Gy). The minimum technical standard for radiotherapy is IMRT |
| • Adequate bone marrow, renal function, and hepatic functions |
| Exclusion criteria |
| • With EGFR, ALK, or ROS1 mutations |
| • Prior treatment with immunotherapeutic or anti-angiogenic drugs |
| • CT or MRI showed that the tumor had invaded major vessels, and was likely to cause fatal hemorrhage during the follow-up study |
| • AEs of CTCAE v5.0 ≥2 that had not completely relieved after previous radiotherapy or chemotherapy |
| • Presence of active bleeding or bleeding tendency |
| • Active or prior documented autoimmune or inflammatory disorders or history of active primary immunodeficiency |
| • History of another primary malignancy except for malignancies treated with curative intent and no known active disease ≥5 years before first dose of study medication |
| • Any co-existing medical condition that in the investigator’s judgement will substantially increase the risk associated with the patient’s participation in the study |
R-ALPS, Radiotherapy and Anlotinib Let PD-L1 Superb; PD-L1, programmed death-ligand 1; NSCLC, non-small cell lung cancer; ECOG-PS, Eastern Cooperative Oncology Group performance status; CRT, chemoradiotherapy; PD, progressive disease; IMRT, intensity-modulated radiation therapy; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1; CT, computed tomography; MRI, magnetic resonance imaging; AEs, adverse events; CTCAE, Common Terminology Criteria for Adverse Events.
In total, 534 patients will be enrolled at 42 sites in China. A complete list of the sites is available on ClinicalTrials.gov (NCT04325763). Recruitment began in May 2020 and is expected to be completed by March 2025.
Study procedures
All patients will be required to provide written informed consent before being enrolled in the clinical trial (available at https://cdn.amegroups.cn/static/public/tlcr-24-362-1.pdf). The randomization will be done through the Interactive Web Response System (IWRS) integrated into the electronic case report form (eCRF). All randomization design aspects and patient allocation status will be conducted in a strict double-blind manner. After randomization, participants will receive either TQB2450 [1,200 mg infused on day 1, every 3 weeks (Q3W) for 36 cycles] and anlotinib (8 mg orally once daily on days 1–14, Q3W for 36 cycles) in experimental arm A; or TQB2450 (1,200 mg infused on day 1, Q3W for 36 cycles) and anlotinib placebo in experimental arm B, or placebos of both TQB2450 and anlotinib in the placebo control arm. The treatment will be continued until the patient develops intolerable toxicities, progressive disease (PD), death, withdrawal, or the end of the trial.
Over the course of the study, treatment response will be examined according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) (27) using enhanced computed tomography (CT) of the chest and upper abdomen, and magnetic resonance imaging (MRI) of the brain at baseline, and then every 2 cycles (6 weeks) throughout the 36 cycles, and every 12 weeks thereafter until the onset of PD. The efficacy will be further confirmed with the immune RECIST (iRECIST), which means that patients who have PD according to the RECIST v1.1 criteria will be further evaluated to confirm the same according to the iRECIST criteria. In patients who discontinue treatment for reasons other than PD, imaging evaluation will be performed based on the above-mentioned timeline until progression, death, initiation of another antitumor treatment according to the care standards, or the end of the study. During and after the study, follow-up will be proactively conducted regarding treatment-related adverse events (AEs) of the participants until they are resolved, return to baseline or are deemed irreversible, loss to follow-up, or withdraw study consent. For participants who refuse to return to the site for evaluation, routine follow-ups by phone will be performed every 3 months. The investigators of this trial will be responsible for further treatment after the end of the study of participants with PD.
The safety assessment during the R-ALPS study will include physical examination, Eastern Cooperative Oncology Group performance status (ECOG-PS), continuous evaluation of AEs, and clinical laboratory profiles. Table 2 summarizes the study procedures. The principal researchers or authorized research assistants will complete the eCRF for each participant to collect data throughout the trial. All observed AEs and toxicities will be recorded in the eCRF and graded according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0) to summarize their relationship to all study treatments and procedures.
Table 2
| Procedure | Point in time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening inclusion | Treatment (36 cycles) | Post-treatment | |||||||||
| Day −14 to 0 | C1D1 | C1D21 | C2D1 | C2D21 | C(2n+1)D21 | C(2n)D21 | Withdrawal | C(n+4)D21 | |||
| Informed consent, eligibility criteria, demographics, medical and disease history | × | × | |||||||||
| Collection of biomaterials | × | ||||||||||
| EGFR, ALK, and ROS1 detection | × | ||||||||||
| CBC for TMB | × | × | |||||||||
| ADA analysis | × | × | × | ||||||||
| Physical examination | × | × | × | × | × | × | × | × | × | ||
| QoLQ | × | × | × | × | × | ||||||
| ECOG-PS | × | × | × | × | × | ||||||
| Pregnancy test | × | × | |||||||||
| Tumor imaging | × | × | × | × | × | ||||||
| 12-lead ECG | × | × | × | × | × | × | × | ||||
| Pulmonary function tests | × | × | × | × | × | ||||||
| CEA, SCC antigen, and Cyfra21-1 | × | × | × | × | × | ||||||
| CBC, serum chemistry panel, CK-MB, and cTn | × | × | × | × | × | × | × | ||||
| Thyroid and coagulation function test | × | × | × | × | × | × | |||||
| Assessment of AEs | × | × | × | × | × | × | × | × | × | ||
CnDn, cycle number and day number; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1; CBC, complete blood count; TMB, tumor mutation burden; ADA, anti-drug-antibody; QoLQ, Quality of Life Questionnaire; ECOG-PS, Eastern Cooperative Oncology Group performance status; ECG, electrocardiograph; CEA, carcinoembryonic antigen; SCC, squamous cell carcinoma; CK-MB, creatine kinase and its MB isoenzyme; cTn, cardiac troponin; AEs, adverse events.
Maintenance treatment after dCRT
All participants should complete the first medication on day 14–42 after the last radiotherapy.
- Patients in the experimental arm A (Group T + A) will be administered: TQB2450 1,200 mg, intravenously, once every 21 days; anlotinib 8 mg, orally, once daily for 14 days and 7 days off. Therefore, anlotinib will be administered regularly. If a dose is missed during the course of the medication and the time from the next medication is less than 12 hours, no additional replacement dose will be taken.
- Patients in the experimental arm B (Group T) will be administered: TQB2450 1,200 mg, intravenously, once every 21 days; and anlotinib placebo (8 mg), orally, once daily for 14 days and 7 days off.
- Patients in the placebo control arm (Group P) will be administered: TQB2450 placebo 1,200 mg, intravenously, once every 21 days; anlotinib placebo (8 mg), orally, once daily for 14 days and 7 days off.
One treatment cycle will take 21 days, and participants will receive no more than 36 cycles (108 weeks). Participants who have disease control [complete response (CR), partial response (PR), or stable disease (SD)] as assessed by the investigator and tolerable AEs may continue to participate in the R-ALPS study voluntarily until the clinical benefit is lost, or the adverse effects of treatment become intolerable. No other antitumor therapy will be administered during this trial.
Collection of biomaterials for translational research
An accompanying translational research project will investigate the mechanisms underlying potential tumor-specific immune effects that might be induced by the combination of PD-L1 and anti-angiogenic therapy and will explore potential biomarkers for such a treatment combination. To this end, the investigators will collect blood samples at baseline and at the time of PD. Tumor tissue samples will be collected at baseline and in cases of re-biopsy after PD during the study treatment, if possible. Although the collection of baseline tissue is mandatory, the collection of all other biomarker samples is voluntary, meaning that eligible patients can still be enrolled in this study if they do not consent to the collection of biomaterials.
Efficacy endpoints
The primary endpoint of the R-ALPS study is PFS assessed by the independent review committee (IRC) calculated as the time from randomization to PD or death from any cause, whichever occurs first. The secondary endpoints are OS, calculated from the date of randomization to death of any cause; ORR (proportion of patients who achieve CR or PR), DCR (proportion of patients who achieve CR, PR, or SD); duration of response (DOR), defined as the interval from the date of first recorded CR or PR to the date of the first recorded PD according to the RECIST v1.1 criteria or death of any cause, whichever occurs first; AEs, and 6-, 12-month PFS assessed by the investigator. In addition, the frequency, nature, severity, and causal relationship of AEs will be evaluated using the CTCAE v5.0 criteria to assess the safety of the treatment regimens. The planned biomarker analysis of biomaterials collected during the trial is an exploratory endpoint of this study.
Statistical analysis
The data management and statistical analysis of the R-ALPS study will be carried out by Department of Biostatistics, School of Public Health, Nanjing Medical University, using SAS v9.4 (SAS Inc., Cary, NC, USA). All statistical analyses will be carried out in line with International Conference on Harmonisation (ICH) Guidelines “Statistical Principles for Clinical Trials”, and “Structure and Content of Clinical Study Reports”.
Sample size calculation
The sample size calculations have been revised since the trial was registered owing to the requirements of the regulatory agencies. The original sample size calculations were based on a comparison of experimental arm B versus the placebo control arm. The sample size calculations were subsequently revised at the request of the regulatory agencies to enable a comparison of experimental arm A versus experimental arm B. Both the original and updated sample size calculations are detailed below.
Based on previously published results (1), the expected median PFS in the placebo control arm is 6 months, and the PFS in the experimental arm B is assumed to extended to 9 months compared with the placebo control arm. A difference between treatment groups regarding the primary endpoint, PFS, will be detectable with a power of 80% using a log-rank test at a two-sided α of 0.05, assuming a dropout rate of 10%, follow-up phase of 18 months, and an accrual period of 16 months. Based on these assumptions, the estimated total sample size yielding the necessary number of 200 events is 222 cases (111 per arm). To adjust for dropouts, the target sample size was set as 124 cases in each of the two arms.
The original protocol was to follow the ratio of 1:1:1 among the three groups, that is, a total of 372 cases in three arms (with 124 cases per arm). However, to meet the review requirements of regulatory agencies, an analysis of experimental arm A versus experimental arm B was added to ensure sufficient power in the study protocol v4.0. Assuming that the PFS in experimental arm A is extended to 12.5 months, the sample size ratio is 1:1, the power is 80%, the final sample size of the two arms (experimental arms A and B) is 410 cases. That is, based on the original 124 cases in each group, and additional 81 cases will be needed in each experimental group. In summary, the revised sample size of this study is 534 cases, with 205 cases in each of the experimental arms A and B and 124 cases in the placebo control arm.
Interim analysis
An independent data monitoring committee (IDMC) consisting of two independent oncologists and one statistician has been established to conduct interim data analyses and assess patient risks and benefits. A safety and effectiveness analysis will be performed by IDMC during this trial. The effectiveness analysis will focus primarily on PFS and will be triggered when 70% (286 cases) of the total PFS events have occurred. Based on the results of the interim analysis, the IDMC will provide a recommendation to the CRO or its delegates, who will be responsible for formally deciding whether the study should be terminated or continued.
Methods of statistical analysis
All statistical analyses will be prespecified and will follow the intention-to-treat (ITT) principle. The primary endpoints will be analyzed using the Kaplan-Meier method. The two-sided significance level is set to α =0.05. Descriptive analyses will be performed of secondary endpoints. Chi-squared tests and t-tests will be used for intergroup comparisons of ordinal or dichotomous variables and continuous variables, respectively. Cox proportional hazards regression will be used to estimate the hazard ratios of factors associated with survival outcomes. Safety analysis will be performed for all participants who have received at least one dose of the study medication, including a description of relative and absolute incidence of AEs and severity grade based on the CTCAE v5.0 criteria. The AE summary tables will provide the number and percentage of participants with AEs and the 95% Clopper-Pearson type confidence intervals for the event rates.
Trial status
Considering that durvalumab has not been covered by health insurance since it was launched in China in December 2019 and requires a high co-payment, its accessibility is currently far from meeting clinical needs. All eligible patients had declined treatment with durvalumab prior to being recruited and providing informed consent to participate in this study. The first patient was enrolled on May 26, 2020. Recruitment of the placebo control arm was completed in September 2022, and recruitment of the other two arms was completed on May 12, 2023.
Discussion
Despite recent advances in the treatment of stage III NSCLC, survival rates need to be improved. Combination therapy, including ICIs and anti-angiogenic agents, is expected to improve the prognosis. A growing number of preclinical studies have demonstrated that PD-L1 and anlotinib complement each other and play synergistic roles in antitumor therapy. Anlotinib, a multitargeted anti-angiogenic agent can normalize tumor blood vessels and improve the immune microenvironment of tumors (28). Furthermore, decreased expression of PD-L1 by endothelial cells leads to an increase in VEGFR2 expression, suggesting a potential regulatory role for PD-L1 in tumor angiogenesis (29-31). However, the clinical potency of the combination of anti-angiogenic therapy and PD-L1 in stage III NSCLC is still unclear.
This phase III trial is designed to examine the efficacy and safety of TQB2450 with or without anlotinib maintenance therapy in patients with stage III NSCLC who have not progressed after prior dCRT. The development of the R-ALPS study will provide new insight into the interaction between immunotherapy and anti-angiogenic therapy, thereby expanding the treatment options for patients with locally advanced or unresectable NSCLC. Participant recruitment for the trial was completed in May 2023, and an interim analysis will be conducted in the future.
Acknowledgments
We thank the patients who participated in this study, their families, and all personnel at each study site who cared for the patients and coordinated with the sponsor to make this trial possible.
Funding: This study was sponsored by
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-362/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-362/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-362/coif). X.W. and Q.C. report being employed by Chia Tai Tianqing Pharmaceutical Group Co., Ltd., and participating in the design and administrative support of this study. However, they do not involve in collection, assembly of data, as well as subsequent data analysis and interpretation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol will be established according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013). The Institutional Review Boards (IRBs) of each center have approved the trial, including Sun Yat-sen University Cancer Center (No. A2021-080-01), Zhejiang Cancer Hospital {No. IRB-[2020]62}, Guangxi Medical University Cancer Hospital {No. CS2020[11]}, Tianjin Medical University Cancer Institute & Hospital (No. E2020090), The Second People’s Hospital of Neijiang (No. 2020-024-001), Henan Cancer Hospital (No. 2020022609), Qilu Hospital of Shandong University (No. 2020011), Xiangya Hospital Central South University (No. 202004075), Shandong Cancer Hospital & Institute (No. SDZLEC2021-041-01), and Shanxi Cancer Hospital (No. YW2020034). The complete list of the IRB names of 42 sites participating in the study and their registration numbers can be found at Table S1. Written informed consent will be obtained from all patients or guardian participants before being enrolled in the clinical trial.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Ahn JS, Ahn YC, Kim JH, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol 2015;33:2660-6. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Ren S, Xiong X, You H, et al. The Combination of Immune Checkpoint Blockade and Angiogenesis Inhibitors in the Treatment of Advanced Non-Small Cell Lung Cancer. Front Immunol 2021;12:689132. [Crossref] [PubMed]
- Han Y, Wang J, Sun T, et al. Predictive biomarkers of response and survival following immunotherapy with a PD-L1 inhibitor benmelstobart (TQB2450) and antiangiogenic therapy with a VEGFR inhibitor anlotinib for pretreated advanced triple negative breast cancer. Signal Transduct Target Ther 2023;8:429. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]
- Tolaney SM, Boucher Y, Duda DG, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci U S A 2015;112:14325-30. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77-86. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond) 2019;39:36. [Crossref] [PubMed]
- Liang L, Hui K, Hu C, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res 2019;38:71. [Crossref] [PubMed]
- Chinese Association for Clinical Oncologists. Cancer Targeted Therapy Professional Committee of China Anti-Cancer Association. Chinese expert consensus on Anlotinib Hydrochloride for advanced lung cancer (2020 edition). Zhonghua Zhong Liu Za Zhi 2020;42:807-16. [Crossref] [PubMed]
- Zhu Q, Ni R, Guan X. Cost-effectiveness analysis of anlotinib as a third-line or further treatment for advanced non-small cell lung cancer in China. Transl Lung Cancer Res 2023;12:1782-9. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Zhang W, Wang J, Wang Q, et al. A randomized double-blind trial of TQB2450 with or without anlotinib in pretreated driver-negative non-small cell lung cancer. Lung Cancer 2023;184:107353. [Crossref] [PubMed]
- Lan CY, Zhao J, Yang F, et al. Anlotinib combined with TQB2450 in patients with platinum-resistant or -refractory ovarian cancer: A multi-center, single-arm, phase 1b trial. Cell Rep Med 2022;3:100689. [Crossref] [PubMed]
- Zhou J, Sun Y, Zhang W, et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology 2023;77:65-76. [Crossref] [PubMed]
- Liu J, Gao T, Tan Z, et al. Phase II Study of TQB2450, a Novel PD-L1 Antibody, in Combination with Anlotinib in Patients with Locally Advanced or Metastatic Soft Tissue Sarcoma. Clin Cancer Res 2022;28:3473-9. [Crossref] [PubMed]
- Du Y, Dai J, Mao L, et al. Phase Ib study of anlotinib in combination with anti-PD-L1 antibody (TQB2450) in patients with advanced acral melanoma. J Eur Acad Dermatol Venereol 2024;38:93-101. [Crossref] [PubMed]
- Cheng Y, Chen J, Zhang W, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med 2024;30:2967-76. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Zhang C, Chen J, Wu H, et al. Efficacy and safety of anlotinib plus penpulimab as second-line treatment for small cell lung cancer: A multicenter, open-label, single-arm phase II trial. Cancer Pathogenesis and Therapy 2024;2:268-75. [Crossref] [PubMed]
- Jiang W, Huang Y, An Y, et al. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano 2015;9:8689-96. [Crossref] [PubMed]
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185-204. [Crossref] [PubMed]
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679. [Crossref] [PubMed]


