Management of elderly patients
Introduction
Oncogeriatric medicine has now come of age. It involves a comprehensive, multidimensional and multidisciplinary approach to the elderly cancer patient (1). Life expectancy is increasing in all western countries, and projections show that, in France in 2020, more than 10% of inhabitants will be over 70 years old (2). However, elderly individuals are very heterogeneous, and their management must take into account both medical and social problems and specific cancer therapy (3). Elderly patients are generally excluded from clinical trials, however, representing only 8-13% of patients (4). Medical evaluation of elderly cancer patients is complicated not only by their age but also by comorbidities (5), which are independent prognostic factors.
In the United States, cancer registries show that patients over 65 years of age represent two-thirds of all lung cancer patients, and median age at diagnosis is around 70 years (6). A French observational study (7) showed that, in 2000, 32% of patients treated for lung cancer were over 70 years old, and that 18.1% were over 80.
Yet clinical trials specifically focusing on elderly patients are rare in the field of thoracic oncology, even though their value is now clear (8). Lung cancer management guidelines now include specific recommendations on the treatment of elderly patients (9,10). The international society of geriatric oncology has also issued similar guidelines (11).
This article examines the specific assessment of elderly cancer patients, the use of certain tools for lung cancer treatment, and likely future developments.
Specificities of lung cancer management in seniors
The selection criteria are the same whether the elderly patient is a candidate for surgery or radiotherapy, and whether the lung cancer is locally advanced or metastatic.
Aging is accompanied by a number of physiological changes, including a decreased glomerular filtration rate, impaired hepatic metabolism, decreased serum albumin, and a decreased absorption-distribution ratio (3). Elderly patients often have comorbidities: Yancik’s study (12) showed that 13% of patients aged between 55 and 65 years had more than 5 comorbidities, a figure rising to 24% between 66 and 74 years and 40% after 75 years. As stressed by Extermann (13), performance status, a prognostic factor in lung cancer, does not have the same impact on patient management as comorbidities, or on tolerance of either the disease or its treatment. Validated tools are available for assessing such comorbidities, such as the Charlson index (14) and the cumulative illness rating scale - geriatric (15). However, comorbidities, performance status are independent from age in the disease prognosis (14,15).
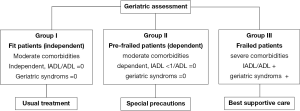
It is crucial to assess the impact of aging by using geriatric indexes (16,17). These multidimensional tools explore cognitive functions (18), depression (19), and other geriatric disorders (20) such as falls and incontinence, nutritional status, polypharmacy, mobility and environmental conditions. These disorders are combined in the standardized comprehensive geriatric assessment (CGA) proposed by Balducci (21-23). However, as the CGA was particularly time-consuming, a short questionnaire was developed and validated (24,25). This work allowed us to classify the elderly into three groups, as shown in Figure 1.
Recent studies have shown that the use of these indices influences the choice of initial care by multidisciplinary panels in about 1 in 5 cases (26-28).
Quality of life, which is widely assessed in lung cancer patients regardless of age, is particularly important in the elderly. Whatever the tool used, clinical trials must include QOL assessments to ensure that treatment does not have a major negative impact (29).
Management of early-stage lung cancer
Age itself does not contraindicate surgery (30), but elderly patients are less likely to be referred to a surgeon (31). There is a positive correlation between the survival rate and the use of limited surgery or video-assisted thoracoscopic surgery (32).
Management of patients with locally advanced lung cancer
There are currently no published trials of concurrent chemoradiotherapy in elderly patients, but trials not specifically devoted to seniors suggested that toxicity was greater in older patients (33). An ongoing French trial is studying the feasibility of using geriatric assessment for patient and treatment selection (34).
Management of patients with metastatic lung cancer
These are the patients who raise the most difficult issues. Standard treatment has consisted essentially of monotherapy, as trials conducted in the 2000s failed to show any improvement in survival with doublets. In 2010, however, Quoix et al. (35) showed the superiority of a weekly carboplatin-paclitaxel combination over gemcitabine or vinorelbine monotherapy, albeit at a cost of more severe hematological toxicity.
Full Table
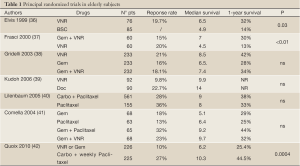
Table 1 summarizes the main phase III trials of single-agent and combination therapy in elderly patients.
It is important, in addition to traditional outcomes, to assess quality of life and particularly the impact of toxicities (29).
The choice between monotherapy and doublet therapy is still controversial, although the trial conducted by Quoix et al. (35) clearly marked a turning point. Des Guetz et al. (43) recently published a meta-analysis comparing the efficacy and safety of monotherapy versus doublet therapy in patients with metastatic lung cancer. This meta-analysis included 10 studies and 2,605 patients with an average age of 74 years. Overall survival at one year was not improved by the use of doublets versus monotherapy (HR 0.92, CI: 0.82-1.03, P=0.016). In contrast, the response rate was significantly better with doublet therapy (HR 1.51, 1.22-1.86, P>0.001). Gastrointestinal toxicity was similar in the two populations, but neutropenia, thrombocytopenia and anemia were more problematic with doublet therapy. Among grade III/IV adverse effects, thrombocytopenia and anemia were more frequent with doublet therapy. The authors concluded that there was little additional benefit to the use of doublets versus monotherapy in these patients. Further studies are required to confirm these results (35). In addition, as the authors pointed out, these findings are applicable to independent older patients and cannot be extrapolated to frailed patients, for whom the best treatment strategy remains to be defined.
In September 2012, ESMO (44) published its new guidelines favoring platinum-based doublets for elderly patients with PS =0-1 and for some selected patients with PS =2, while monotherapy should be offered to vulnerable patients and those with multiple comorbidities, owing to the higher risk of adverse effects. The “vulnerable” elderly patient was not defined.
While most of the studies presented in Table 1 selected patients on the basis of standard criteria (age and performance status) (36-42), other teams attempted to define their geriatric patient population more precisely, based on a combination of age, performance status and a comorbidity index (Charlson score). Two open-label phase II (45,46) trials involved two distinct populations: patients who were considered to be in good general condition with few comorbidities were treated with docetaxel and gemcitabine, while the most fragile patients were treated with docetaxel alone. Both trials were designed to assess the feasibility of the rating tools. Effectiveness was moderate in the monotherapy group, while patients treated with the combination had results similar to those observed in younger patients.
Two randomized phase II trials (47,48) were secondary published with the same selection and a targeted therapy with erlotinib into the treatment strategy. The docetaxel-gemcitabine combination followed by erlotinib gave the best results. Patients were selected on the basis of age, PS, the Charlson score, the number of comorbidities, and geriatric symptoms (falls, incontinence and dependency for ADL and IADL). The results were modest in the fragile patients treated with monotherapy (gemcitabine followed by erlotinib, or vice versa).
These latter two studies showed that geriatric assessment was feasible in clinical trials. Early use of geriatric criteria led to better-defined groups and favored the selection of patients for combination therapy or monotherapy.
Although quality of life was preserved in some clinical trials, such as that conducted by Quoix et al., the risk-benefit assessment must take adverse effects into account (49).
Gradually, targeted therapies have started to be used in these patients. Numerous studies (50-53) have shown that, in Asian patients with activating EGFR mutations, EGFR-TKI significantly improved progression-free survival after frontline treatment, compared to platinum-based chemotherapy. These results were found with gefitinib in an Asian population [HR: 0.36 (0.25-0.51) (52); HR: 0.16 (0.10-0.26) (54)], and with erlotinib in a Caucasian population, HR: 0.37 (0.25 to 0.54) (53).
Following these results, gefitinib and erlotinib obtained marketing authorization for first-line treatment of advanced NSCLC in patients with activating EGFR mutations, even though these studies included very few elderly patients. The age limit for inclusion was 75 years in the studies by Maemondo et al. (52) and Zhou et al. (54), and median age was 65 years in the study by Rosell et al. (53). These activating mutations were a powerful predictor of intense and rapid responses [ORR 58% (53) to 73.7% (52)] to EGFR TKI, a drug with a favorable safety profile. Most elderly EGFR-mutated patients with symptoms or altered general condition (due mainly due to cancer extension) derive a major benefit. Inoue et al. (55) showed that some patients with activating EGFR mutations who were considered ineligible for chemotherapy because of poor PS (3 or 4) could regain a PS of 0 or 1, and that some even became eligible for second-line chemotherapy on disease progression.
There are no specific trials of angiogenesis inhibitors in elderly lung cancer patients.
In the ECOG 4599 trial (56), comparing carboplatin-paclitaxel to carboplatin-paclitaxel-bevacizumab. Bevacizumab did not improve survival in the subgroup of patients aged 70 years or more (median 74 years), although there was a trend towards a better response rate and longer progression-free survival in the bevacizumab group. Toxicity, and especially hematologic adverse effects, was higher in the bevacizumab arm. In the AVAIL study (57) of cisplatin-gemcitabine with or without bevacizumab, progression-free survival was significantly better with bevacizumab and was similar in the older and younger subgroups, without specific toxicity in the older group; however, the median age of patients over 65 was only 68 years. In the ARIES prospective cohort study (58) evaluating the use of bevacizumab in combination with first-line chemotherapy, PFS was respectively 6.6 and 6.7 months in patients <70 years (n=1,320) and ≥70 years (n=647), and overall survival was respectively 14.2 and 12.2 months, i.e. largely inferior in patients ≥70 years. There was no excess toxicity in these latter patients.
The role of bevacizumab in combination with platinum-based chemotherapy in patients ≥70 years of age needs to be determined in a phase III trial specifically dedicated to these patients.
Future developments
While clinical practice guidelines favored the use of monotherapy in elderly lung cancer patients, recent studies supported the use of doublets in selected patients.
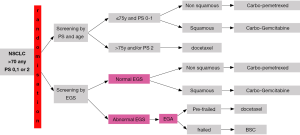
A phase III trial is now needed to validate the use of a geriatric index as a criterion for patient selection. Enrolment in the Esogia trial (Figure 2) is now complete and the results should be available in 2013. If the results are positive, the short geriatric assessment could become a standard selection tool for the elderly population. The use of a complete or an abbreviated form might facilitate its application (59).
Elderly lung cancer patients cannot be selected on the basis of clinical criteria alone: biological factors must also be taken into account. Rosell et al. (60) have shown that the prevalence of EGFR mutations is higher (41%) among patients over 70, supporting the use of EGFR inhibitors.
A recent report of the BATTLE trial (61) showed similar results in seniors and younger patients in an open trial in which treatment selection was based on a biomarker profile (EGFR, K-RAS, B-RAF, cyclin D1, VEGF receptor, and retinoid x receptor).
The future clearly lies in a combination of all these factors. Given the favorable harm-benefit ratio of targeted therapies (EGFR TKI and ALK inhibitors), these drugs might be used as first-line treatments for patients whose tumors bear the molecular target, including patients whose general condition is degraded by the disease. It is possible that, as new therapeutic targets and more effective and well-tolerated drugs are developed, the scope of geriatric assessment may change. Oncogeriatric tools will need to be adapted to these new treatments, including optimal use of biological markers and selection of eligible subpopulations on the basis of clinical criteria, including a geriatric assessment.
Acknowledgements
A. Vergnenegre has received honoraria from Roche, Amgen, Lilly and has received funding for clinical research from Astra-Zeneca, Chugaï, Lilly, Amgen, Roche and Boehringer-Ingelheim; R. Corre has funding for clinical research from Lilly, Roche, Chugai and Sanofi Aventis; H Léna has received honoraria from Lilly for board activity and from Astra Zeneca for speaker activity; H Le Caer has received honoraria from Roche and Lilly.
Disclosure: The authors declare no conflict of interest.
References
- Zulian G, Terret C, Droz JP. Interdependance of oncologist and geriatrician. Médecine et hygiène 2003;61:1098-106.
- Brutel C. Projections de population à l’horizon 2050 - un vieillissement inéluctable. Insee Première 2001:762:1 vol. Available online: http://www.insee.fr/fr/ffc/docs_ffc/ip762.pdf
- Lichtman SM, Balducci L, Aapro M. Geriatric oncology: a field coming of age. J Clin Oncol 2007;25:1821-3. [PubMed]
- Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004;22:4626-31. [PubMed]
- Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54-9. [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [PubMed]
- Piquet J, Blanchon F, Grivaux M, et al. Primary bronchial carcinoma in elderly subjects in France. Rev Mal Respir 2003;20:691-9. [PubMed]
- Jatoi A, Hillman S, Stella P, et al. Should elderly non-small-cell lung cancer patients be offered elderly-specific trials? Results of a pooled analysis from the North Central Cancer Treatment Group. J Clin Oncol 2005;23:9113-9. [PubMed]
- Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [PubMed]
- Felip E, Gridelli C, Baas P, et al. Metastatic non-small-cell lung cancer: consensus on pathology and molecular tests, first-line, second-line, and third-line therapy: 1st ESMO Consensus Conference in Lung Cancer; Lugano 2010. Ann Oncol 2011;22:1507-19. [PubMed]
- Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients--an analysis of the medical literature. J Clin Oncol 2007;25:1832-43. [PubMed]
- Yancik R, Ganz PA, Varricchio CG, et al. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol 2001;19:1147-51. [PubMed]
- Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 1998;16:1582-7. [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [PubMed]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622-6. [PubMed]
- Repetto L, Venturino A, Fratino L, et al. Geriatric oncology: a clinical approach to the older patient with cancer. Eur J Cancer 2003;39:870-80. [PubMed]
- Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: a critical issue in geriatric oncology. Crit Rev Oncol Hematol 2003;46:127-37. [PubMed]
- Lichtman SM. Guidelines for the treatment of elderly cancer patients. Cancer Control 2003;10:445-53. [PubMed]
- Roose SP, Katz IR, Pollock BG, et al. Contemporary issues in the diagnosis and treatment of late-life depression. J Am Med Dir Assoc 2002;3:H26-9. [PubMed]
- Hardy C, Wallace C, Khansur T, et al. Nutrition, cancer, and aging: an annotated review. II. Cancer cachexia and aging. J Am Geriatr Soc 1986;34:219-28. [PubMed]
- Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000;5:224-37. [PubMed]
- Balducci L. Geriatric oncology. Crit Rev Oncol Hematol 2003;46:211-20. [PubMed]
- Balducci L. Management of cancer in the elderly. Oncology (Williston Park) 2006;20:135-43; discussion 144, 146, 151-2.
- Overcash JA, Beckstead J, Extermann M, et al. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol 2005;54:129-36. [PubMed]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. [PubMed]
- Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol 2011;29:3636-42. [PubMed]
- Aliamus V, Adam C, Druet-Cabanac M, et al. Geriatric assessment contribution to treatment decision-making in thoracic oncology. Rev Mal Respir 2011;28:1124-30. [PubMed]
- Yonnet S, Gazaille V, Grasset-Dupuy M, et al. Age and management decisions in patients with primary lung cancer. Rev Mal Respir 2008;25:295-302. [PubMed]
- LeCaer H, Delhoume JY, Thomas PA, et al. Multicenter phase II trial of carboplatin/vinorelbine in elderly patients with advanced non-small-cell lung cancer efficacy and impact on quality of life: Groupe Francais de Pneumo-Cancerologie Study 9902. Clin Lung Cancer 2005;7:114-20. [PubMed]
- Riquet M, Medioni J, Manac’h D, et al. Non-small cell lung cancer: surgical trends as a function of age. Rev Mal Respir 2001;18:173-84. [PubMed]
- Riquet M, Le Pimpec Barthes F. Chirurgie thoracique du sujet âgé. ed. In: Morère JF, Rainfray M. eds. Cancer du sujet âgé. Paris: Springer Verlag France, 2002;1:33-45.
- Jaklitsch MT, Mery CM, Audisio RA. The use of surgery to treat lung cancer in elderly patients. Lancet Oncol 2003;4:463-71. [PubMed]
- Langer C, Hsu C, Curran W, et al. Elderly patients (pts) with locally advanced non-small cell lung cancer (LA-NSCLC) benefit from combined modality therapy: secondary analysis of Radiation Therapy Oncology Group (RTOG) 94-10. Proc Am Soc Clin Oncol 2002;21:abstr 1193.
- Locher C, Pourel N, Marin B, et al. A phase II study of weekly cisplatine plus oral vinorelbine with concomittant radiotherapy in non-dependent elderly patients with localized inoperable non small cell lung carcinoma (Essai GFPC 08-06, Raccosa). Rev Mal Respir 2011;28:58-65. [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [PubMed]
- Des Guetz G, Uzzan B, Nicolas P, et al. Comparison of the efficacy and safety of single-agent and doublet chemotherapy in advanced non-small cell lung cancer in the elderly: a meta-analysis. Crit Rev Oncol Hematol 2012;84:340-9. [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii56-64. [PubMed]
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 1999;91:66-72. [PubMed]
- Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2000;18:2529-36. [PubMed]
- Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003;95:362-72. [PubMed]
- Kudoh S, Takeda K, Nakagawa K, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 2006;24:3657-63. [PubMed]
- Lilenbaum RC, Herndon JE, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190-6. [PubMed]
- Comella P, Frasci G, Carnicelli P, et al. Gemcitabine with either paclitaxel or vinorelbine vs paclitaxel or gemcitabine alone for elderly or unfit advanced non-small-cell lung cancer patients. Br J Cancer 2004;91:489-97. [PubMed]
- Quoix E, Monnet I, Scheid P, et al. Management and outcome of French elderly patients with lung cancer: an IFCT survey. Rev Mal Respir 2010;27:421-30. [PubMed]
- LeCaer H, Barlesi F, Robinet G, et al. An open multicenter phase II trial of weekly docetaxel for advanced-stage non-small-cell lung cancer in elderly patients with significant comorbidity and/or poor performance status: The GFPC 02-02b study. Lung Cancer 2007;57:72-8. [PubMed]
- LeCaer H, Fournel P, Jullian H, et al. An open multicenter phase II trial of docetaxel-gemcitabine in Charlson score and performance status (PS) selected elderly patients with stage IIIB pleura/IV non-small-cell lung cancer (NSCLC): the GFPC 02-02a study. Crit Rev Oncol Hematol 2007;64:73-81. [PubMed]
- LeCaer H, Barlesi F, Corre R, et al. A multicentre phase II randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs the reverse sequence, in elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0504 study). Br J Cancer 2011;105:1123-30. [PubMed]
- LeCaer H, Greillier L, Corre R, et al. A multicenter phase II randomized trial of gemcitabine followed by erlotinib at progression, versus the reverse sequence, in vulnerable elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0505 study). Lung Cancer 2012;77:97-103. [PubMed]
- Janssen-Heijnen ML, Maas HA, Siesling S, et al. Treatment and survival of patients with small-cell lung cancer: small steps forward, but not for patients >80. Ann Oncol 2012;23:954-60. [PubMed]
- Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350-7. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [PubMed]
- Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008;26:60-5. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [PubMed]
- Wozniak A, Garst J, Jahanzeb M, et al. Clinical outcomes (CO) for special populations of patients (pts) with advanced non-small cell lung cancer (NSCLC): Results from ARIES, a bevacizumab (BV) observational cohort study (OCS). J Clin Oncol 2012;28:abstr 7618.
- Provencio M, Camps C, Alberola V, et al. Lung cancer and treatment in elderly patients: the Achilles Study. Lung Cancer 2009;66:103-6. [PubMed]
- Rosell R, Santarpia M, Moran T, et al. Age-related genetic abnormalities: The Achilles Heel for customizing therapy in elderly lung cancer patients. Personal Med 2007;4:59-72.
- Tsao AS, Liu S, Lee JJ, et al. Clinical outcomes and biomarker profiles of elderly pretreated NSCLC patients from the BATTLE trial. J Thorac Oncol 2012;7:1645-52. [PubMed]