
Adding immune checkpoint inhibitors to chemotherapy confers modest survival benefit in patients with small cell lung cancer and brain metastases: a retrospective analysis
Highlight box
Key findings
• Adding immune checkpoint inhibitors to chemotherapy confers modest survival benefits in patients with small cell lung cancer (SCLC) and brain metastases (BM).
What is known and what is new?
• Chemoimmunotherapy is a primary treatment for SCLC. Nevertheless, its efficacy in patients with BM remains a topic of debate.
• Our study presented real-world evidence regarding the efficacy of chemoimmunotherapy in patients with SCLC and BM. Furthermore, we compared the intracranial and extracranial efficacy differences observed in patients with BM.
What is the implication, and what should change now?
• BM are not immune-privileged sites where chemoimmunotherapy fails to exert its therapeutic effect. Nonetheless, the efficacy of chemoimmunotherapy in patients with SCLC and BM remains suboptimal, necessitating further exploration and development of additional therapeutic modalities.
Introduction
Lung cancer is a prevalent malignancy worldwide, with small cell lung cancer (SCLC) representing the most aggressive subtype and accounting for approximately 14% of all cases (1). Due to the highly aggressive nature of SCLC, distant metastases are commonly observed upon diagnosis. Notably, the brain serves as a frequent site for metastatic spread in patients with SCLC (2). Studies have reported a high incidence rate of brain metastases (BM), ranging from 40% to 50%, in individuals with SCLC during diagnosis and treatment (3,4). Furthermore, patients with SCLC and BM exhibit significantly poorer prognosis, characterized by a dismal 2-year survival rate below 2% and a median overall survival (OS) time limited to merely 6 months (5-7).
In the era of immunotherapy, immune checkpoint inhibitors (ICIs) have emerged as a crucial component in the treatment of extensive-stage SCLC (ES-SCLC) (8,9). The addition of ICIs to chemotherapy has been demonstrated to significantly prolong median OS by 2 to 4 months in patients with ES-SCLC (10). As such, the combination of chemotherapy and ICIs has become the standard of care for patients with this disease (11). However, the role of ICIs in patients with SCLC and BM remains controversial. In the IMpower133 (12) trials, the addition of ICIs to SCLC first-line treatment resulted in a median OS extension of approximately 2 months, but did not benefit patients with BM [hazard ratio (HR) =1.07; 95% confidence interval (CI): 0.47–2.43]. On the contrary, the CASPIAN study (13) demonstrated for the first time that adding ICIs to chemotherapy could be beneficial for patients with SCLC and BM. Specifically, combining durvalumab and chemotherapy prolonged both median OS (HR =0.79; 95% CI: 0.44–1.41) and median progression-free survival (PFS) (HR =0.73; 95% CI: 0.42–1.29) compared to chemotherapy alone in these patients. Similarly, in the ASTRUM-005 trial (14), ICIs also improved outcomes for patients with SCLC and BM by prolonging their median OS compared to those receiving chemotherapy alone (HR =0.61; 95% CI: 0.33–1.13). Of note, the numbers of patients with baseline BM in the several pivotal studies were small, and adding ICIs showed only a trend toward improving survival without reaching statistical significance. In addition, several studies (CAPSTONE-1, RATIONALE-312 and EXTENTORCH) included too few patients with BM to perform efficacy analysis (15-17). Therefore, the available evidence regarding the efficacy of ICIs in patients with SCLC and BM is currently insufficient, necessitating further exploration.
Therefore, this retrospective study aims to enroll patients with SCLC and BM at diagnosis and compare the treatment efficacy between chemotherapy combined with ICIs and chemotherapy alone. The objective is to evaluate whether the addition of ICIs confers survival benefits for patients with SCLC and BM. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-335/rc).
Methods
Study design
This retrospective study enrolled patients with histologically confirmed SCLC at Sun Yat-sen University Cancer Center from January 2018 to December 2022, with their BM being verified through magnetic resonance imaging at baseline. The clinical characteristics of patients were extracted from medical records. Based on whether the first-line treatment regimen included ICIs, the patients were stratified into two groups: the chemoimmunotherapy group and the chemotherapy group. In both groups, patients received the same chemotherapy regimen consisting of etoposide in combination with platinum. Within the chemoimmunotherapy group, the ICIs utilized included durvalumab (n=39), atezolizumab (n=24), pembrolizumab (n=2), sintilimab (n=5), tislelizumab (n=3), camrelizumab (n=3), serplulimab (n=3), and toripalimab (n=1).
This study was reviewed and approved by the ethics and research committee of Sun Yat-sen University Cancer Center (approval number: B2023-489-01), and it was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients’ individual consent for this retrospective analysis was waived.
Efficacy assessments
Extracranial lesions (lesions in the lungs and liver) were evaluated using enhanced computed tomography scans, while intracranial lesions were evaluated using enhanced magnetic resonance imaging at baseline and after every second treatment cycle (6–8 weeks). Treatment efficacy assessments were conducted by experienced oncologists. According to the revised Response Evaluation Criteria in Solid Tumors version 1.1 guidelines, the number of target lesions for intracranial disease has been expanded to up to 5, and the number of target lesions for extracranial disease has also been expanded to up to 5.
Endpoint
The primary endpoint of this study was OS, defined as the duration from treatment initiation to death due to any cause. The secondary endpoints were PFS for intracranial tumor lesions and PFS for extracranial tumor lesions. PFS for intracranial tumor lesions was defined as the period from the first treatment for intracranial tumor lesions until the occurrence of lesion progression, whereas PFS for extracranial tumor lesions referred to the period between initiation of systemic treatment and onset of lesion progression.
Statistical analysis
Descriptive statistics were used to summarize all variables in the study. Categorical variables were analyzed using appropriate statistical tests, such as the chi-square test or Fisher’s exact test. Survival data was estimated using the Kaplan-Meier curve and compared using the log-rank test. Cox proportional hazards regression models were utilized to assess the risk of disease progression and death, with HRs and 95% CIs calculated. Univariate and multivariate Cox proportional hazards regression analyses were conducted to evaluate the prognostic value of each factor for OS. Variables with P<0.20 in the univariable Cox regression were included in the multivariable Cox regression. The survival package in R software (version 4.0) was used for fitting survival regression, while visualization of results was achieved through the surviviner package and ggplot2 package. Statistical analyses were performed using SPSS (version 25). A two-sided P value <0.05 was considered statistically significant. The last follow-up date was December 15, 2023.
Results
Baseline characteristics
A total of 165 patients diagnosed with SCLC and BM were enrolled in this study. The median age of patients was 62 years. Most of the patients were male and had a history of smoking. Depending on whether the first-line treatment regimen included ICIs, patients were divided into the chemotherapy group and the chemoimmunotherapy group. The baseline clinical characteristics between the two treatment groups were well-matched, and the specific baseline characteristics are summarized in Table 1.
Table 1
| Characteristics | Patients | P value | ||
|---|---|---|---|---|
| Total (n=165) | Chemotherapy (n=85) | Chemoimmunotherapy (n=80) | ||
| Age (years), median | 62 | 61 | 63 | 0.63 |
| <60 | 65 (39.4) | 35 (41.2) | 30 (37.5) | |
| ≥60 | 100 (60.6) | 50 (58.8) | 50 (62.5) | |
| Sex | 0.15 | |||
| Female | 11 (6.7) | 8 (9.4) | 3 (3.8) | |
| Male | 154 (93.3) | 77 (90.6) | 77 (96.3) | |
| Smoking history | >0.99 | |||
| Non-smoker | 33 (20.0) | 17 (20.0) | 16 (20.0) | |
| Smoker | 132 (80.0) | 68 (80.0) | 64 (80.0) | |
| ECOG PS | 0.64 | |||
| 0–1 | 65 (39.4) | 32 (37.6) | 33 (41.3) | |
| ≥2 | 100 (60.6) | 53 (62.4) | 47 (58.8) | |
| Lung metastases | 0.43 | |||
| No | 102 (61.8) | 55 (64.7) | 47 (58.8) | |
| Yes | 63 (38.2) | 30 (35.3) | 33 (41.3) | |
| Liver metastases | 0.70 | |||
| No | 130 (78.8) | 68 (80.0) | 62 (77.5) | |
| Yes | 35 (21.2) | 17 (20.0) | 18 (22.5) | |
| Bone metastases | 0.95 | |||
| No | 111 (67.3) | 57 (67.1) | 54 (67.5) | |
| Yes | 54 (32.7) | 28 (32.9) | 26 (32.5) | |
| Intracranial tumor† | 0.79 | |||
| 1–3 | 89 (53.9) | 45 (52.9) | 44 (55.0) | |
| ≥4 | 76 (46.1) | 40 (47.1) | 36 (45.0) | |
| Intracranial tumor size‡ (mm) | 0.56 | |||
| <20 | 74 (44.8) | 40 (47.1) | 34 (42.5) | |
| ≥20 | 91 (55.2) | 45 (52.9) | 46 (57.5) | |
| Symptoms of brain metastases | 0.26 | |||
| Asymptomatic | 90 (54.5) | 50 (58.8) | 40 (50.0) | |
| Symptomatic | 75 (45.5) | 35 (41.2) | 40 (50.0) | |
| Local treatment for brain metastases‡ | 0.46 | |||
| No | 75 (45.5) | 41 (48.2) | 34 (42.5) | |
| Yes | 90 (54.5) | 44 (51.8) | 46 (57.5) | |
Data are presented as n (%). †, intracranial tumor characteristics were evaluated according to baseline-enhanced brain magnetic resonance imaging; ‡, local treatment for brain metastases includes surgery (n=4), SRS (n=23), and WBRT (n=63). In the chemotherapy group, 2 patients received surgery, 13 received SRS, and 29 received WBRT for their brain metastases. In the chemoimmunotherapy group, 2 received surgery, 10 received SRS, and 34 received WBRT for their brain metastases. SCLC, small cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Survival outcomes
During a median follow-up period of 34.7 months, 75 out of 85 patients in the chemotherapy cohort died, whereas 62 out of 80 patients in the chemoimmunotherapy cohort died. For intracranial lesions, there was a trend toward prolonged median PFS in the chemoimmunotherapy group than in the chemotherapy group (6.6 vs. 5.9 months, HR =0.77; P=0.14) (Figure 1A). Similarly, for extracranial lesions, there was a trend towards an extended median PFS in the chemoimmunotherapy group relative to the chemotherapy group (6.9 vs. 6.5 months, HR =0.73; P=0.12) (Figure 1B). Furthermore, no significant difference in median OS was observed between the cheotherapy and chemoimmunotherapy groups (15.6 vs. 14.5 months, HR =0.98; P=0.93) (Figure 1C). Within the chemotherapy cohort, 26 patients underwent subsequent treatment with ICI. After excluding these 26 patients, the chemoimmunotherapy group demonstrated a trend of prolonged OS compared to the chemotherapy group (15.6 vs. 11.6 months, HR =0.79; P=0.21) (Figure 1D). Additionally, subgroup analysis based on clinical characteristics indicated that the addition of ICIs to the first-line treatment regimen resulted in a favorable trend of OS benefit across the majority of subgroups (Figure S1).

Response
When it comes to the intracranial response of patients, among the 85 patients in the chemotherapy group, the objective response rate (ORR) was 68.2% (58/85), while the disease control rate (DCR) was 89.4% (76/85). Furthermore, 8 patients (9.4%) achieved a complete response (CR), 50 patients (58.8%) achieved a partial response (PR), 18 patients (21.2%) had stable disease (SD), and 9 patients (10.6%) experienced progressive disease (PD). In contrast, among the 80 patients in the chemoimmunotherapy group, the ORR was 75.0% (60/80), while the DCR was 92.5% (74/80). Specifically, 11 patients (13.8%) achieved a CR, 49 patients (61.3%) achieved a PR, 14 patients (17.5%) had SD, and 6 patients (7.5%) experienced PD (Table 2).
Table 2
| Variables | Intracranial response | Extracranial response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT, n (%) | CIT, n (%) | OR (95% CI) | P value | CT, n (%) | CIT, n (%) | OR (95% CI) | P value | ||
| Complete response | 8 (9.4) | 11 (13.8) | NA | NA | 0 | 0 | NA | NA | |
| Partial response | 50 (58.8) | 49 (61.3) | NA | NA | 62 (72.9) | 63 (78.8) | NA | NA | |
| Stable disease | 18 (21.2) | 14 (17.5) | NA | NA | 19 (22.4) | 15 (18.8) | NA | NA | |
| Progressive disease | 9 (10.6) | 6 (7.5) | NA | NA | 4 (4.7) | 2 (2.5) | NA | NA | |
| Objective response rate | 58 (68.2) | 60 (75.0) | 0.72 (0.36–1.42) | 0.34 | 62 (72.9) | 63 (78.8) | 0.73 (0.36–1.49) | 0.39 | |
| Disease control rate | 76 (89.4) | 74 (92.5) | 0.69 (0.23–2.02) | 0.49 | 81 (95.3) | 78 (97.5) | 0.52 (0.09–2.92) | 0.46 | |
Treatment efficacy was evaluated according to Response Evaluation Criteria in Solid Tumors v1.1 criteria. CT, chemotherapy; CIT, chemoimmunotherapy; OR, odds ratio; CI, confidence interval; NA, not applicable.
As for the extracranial response of patients, among the 85 patients in the chemotherapy group, an ORR of 72.9% (62/85) and a DCR of 95.3% (81/85) were observed. Furthermore, 62 patients (72.9%) achieved a PR, 19 patients (22.4%) had SD, and 4 patients (4.7%) experienced PD. In contrast, among the 80 patients in the chemoimmunotherapy group, an ORR of 78.8% (63/80) and a DCR of 97.5% (78/80) were noted. Specifically, 63 patients (78.8%) achieved a PR, 15 patients (18.8%) had SD, and 2 patients (2.5%) experienced PD (Table 2).
Predictive factor
Cox regression analysis was performed to further distinguish the predictors associated with OS in patients with SCLC and BM. It was found that Eastern Cooperative Oncology Group performance status (ECOG PS), liver metastases, bone metastases, and local treatment for BM were prognostic factors for OS in univariate analysis. Furthermore, multivariate analysis confirmed that local treatment for BM was an independent prognostic factor for OS in patients with BM (Table 3).
Table 3
| Characteristics | N | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ||||||
| <60 | 55 | Reference | – | – | ||
| ≥60 | 84 | 0.98 (0.67–1.43) | 0.92 | – | – | |
| Sex | ||||||
| Female | 5 | Reference | – | – | ||
| Male | 134 | 0.74 (0.30–1.81) | 0.50 | – | – | |
| Smoking history | ||||||
| Non-smoker | 25 | Reference | – | – | ||
| Smoker | 114 | 0.78 (0.49–1.26) | 0.32 | – | – | |
| ECOG PS | ||||||
| 0–1 | 55 | Reference | Reference | |||
| ≥2 | 84 | 1.30 (0.89–1.91) | 0.17* | 1.34 (0.91–1.97) | 0.14 | |
| Lung metastases | ||||||
| No | 86 | Reference | – | – | ||
| Yes | 53 | 0.93 (0.63–1.36) | 0.70 | – | – | |
| Liver metastases | ||||||
| No | 107 | Reference | Reference | |||
| Yes | 32 | 1.49 (0.97–2.29) | 0.07* | 1.55 (0.97–2.46) | 0.07 | |
| Bone metastases | ||||||
| No | 93 | Reference | Reference | |||
| Yes | 46 | 1.38 (0.94–2.03) | 0.11* | 1.09 (0.71–1.66) | 0.70 | |
| Intracranial tumor | ||||||
| 1–3 | 76 | Reference | – | – | ||
| ≥4 | 63 | 1.08 (0.74–1.56) | 0.70 | – | – | |
| Intracranial tumor size (mm) | ||||||
| <20 | 60 | Reference | – | – | ||
| ≥20 | 79 | 1.08 (0.74–1.56) | 0.71 | – | – | |
| Symptoms of brain metastases | ||||||
| Asymptomatic | 77 | Reference | – | – | ||
| Symptomatic | 62 | 1.05 (0.72–1.51) | 0.81 | – | – | |
| Local treatment for brain metastases | ||||||
| No | 65 | Reference | Reference | |||
| Yes | 74 | 0.69 (0.48–0.99) | 0.046* | 0.64 (0.44–0.95) | 0.03* | |
| With ICIs | ||||||
| No | 80 | Reference | – | – | ||
| Yes | 59 | 0.79 (0.54–1.14) | 0.21 | – | – | |
*, statistically significant values. P values were calculated with the log-rank test. Variables with P<0.20 in the univariable Cox regression were included in the multivariable Cox regression. HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ICIs, immune checkpoint inhibitors.
The survival analysis demonstrated a significant improvement in OS for patients with BM from SCLC who received local treatment for BM compared to those who did not receive such treatment (Figure S2A). Furthermore, there was no statistically significant difference in survival outcomes observed between patients receiving first-line treatment compared to those receiving second-line or subsequent-line local treatment (17.1 vs. 15.7 months, HR =0.97; P=0.90) (Figure S2B). Subgroup analysis in Figure S3 indicated that local treatment for BM improved OS in patients aged over 60 years, males, smokers, those with liver metastases, those without bone metastases, those with brain lesions larger than 20 mm, and symptomatic patients.
Pattern of initial progression
During the follow-up period, 5 patients showed no signs of disease progression. Among the remaining 160 patients who experienced disease progression, 82 (51.3%) had simultaneous initial progression of both intracranial and extracranial lesions, 55 (34.4%) demonstrated initial progression limited to intracranial lesions, and 23 (14.4%) showed initial progression confined to extracranial lesions (Figure 2A and Table S1). Of the 82 patients with disease progression in the chemotherapy group, 46 (56.1%) displayed simultaneous initial progression of both intracranial and extracranial lesions, 26 (31.7%) had initial progression of intracranial lesions only, and 10 (12.2%) had initial progression of extracranial lesions only. On the other hand, of the 78 patients with disease progression in the chemoimmunotherapy group, 36 (46.2%) had simultaneous initial progression of both intracranial and extracranial lesions, 29 (37.2%) had initial progression of intracranial lesions only, and 13 (16.7%) had initial progression of extracranial lesions only (Figure 2B and Table S1).
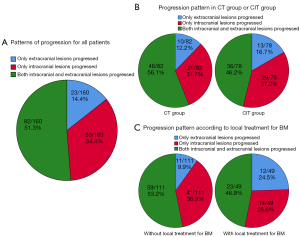
Among patients who received first-line treatment with local therapy targeting brain lesions, disease progression was observed in 49 out of 52 cases. Among these cases, 23 (46.9%) experienced initial progression involving both intracranial and extracranial lesions, 14 (28.6%) experienced initial progression limited to intracranial lesions, and 12 (24.5%) experienced initial progression limited to extracranial lesions. In contrast, among patients who received first-line treatment without local therapy targeting brain lesions, disease progression occurred in 111 out of 113 cases. Among these cases, 59 (53.2%) showed initial progression involving both intracranial and extracranial lesions, 41 (36.9%) displayed initial progression limited to intracranial lesions only, and 11 (9.9%) manifested initial progression restricted to extracranial lesions (Figure 2C and Table S1).
Discussion
BM is frequently observed in patients with ES-SCLC (3,4). However, due to the association of BM with poor outcomes, several SCLC-related trials have either excluded or limited the inclusion of patients with BM, resulting in a lack of evidence regarding the efficacy of ICIs in this population. Our study aims to provide valuable real-world insight into the efficacy of ICIs in patients with SCLC and BM. It was found that the incorporation of ICIs into first-line chemotherapy regimens in patients with SCLC and BM might lead to a trend of prolonged PFS and OS benefits.
In recent years, it has become widely recognized that BM in cancer patients (not specifically SCLC) can also derive benefits from immunotherapy. Theoretically, ICIs exert their anti-tumor effects by activating peripheral T cells (18), which can traverse the blood-brain barrier or enter the cerebrospinal fluid, thereby enabling control of intracranial tumors (19). Several clinical studies have also confirmed the anti-tumor efficacy of ICIs in tumors such as non-small cell lung cancer (NSCLC) and melanoma that harbor BM (20,21). In the context of SCLC, our study revealed a trend toward improved PFS in both intracranial and extracranial lesions when utilizing first-line chemoimmunotherapy compared to chemotherapy. Similarly, the intracranial ORR in the chemoimmunotherapy group exhibited a slight increase relative to the chemotherapy group. These findings demonstrate that ICIs also possess appropriate anti-tumor activity in intracranial lesions within SCLC patients.
In our study, although the addition of ICIs showed a trend toward survival benefit, there was no statistically significant difference observed (Figure 1). A plausible explanation is that the benefit conferred by ICIs in patients with SCLC and BM may not be substantial, thus requiring a larger sample size to attain statistically significant results. Similarly, some studies have concluded that ICIs do not confer a survival benefit in patients with SCLC and BM (12,17), which may be attributed to the limited number of cases included in these studies.
The limited benefits of ICIs in patients with SCLC and BM can be attributed to several potential reasons. Firstly, the efficacy of ICIs in SCLC, whether with or without BM, is not particularly remarkable. For instance, the addition of ICIs to first-line chemotherapy in advanced NSCLC has been shown to extend OS by 6–12 months (22-24), whereas in advanced SCLC, the extension is only 2–4 months (10). The diminished benefit of the chemoimmunotherapy approach in SCLC compared to metastatic NSCLC can be attributed to the intrinsic immunosuppressive phenotype of SCLC (25). Secondly, the brain represents a highly immune-specific environment, and there exists a unique immunosuppressive microenvironment in BM lesions (26,27). The intricate interplay between tumor cells and the brain microenvironment creates a complex and challenging immune landscape, potentially limiting the full potential of ICIs. Last but not least, SCLC inherently exhibits a high degree of heterogeneity, with tumor cells within the intracranial and extracranial lesions potentially possessing distinct characteristics and immune evasion mechanisms (28,29), influencing the efficacy of immunotherapy.
In SCLC (regardless of the presence of BM), whether the combination of programmed cell death 1 ligand 1 (PD-L1) inhibitors or programmed death receptor 1 (PD-1) inhibitors is more effective has always been a hot topic. A meta analysis suggests that chemotherapy plus PD-1 inhibitors seem to outperform chemotherapy plus PD-L1 inhibitors in PFS (30). However, in patients with SCLC and BM, there is a lack of data on the superiority of PD-1 inhibitors or PD-L1 inhibitors. Through our data analysis, we found that chemotherapy plus PD-1 inhibitors seem to have a trend towards prolonged PFS (both intracranial and extracranial PFS) compared to chemotherapy plus PD-L1 inhibitors (Figure S4A,S4B), but there was no difference in OS between the two groups (Figure S4C). In the future, some prospective clinical studies will be needed to further explore this topic. Notably, our study highlighted the critical role of local therapy for BM in SCLC patients. The incorporation of local therapy for BM into the first-line treatment strategy resulted in noteworthy alterations in the initial progression pattern, leading to a substantial decrease in the occurrence of brain progression (Figure 2C). Furthermore, local therapy for BM demonstrated a significant enhancement in OS and emerged as an independent prognostic factor for OS in multivariate analysis (Table 3). Research has unequivocally established the efficacy of local treatment for BM, encompassing surgical interventions and radiotherapy, in significantly reducing the progression rate of brain lesions and establishing better conditions for subsequent therapies (31,32). Moreover, local radiotherapy exhibits a synergistic interaction with immunotherapy. In our study, among patients who received local intracranial therapy, there was a trend towards prolonged intracranial PFS in the chemoimmunotherapy group compared to the chemotherapy group (HR =0.55, P=0.10) (Figure S5A). In terms of extracranial efficacy, the survival curves remained relatively close between the two treatment groups (HR =0.77, P=0.47) (Figure S5B). which also indirectly reflects the synergistic effect between radiation therapy and immunotherapy. Mechanistically, radiotherapy amplifies the release and presentation of tumor antigens, facilitates the infiltration of effector T cells into tumor tissues, and upregulates the expression of tumor PD-L1 and major histocompatibility complex class I (MHC-I), thereby augmenting the efficacy of immunotherapy (33-35). This provides a solid theoretical foundation for the concurrent utilization of immunotherapy and radiotherapy in the management of cancer. Consequently, we strongly advocate for the comprehensive implementation of diverse treatment modalities for patients with SCLC and BM, along with timely consideration of local therapy for brain lesions based on individual conditions.
Based on the findings of our study, we would like to underscore several key points. Firstly, in the management of patients with SCLC and BM, the combination of chemotherapy and ICIs should be regarded as the preferred systemic treatment. Nevertheless, in situations where access or affordability pose concerns, chemotherapy alone may serve as a viable alternative due to the limited incremental benefit associated with the addition of ICIs. Secondly, it is imperative to actively contemplate local brain-directed therapies, such as whole-brain radiotherapy or stereotactic radiosurgery, for these patients. Thirdly, there exists a pressing need to pursue biomarkers that can effectively predict the efficacy of immunotherapy in patients with SCLC and BM. Lastly, given the unfavorable prognosis and constrained efficacy of current interventions for this patient cohort, the ongoing development of novel therapies designed specifically for targeting SCLC with BM is of paramount importance.
There are some limitations to be acknowledged in this study. Firstly, due to its retrospective design, the presence of missing information and potential analytical biases could not be completely eliminated. Secondly, patients who receive chemoimmunotherapy might have better economic status compared to those who receive chemotherapy alone, and thus receive better care, which might also affect patient prognosis. Finally, the ICIs utilized in the chemoimmunotherapy group were not the same ones, and different agents may yield distinct survival outcomes. Notwithstanding these limitations, our study offered valuable insight into the real-world treatment efficacy for patients with SCLC and BM. Future prospective studies involving larger cohorts should be undertaken to thoroughly assess the efficacy of immunotherapy in this specific patient population.
Conclusions
In summary, our study findings indicate that the current first-line regimen consisting of the combination of chemotherapy and ICIs provides modest survival benefits for patients with SCLC and BM. Nonetheless, given the abysmal prognosis associated with BM in SCLC, there remains room for further enhancements in the efficacy of existing treatment protocols. Hence, it is imperative to undertake the exploration of novel therapeutic agents tailored specifically to this particular patient cohort, while simultaneously employing a comprehensive treatment strategy, in order to substantively ameliorate their prognostic outcomes.
Acknowledgments
The authors would like to thank all the patients and their families for their cooperation and participation. Additionally, the authors are thankful to all research staff and coinvestigators involved in this investigation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-335/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-335/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-335/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-335/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the ethics and research committee of Sun Yat-sen University Cancer Center (approval number: B2023-489-01), and it was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients’ individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin 2023;73:620-52. [Crossref] [PubMed]
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Hirsch FR, Paulson OB, Hansen HH, et al. Intracranial metastases in small cell carcinoma of the lung. Prognostic aspects. Cancer 1983;51:529-33. [Crossref] [PubMed]
- Nugent JL, Bunn PA Jr, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer 1979;44:1885-93. [Crossref] [PubMed]
- Reveiz L, Rueda JR, Cardona AF. Chemotherapy for brain metastases from small cell lung cancer. Cochrane Database Syst Rev 2012;2012:CD007464. [Crossref] [PubMed]
- Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663-71. [Crossref] [PubMed]
- Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy--a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 2000;18:3400-8. [Crossref] [PubMed]
- Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol 2023;9:419-29. [Crossref] [PubMed]
- Konala VM, Madhira BR, Ashraf S, et al. Use of Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Oncology 2020;98:749-54. [Crossref] [PubMed]
- Wang S, Li Y, Liu Z, et al. Efficacy and safety of first-line immune checkpoint inhibitors combined with chemotherapy for extensive-stage small cell lung cancer: A network meta-analysis. Lung Cancer 2023;178:47-56. [Crossref] [PubMed]
- Mathieu L, Shah S, Pai-Scherf L, et al. FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer. Oncologist 2021;26:433-8. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Cheng Y, Fan Y, Zhao Y, et al. Tislelizumab Plus Platinum and Etoposide Versus Placebo Plus Platinum and Etoposide as First-Line Treatment for Extensive-Stage SCLC (RATIONALE-312): A Multicenter, Double-Blind, Placebo-Controlled, Randomized, Phase 3 Clinical Trial. J Thorac Oncol 2024;19:1073-85. [Crossref] [PubMed]
- Cheng Y, Liu Y, Zhang W, et al. LBA93 EXTENTORCH: A randomized, phase III trial of toripalimab versus placebo, in combination with chemotherapy as a first-line therapy for patients with extensive stage small cell lung cancer (ES-SCLC). Ann Oncol 2023;34:S1334.
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-41. [Crossref] [PubMed]
- Ahmad A, Khan P, Rehman AU, et al. Immunotherapy: an emerging modality to checkmate brain metastasis. Mol Cancer 2023;22:111. [Crossref] [PubMed]
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet 2021;398:1002-14. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 2023;41:1992-8. [Crossref] [PubMed]
- Wang Z, Wu L, Li B, et al. Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non-Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01). J Clin Oncol 2023;41:651-63. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab Plus Carboplatin and Pemetrexed as First-Line Treatment for Advanced Nonsquamous NSCLC: Extended Follow-Up of CameL Phase 3 Trial. J Thorac Oncol 2023;18:628-39. [Crossref] [PubMed]
- Remon J, Aldea M, Besse B, et al. Small cell lung cancer: a slightly less orphan disease after immunotherapy. Ann Oncol 2021;32:698-709. [Crossref] [PubMed]
- Boire A, Brastianos PK, Garzia L, et al. Brain metastasis. Nat Rev Cancer 2020;20:4-11. [Crossref] [PubMed]
- Rios-Hoyo A, Arriola E. Immunotherapy and brain metastasis in lung cancer: connecting bench side science to the clinic. Front Immunol 2023;14:1221097. [Crossref] [PubMed]
- Jin Y, Chen Y, Qin Z, et al. Understanding SCLC heterogeneity and plasticity in cancer metastasis and chemotherapy resistance. Acta Biochim Biophys Sin (Shanghai) 2023;55:948-55. [Crossref] [PubMed]
- Nabet BY, Hamidi H, Lee MC, et al. Immune heterogeneity in small-cell lung cancer and vulnerability to immune checkpoint blockade. Cancer Cell 2024;42:429-443.e4. [Crossref] [PubMed]
- Li H, Han H, Li C, et al. Efficacy and safety of first-line PD-1/PD-L1 inhibitor combinations for extensive-stage small-cell lung cancer: a Bayesian network meta-analysis. Ther Adv Med Oncol 2023;15:17588359231189430. [Crossref] [PubMed]
- Li N, Chu Y, Song Q. Brain Metastasis in Patients with Small Cell Lung Cancer. Int J Gen Med 2021;14:10131-9. [Crossref] [PubMed]
- Videtic GM. The role of radiation therapy in small cell lung cancer. Curr Oncol Rep 2013;15:405-10. [Crossref] [PubMed]
- Huang W, Chen JJ, Xing R, et al. Combination therapy: Future directions of immunotherapy in small cell lung cancer. Transl Oncol 2021;14:100889. [Crossref] [PubMed]
- Kang K, Wu Y, Yao Z, et al. Tackling the current dilemma of immunotherapy in extensive-stage small cell lung cancer: A promising strategy of combining with radiotherapy. Cancer Lett 2023;565:216239. [Crossref] [PubMed]
- Tian Y, Ma J, Jing X, et al. Radiation therapy for extensive-stage small-cell lung cancer in the era of immunotherapy. Cancer Lett 2022;541:215719. [Crossref] [PubMed]


