
Prognostic impact of targetable driver alterations in resected early-stage lung cancer
Highlight box
Key findings
• This study showed that non-small cell lung cancer (NSCLC) patients with resected tumors that harbor molecular alterations have the same recurrence risk as patients with tumors without molecular alterations.
• What is known and what is new?
• For EGFR and ALK positive tumors adjuvant targeted therapy improves outcome for patients.
• For other oncogene-driven tumors adjuvant targeted therapy has not been approved yet. It is unclear if they have the same outcome when treated as tumors without molecular alterations.
What is the implication, and what should change now?
• Our study results suggest that adding chemotherapy to targeted therapies in the adjuvant setting should be investigated in future clinical trials.
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC) accounts for up to 80% of lung cancer. Early-stage disease is resected surgically with curative intent. However, 5-year survival rates differ between 70–93% for stage I disease and 10–40% for stage III disease (2). Adjuvant radiotherapy is no longer routinely recommended for N2 disease after surgery because it has been shown to have a deleterious effect on long-term survival (3). Adjuvant platinum-based chemotherapy yields an overall survival (OS) benefit of 5% at 5 years (4). In recent years, a lot of molecular subtypes of NSCLC have emerged, leading to targeted therapies in the metastatic setting. Until a few years ago, the different molecular features were irrelevant for adjuvant treatment decision-making until the phase 3 randomised trial ADAURA showed a significant disease-free survival (DFS) and OS benefit for the epidermal growth factor receptor (EGFR)-mutated subtype treated with osimertinib (5). Only very recently results from the phase 3 randomised ALINA trial have been presented for completely resected ALK-positive NSCLC which has now become the second molecular subtype to be treated with targeted therapy in the adjuvant setting (6). Treatment with alectinib was associated with significantly improved DFS compared with platinum-based chemotherapy in this cohort of patients. Accordingly, guidelines recommend molecular testing, at a minimum, for EGFR mutations and ALK rearrangements in the early-stage setting. For the other molecular subtypes, cisplatinum-based chemotherapy is up to now the mainstay of adjuvant treatment.
As it is difficult to carry out randomized controlled trials for rare druggable alterations and recruitment into such trials is slow, we aimed to retrospectively investigate if there is a difference in recurrence-free survival in stage I–III NSCLC harboring druggable molecular alterations compared to subtypes without targetable molecular alterations. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-433/rc).
Methods
Study cohort
Since 2015 NSCLC patients with early-stage disease have been analyzed for driver mutations and programmed cell death ligand 1 (PD-L1) expression at our institutions. For this study, all consecutive patients who underwent surgery with curative intent for NSCLC (stage I–III) with targetable mutations between January 2015 and December 2020 at the Medical University of Graz, the Medical University of Innsbruck, and the Klinik Floridsdorf were retrospectively identified. Tumors with the EGFR-mutated subtype were excluded due to already existing results from prospective trials. ALK-translocated tumors were not excluded as results from prospective trials for this subtype were unknown at the time of study initiation. The same approach and testing platforms were used at the Medical University of Graz and the Medical University of Vienna. Namely, reflex testing for all non-squamous NSCLC was performed using separate DNA and RNA-based analyses. DNA-based analysis was performed using the Ion Ampliseq Colon and Lung Cancer Research Panel panel covering hotspot mutations in 22 genes (KRAS, EGFR, BRAF, PIK3CA, AKT1, ERBB2, PTEN, NRAS, STK11, MAP2K1, ALK, DDR2, CTNNB1, MET, TP53, SMAD4, FBX7, FGFR3, NOTCH1, ERBB4, FGFR1 and FGFR2). For the RNA-based analysis, from 2015–2019, the Ion Torrent Ampliseq Lung Fusion Panel was used. It included over 70 ALK, RET, ROS1, and NTRK1 transcripts. Its improved version, Archer FusionPlex Panel (Integrated DNA Technologies, USA), was introduced in 2020. It analyses 17 genes (ALK, BRAF, EGFR, ERBB2, FGFR1, FGFR2, FGFR3, KRAS, MET, NRG1, NTRK1, NTRK2, NTRK3, NUTM1, PIK3CA, RET and ROS1) using open-ended targeted amplification enabling detection of both novel and known fusions. Sequencing was performed on an Ion Torrent sequencer (Thermo Fisher Scientific, Waltham, MA, USA).
Samples coming from Innsbruck (13% of patients with molecular alterations) were tested from 2015 to 2016 with RT-PCR for EGFR mutations, and from 2016 to 2018 Sanger Sequencing and PyroSequencing were used for EGFR, KRAS and TP53 mutations. ALK, ROS, MET and from 2018 also RET fusions were tested with FISH until 2020, when in Innsbruck Archer FusionPlex Panel was introduced. Since March 2020, detection of variants on DNA-level was primarily performed using the AmpliSeq for Illumina Cancer Hotspot Panel v2 or AmpliSeq for Illumina Focus Panel. Both panels target hotspot regions of 50 genes including KRAS, EGFR, BRAF, PIK3CA, ERBB2, PTEN, NRAS, STK11, ALK, CTNNB1, MET, TP53, SMAD4, NOTCH1, ERBB4, FGFR1, FGFR2 and FGFR3. Patients with resected NSCLC without targetable molecular alterations treated at the Medical University of Graz served as control cohort. Of note, KRAS mutations other than KRAS G12C were not excluded from the control cohort. In total, 529 patients were identified (all Caucasians). One hundred and sixty subjects had tumors with molecular alterations and 355 subjects served as control cohort. Fourteen patients had to be excluded from further analysis (5 perioperative deaths, 9 with unclear follow-up status). Patients were monitored for recurrence according to international guidelines with continuous CT scans.
Statistical analysis
All statistical analyses were performed with Stata 18.0 (Stata Corp., Houston, TX, USA). Continuous variables were summarized with medians (25th–75th percentile), and count data as absolute frequencies (column %). The distribution of variables between patients with and without molecular alterations was compared with rank-sum-tests, χ2-tests, and Fisher’s exact tests, as appropriate. The primary endpoint of the time-to-event analysis was the cumulative incidence of recurrence with death from other or unknown causes as the competing event of interest. An exploratory analysis was also performed for OS. The baseline date for the recurrence endpoint was the date of cancer diagnosis and in 2 patients with missing date of diagnosis the date of surgery. The baseline date for the OS analysis was the date of recurrence. Follow-up was truncated at 5 years after baseline. Median follow-up was estimated with a reverse Kaplan-Meier estimator (7). The cumulative incidence of recurrence was computed with a competing risk cumulative incidence estimator (8), compared between two or more groups with Gray’s tests (9), and modeled with uni- and multivariable Fine & Gray competing risk regression models (10), respectively. OS was analyzed with Kaplan-Meier estimators and log-rank tests. Missing data are reported in Table 1, and a multiple imputation with a chained equations algorithm and 10 imputation datasets was used to account for missing baseline data in time-to-event models (11).
Table 1
| Variable | N (%miss.) | Overall (n=515) | No molecular alteration (n=355) | Molecular alteration (n=160) | P* |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 513 (<1%) | 66 [59–73] | 65 [59–73] | 66 [59–73] | 0.58 |
| Female sex | 515 (0%) | 220 (43%) | 130 (37%) | 90 (56%) | <0.001 |
| ECOG ≥1 point | 222 (57%) | 104 (47%) | 54 (54%) | 50 (41%) | 0.053 |
| Smoking status | 235 (54%) | – | – | – | 0.01 |
| Never smoker | – | 36 (15%) | 6 (7%) | 30 (21%) | – |
| Former smoker | – | 111 (47%) | 43 (48%) | 68 (47%) | – |
| Current smoker | – | 88 (37%) | 40 (45%) | 48 (33%) | – |
| Adenocarcinoma | 515 (0%) | 354 (69%) | 198 (56%) | 156 (98%) | <0.001 |
| Type of primary treatment | 515 (0%) | – | – | – | <0.001 |
| Lobectomy | – | 413 (84%) | 305 (86%) | 126 (79%) | – |
| Pneumonectomy | – | 36 (7%) | 33 (9%) | 3 (2%) | – |
| Atypical resection | – | 16 (3%) | 12 (3%) | 4 (3%) | – |
| SBRT | – | 8 (2%) | 2 (1%) | 6 (4%) | – |
| Definitive CRT | – | 6 (1%) | 3 (1%) | 3 (2%) | – |
| Surgery NOS | – | 18 (4%) | 0 (0%) | 18 (11%) | – |
| Postoperative stage** | 513 (<1%) | – | – | – | 0.09 |
| I | – | 227 (44%) | 146 (41%) | 81 (51%) | – |
| II | – | 136 (27%) | 103 (29%) | 33 (21%) | – |
| III | – | 147 (29%) | 103 (29%) | 44 (28%) | – |
| IV | – | 3 (1%) | 3 (1%) | 0 (0%) | – |
| Neoadjuvant treatment | 515 (0%) | 58 (11%) | 39 (11%) | 19 (12%) | 0.77 |
| Adjuvant treatment | 513 (<1%) | 123 (24%) | 88 (25%) | 35 (22%) | 0.45 |
n (%miss.) reports the number of patients with observed variable (% missing). Reported data are medians [25th–75th percentile] for continuous data and absolute frequencies (column %) for count data. *, P values are from ranksum-tests, χ2-tests, and Fisher’s exact tests, as appropriate. **, in 7 patients with missing postoperative stage we imputed the missingness with the preoperative stage. ECOG, Eastern Cooperative Oncology Group; SBRT, stereotactic body radiotherapy; CRT, chemoradiation; NOS, not otherwise specified.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of the Medical University of Graz (EK number 33-066 ex 20/21), the Medical University of Innsbruck (EK number 1179/2022) and the Ethics Committee of Vienna (EK-20-061-VK). Individual consent for this retrospective analysis was waived.
Results
Cohort description
One-hundred-and-sixty patients (31%) had molecular alterations. The three most common alterations were the Kirsten rat sarcoma proto-oncogen (KRAS) G12C mutation (n=92), ALK fusions (n=21), and the BRAF V600E mutation (n=15) (Figure 1). Baseline characteristic are shown in Table 1. Sex distribution was significantly different between the cohort with mutations and the control cohort. Fifty-six percent of patients with molecular alterations were female compared to 37% in the control cohort. There were also significantly more non-smokers (never-smokers and ex-smokers, defined as someone who has smoked more than 100 cigarettes in life-time but has not smoked in the last 28 days) among patients with molecular alterations compared to controls (21% vs. 7%, respectively). Adenocarcinoma was the histology in 98% of tumors with molecular alterations compared to 56% of controls. We could not find a significant difference between the two groups concerning age (Table 1).

Patients had a median follow-up time of 3.1 years after cancer diagnosis, and 75% and 25% of the cohort were followed for at least 1.8 and 4.4 years, respectively. During this follow-up period, we observed 179 recurrences (35%), and 149 deaths (29%). These deaths were attributed to tumor progression (n=81), treatment (n=12), other causes (n=23), and unknown causes (n=33). The corresponding 6-month, 1-year, 3-year, and 5-year competing risk cumulative incidence estimates of recurrence were 8% [95% confidence interval (CI): 6–10%], 16% (95% CI: 13–19%), 37% (95% CI: 33–42%), and 46% (95% CI: 40–52%), respectively (Figure S1).
Molecular alterations and recurrence risk
Patients with and without molecular alterations had a highly similar recurrence risk experience. In detail, the 1-, 3-, and 5-year cumulative incidence of recurrence estimates were 16% (95% CI: 13–21%), 38% (95% CI: 32–43%), and 46% (95% CI: 39–52%) in patients without molecular alterations (n=355); 16% (95% CI: 9–25%), 38% (95% CI: 26–49%), and 48% (95% CI: 30–64%) in patients with the KRAS G12C mutation (n=92); and 12% (95% CI: 5–22%), 33% (95% CI: 20–47%), and 55% (95% CI: 21–79%) in patients with other molecular alterations (n=68), respectively (Gray’s test P=0.89, Figure 2). This finding of a lack of association between molecular alteration status and recurrence risk was also observed in a hypothesis-generating sub-analysis of recurrence risk according to the most common other molecular alterations including the BRAF V600E mutation, ALK fusions, MET exon 14 skipping, and EGFR exon 20 insertions (Gray’s test P=0.47, Figure S2). When looking at patients with ALK-fusions who received adjuvant treatment [adjuvant chemotherapy 2, adjuvant tyrosine kinase inhibitor (TKI) 3] no recurrences were observed. However, due to the small number of patients (n=5), no statistical testing was performed.

In univariable competing risk regression, recurrence risk was similar in patients with and without molecular alterations (Table 2). Univariable predictors of an increased recurrence risk were extensive local treatment (pneumonectomy and definitive chemoradiation), stage III disease, receipt of neoadjuvant treatment, and receipt of adjuvant treatment. Molecular alteration status was neither associated with recurrence risk after adjusting for tumor stage (Figure 3), nor after full multivariable adjustment for all other variables under study (Table 2). Moreover, all other univariable predictors of an adverse recurrence risk experience except adjuvant therapy prevailed upon multivariable adjustment (Table 2). The used perioperative treatment regimens are listed in Table S1. The most frequently used chemo-regimen in patients without molecular alterations was platinum-vinorelbine (used for 60% of treated patients) whereas in patients with molecular alterations the most frequently used chemo-regimen was platinum-pemetrexed (used for 40% of treated patients).
Table 2
| Variable | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | ||
| Presence of any molecular alteration | 0.96 | 0.68–1.33 | 0.79 | N/A | N/A | N/A | |
| No molecular alteration | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| KRAS G12C mutation | 1.00 | 0.67–1.51 | 0.99 | 0.95 | 0.59–1.51 | 0.82 | |
| Any other molecular alteration | 0.89 | 0.55–1.44 | 0.63 | 0.80 | 0.45–1.40 | 0.43 | |
| Age at diagnosis (per 5 years increase) | 0.97 | 0.89–1.04 | 0.39 | 1.02 | 0.93–1.12 | 0.61 | |
| Female sex | 0.98 | 0.73–1.32 | 0.91 | 1.07 | 0.78–1.48 | 0.67 | |
| ECOG ≥1 point | 1.05 | 0.73–1.51 | 0.80 | 1.20 | 0.79–1.83 | 0.38 | |
| Former or current smoker | 1.02 | 0.62–1.69 | 0.94 | 1.06 | 0.55–2.05 | 0.86 | |
| Adenocarcinoma | 0.93 | 0.68–1.27 | 0.64 | 1.19 | 0.82–1.73 | 0.36 | |
| Pneumonectomy + definitive CRT | 2.74 | 1.78–4.23 | <0.001 | 1.90 | 1.13–3.19 | 0.02 | |
| Postoperative stage III–IV | 2.79 | 2.06–3.77 | <0.001 | 2.26 | 1.50–3.39 | <0.001 | |
| Neoadjuvant treatment | 2.32 | 1.59–3.37 | <0.001 | 1.78 | 1.13–2.82 | 0.01 | |
| Adjuvant treatment | 1.45 | 1.04–2.01 | 0.03 | 1.05 | 0.69–1.60 | 0.81 | |
Data are from uni- and multivariable Fine & Gray regression models. SHR, subdistribution hazard ratio; CI, confidence interval; P, Wald test P value; N/A, not applicable (we chose the three-level mutation status variable below for multivariable analysis); Ref., reference category; ECOG, Eastern Cooperative Oncology Group; CRT, chemoradiation therapy.

As it can be argued that patients with stage III disease are in a more advanced disease, we also performed a subanalysis restricted to patients with stage I and II disease. Again, recurrence risk was similar in patients with and without druggable molecular alterations (Figure S3). Because molecular alterations were mainly found in adenocarcinomas (98%), we also performed a subanalysis restricted to patients with adenocarcinomas. Patients with and without molecular alterations had a highly similar recurrence risk experience. In detail, the 1-, 3-, and 5-year cumulative incidence of recurrence estimates were 16% (95% CI: 11–21%), 38% (95% CI: 31–45%), and 44% (95% CI: 36–52%) in patients without molecular alterations (n=198); 16% (95% CI: 9–25%), 39% (95% CI: 27–50%), and 49% (95% CI: 31–65%) in patients with the KRAS G12C mutation (n=90); and 11% (95% CI: 4–21%), 32% (95% CI: 19–47%), and 55% (95% CI: 21–79%) in patients with other molecular alterations (n=66), respectively (Gray’s test P=0.85, Figure S4).
Molecular alterations and OS after recurrence (n=179)
Among the 179 patients who experienced recurrence, 46 (26%) had druggable molecular alterations while 133 (74%) had no druggable molecular alterations. Twenty-eight (30%) of the 92 overall patients with a KRAS G12C mutation relapsed. The majority of patients (n=11) received chemoimmunotherapy as palliative first-line treatment. Two patients received targeted therapy with a KRAS inhibitor in the first-line setting and only two patients in the second-line setting. Nine patients received local treatment (stereotactic beam radiotherapy or surgery) for oligo-progressive disease whereas four patients did not receive any palliative tumor-specific treatment at all. In patients with ALK fusions (n=21), 5 (24%) patients relapsed of whom 4 received a TKI as first-line treatment and one patient received no palliative treatment apart from best supportive care. Seven (47%) out of 15 patients with a BRAF V600E mutation experienced disease recurrence and three patients received targeted therapy as first-line treatment and one patient as second-line treatment. Two patients were treated with stereotactic body radiotherapy for oligo-progressive disease whereas one patient did not receive any palliative treatment. One (20%) out of the five patients with RET fusion relapsed and received targeted therapy as first-line treatment. Two (18%) of the 11 patients with a MET exon 14 skipping mutation relapsed and received a targeted therapy in the first-line setting. Two (50%) of 4 patients with a HER2 mutation relapsed and one of them received targeted therapy with an antibody drug-conjugate as first-line treatment in the palliative setting. One patient with an exon 20 insertion relapsed but did not receive any treatment apart from best supportive care.
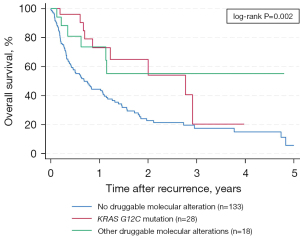
The 1-, 3-, and 5-year OS estimates were 44% (95% CI: 35–53%), 17% (95% CI: 10–27%), and 6% (95% CI: 1–19%) in patients without druggable molecular alterations (n=133); 73% (95% CI: 46–88%), 20% (95% CI: 1–55%), and not defined in patients with the KRAS G12C mutation (n=28); and 74% (95% CI: 43–89%), 55% (95% CI: 25–77%), and not defined in patients with other druggable molecular alterations (n=18), respectively (log-rank P=0.002, Figure 4).
Discussion
In this study, we showed that NSCLC patients with resected tumors that harbor molecular alterations have the same recurrence risk as patients with tumors without molecular alterations if treated with surgery and adjuvant chemotherapy.
The characteristics of NSCLC patients with oncogenic driver mutations from our early-stage cohort are comparable to what we already know from existing data from the advanced stage (12-15). There was a higher prevalence of female sex and never-smokers among patients with oncogenic-driven tumors. The biggest group in our study cohort consisted of patients with KRAS G12C mutations which is also the most common type of mutation in patients with metastatic NSCLC (16-18). The KRAS G12C mutation has been linked to female sex too but typically occurs in the smoking cancer population (19-21). Targeting KRAS has been frustrating for many years until sotorasib became the first approved drug for advanced KRAS G12C-mutated NSCLC after at least one line of therapy (18). There are little but contradictory results for the prognosis of this subtype of tumor in the early stage setting. Nadal et al. reported that KRAS G12C tumors were associated with poor outcome compared to KRAS wildtype tumors. However, the study cohort consisted of 85 resected lung cancers with KRAS mutations but only 35 patients with KRAS G12C mutations (20). The bigger study by Jones et al. comprised 95 patients with a somatic KRAS G12C mutation. The authors concluded that KRAS G12C mutations were associated with worse DFS when compared to other KRAS mutant tumors but not when compared to the KRAS wildtype group (21). In our cohort, we could not find a difference for the recurrence-risk between patients with KRAS G12C mutations and our control cohort, with the difference that other KRAS mutations than the specific KRAS G12C mutation were not excluded from the control cohort. With the limitation of small subsets, we could not identify any other driver alteration that was associated with a better or worse recurrence-free survival when compared to the control cohort.
It is reassuring that the opposite was shown in the OS analysis for our study population. Patients with tumors with targetable alterations had significantly better OS. This was also the case for the subset of patients with KRAS G12C mutations. A recent Danish study, including 328 KRAS G12C mutated advanced NSCLC patients, reported that with the implementation of checkpoint inhibitors, the survival in KRAS G12C mutated patients is comparable to patients with any other KRAS mutation or to wildtype patients (22). The fact that the subgroup of KRAS G12C mutated patients from our cohort had a better OS might be attributed to the fact that 32% had oligo-progressive disease and received local treatment at the metastatic site.
With respect to ALK fusions, a retrospective analysis by Schmid et al. has come to the same conclusion as our study that patients with stage I–III ALK-rearranged NSCLC have the same recurrence risk as patients without molecular driver alterations (23). Tumors with certain molecular alterations are sensitive to cytotoxic chemotherapy, especially to platinum-pemetrexed combinations. A retrospective analysis by Shaw et al. found a statistically significant difference in PFS for patients with ALK positive tumors compared to the wild type group when treated with a platinum-pemetrexed combination as front-line treatment (median PFS of 8.5 vs. 5.4 months, respectively, P<0.001) (24). For patients with tumors harboring a RET fusion, a superiority of platinum-pemetrexed over single agent chemotherapy or immune-checkpoint blockade was found in the retrospective RET-MAP trial (25), whereas tumors with ROS1 fusions seem to benefit as well from single agent pemetrexed as from platinum-pemetrexed combinations compared to wild type tumors. Even with the favorable results of the ALINA trial, using alectinib as adjuvant treatment for tumors with ALK fusions does not address the potential additional usefulness of adding chemotherapy to alectinib. The aim of adjuvant treatment is to eradicate minimal residual disease, but a major concern of targeted therapy is, that it only suppresses rather than eliminates cancer cells and so functions as tumor-static but not tumor-toxic treatment. Despite the significant DFS and OS benefit for adjuvant osimertinib in EGFR-mutated tumors, the DFS curves begin to converge at the end of adjuvant TKI-treatment (26).
Several ongoing trials are investigating neoadjuvant and adjuvant treatment with TKIs for patients with genetic alterations besides common EGFR mutations. The largest ongoing trial is the NAUTIKA1 study (NCT04302025) which includes patients with ALK, ROS1, NTRK, RET or BRAF V600E mutation. In this single-arm trial, patients receive eight weeks of neoadjuvant targeted therapy followed by resection, adjuvant chemotherapy and cohort-specific target treatment. First results in the ALK positive cohort were encouraging with three pathologic complete responses and three major pathologic responses out of eight resected patients. In the ongoing randomized, double-blind placebo-controlled phase III Alchemist trial (NCT02194738) patients receive adjuvant crizotinib vs. placebo for 24 months whereas the Libretto-432 trial investigates the RET inhibitor selpercatinib vs. placebo as adjuvant treatment for RET positive resected stage IB to IIIA NSCLC patients. A major issue of these trials is the slow recruitment due to the rareness of driver mutations.
There are several limitations to this study, firstly its retrospective nature and secondly its molecular testing. Over time, the panels of molecular testing have changed as described above and we might have missed some patients with targetable genetic changes (especially relatively limited DNA-based testing in Innsbruck before March 2020). This is of course one of the limitations of our study. However, molecular testing in all centers was performed according to international recommendations at every stage, therefore we are providing real-world results from a country with an advanced testing approach in early-stage carcinomas. Furthermore, the approval of immune checkpoint inhibitors in the neo- and adjuvant setting is now the new standard in resectable early-stage lung cancer which was not the case for our historic study population. Several trials showed significant improvement of event-free survival for adjuvant, neoadjuvant or perioperative treatment strategies combining chemotherapy and checkpoint inhibition. However, not in all of these early-stage trials molecular testing was mandatory and mostly, only patients with known EGFR mutations or ALK translocations were excluded. Therefore, the benefit of immunotherapy for patients with oncogene-driven NSCLC remains unclear in the early-stage setting (27-30).
Conclusions
Targeted therapies are becoming incorporated into perioperative prospective randomized clinical trials nowadays, but recruitment is slow for very rare druggable alterations, and therefore retrospective studies like this provide important information for clinical decision-making. Furthermore, our study results suggest that adding chemotherapy to targeted therapies in the adjuvant setting should be investigated in future clinical trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-433/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-433/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-433/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-433/coif). M.J.H. received research grants from Takeda, Roche, Lilly, Merck Sharp & Dohme, AstraZeneca and Bristol-Myers Squibb; received honoraria for lectures and presentations from Daiichi Sankyo, Lilly and Merck Sharp & Dohme and travel support from Amgen, Merck Sharp & Dohme and Bristol Myers Squibb. G.A. received honoraria, payments for advisory boards and travel support from Amgen, AstraZeneca, Roche, Takeda, Pfizer. O.I. received consulting fees from BMS, Eli Lilly, Merck Sharp & Dohme, Pfizer, Roche, Boehringer Ingelheim; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Eli Lilly, BMS, Menarini, Merck Sharp & Dohme, Roche, Pfizer; support for attending meetings and/or travel from Eli Lilly, BMS and research grants from Amgen and AstraZeneca outside of the submitted study. A.T. received research grants from AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, Roche Austria and Sanofi-aventis; received honoraria for lectures from Amgen and Merck Shapr & Dohme and travel support from Amgen. G.A. received honoraria and travel support from Amgen, AstraZeneca, Roche, Takeda and Pfizer. L.H. received travel support from Janssen. L.B. received grants from Takeda, AstraZeneca, BMS and Roche; he also received payment for lectures and participated in advisory boards form Invitae, Eli-Lilly, AstraZeneca, Roche, MSD, Merck, BMS, Pfizer, Novartis, Takeda, Janssen; support for attending meeting from Pfizer; he is Int. Secretary-Austrian Society of Pathology, PPS Membership and Awards Committee and Member of the Mesothelioma Committee of IASLC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of the Medical University of Graz (EK number 33-066 ex 20/21), the Medical University of Innsbruck (EK number 1179/2022) and the Ethics Committee of Vienna (EK-20-061-VK). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Le Pechoux C, Pourel N, Barlesi F, et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:104-14. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Wu YL, Dziadziuszko R, Ahn JS, et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:1265-76. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695-706. [Crossref] [PubMed]
- Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 1988;16:1141-54. [Crossref]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496-509. [Crossref]
- Mukherjee K, Gunsoy NB, Kristy RM, et al. Handling Missing Data in Health Economics and Outcomes Research (HEOR): A Systematic Review and Practical Recommendations. Pharmacoeconomics 2023;41:1589-601. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Warth A, Muley T, Dienemann H, et al. ROS1 expression and translocations in non-small-cell lung cancer: clinicopathological analysis of 1478 cases. Histopathology 2014;65:187-94. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [Crossref] [PubMed]
- Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol 2015;16:e342-51. [Crossref] [PubMed]
- Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019;322:764-74. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Isaksson J, Berglund A, Louie K, et al. KRAS G12C Mutant Non-Small Cell Lung Cancer Linked to Female Sex and High Risk of CNS Metastasis: Population-based Demographics and Survival Data From the National Swedish Lung Cancer Registry. Clin Lung Cancer 2023;24:507-18. [Crossref] [PubMed]
- Nadal E, Chen G, Prensner JR, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol 2014;9:1513-22. [Crossref] [PubMed]
- Jones GD, Caso R, Tan KS, et al. KRAS (G12C) Mutation Is Associated with Increased Risk of Recurrence in Surgically Resected Lung Adenocarcinoma. Clin Cancer Res 2021;27:2604-12. [Crossref] [PubMed]
- Frost MG, Jensen KJ, Gotfredsen DR, et al. KRAS G12C mutated advanced non-small cell lung cancer (NSCLC): Characteristics, treatment patterns and overall survival from a Danish nationwide observational register study. Lung Cancer 2023;178:172-82. [Crossref] [PubMed]
- Schmid S, Garcia M, Cheng S, et al. Treatment patterns and outcomes in early-stage ALK-rearranged non-small cell lung cancer. Lung Cancer 2022;166:58-62. [Crossref] [PubMed]
- Shaw AT, Varghese AM, Solomon BJ, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol 2013;24:59-66. [Crossref] [PubMed]
- Aldea M, Marinello A, Duruisseaux M, et al. RET-MAP: An International Multicenter Study on Clinicobiologic Features and Treatment Response in Patients With Lung Cancer Harboring a RET Fusion. J Thorac Oncol 2023;18:576-86. [Crossref] [PubMed]
- Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Heymach JV, Harpole D, Reck M. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:287-8. Reply. [Crossref] [PubMed]
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]


