
Optimizing epidermal growth factor receptor-tyrosine kinase inhibitor treatment in lung cancer: a systematic review and meta-analysis of the influence of gastric acid suppressants
Highlight box
Key findings
• Concurrent use of gastric acid suppressants (GASs), such as proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs), and epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) was significantly associated with decreased overall survival (OS) and progression-free survival (PFS) in patients with lung cancer.
What is known and what is new?
• There is no recent evidence on the adverse effects of concurrent GAS treatment on the efficacy of EGFR-TKIs.
• GASs should be carefully prescribed to patients on EGFR-TKI treatment.
• If the use of GASs is unavoidable, measures, such as adjusting the timing of administration, minimizing the duration of overlap, or opting for H2RAs over PPIs are suggested to mitigate the negative effects on EGFR-TKI efficacy.
What is the implication, and what should change now?
• Drug interactions should be investigated to optimize lung cancer treatment strategies.
• There is an urgent need to identify the optimal timing for minimizing the risk of poor survival associated with concurrent use of GASs during EGFR-TKI treatment.
Introduction
Background
Despite advancements in treatment, lung cancer remains the leading cause of cancer-related mortality worldwide (1). The development of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) has substantially advanced the treatment landscape for late-stage non-small cell lung cancer (NSCLC) (2,3). Compared to traditional chemotherapy, EGFR-TKIs have superior efficacy and a more favorable adverse effect profile, particularly benefiting individuals with EGFR-activating mutations (4,5). This targeted approach represents a critical shift in treatment paradigms, offering new hope for patients with advanced NSCLC (6).
Rationale and knowledge gap
The efficacy of orally administered EGFR-TKIs can be influenced by factors affecting drug absorption, such as gastric acidity. Gefitinib and erlotinib, which are first-generation EGFR-TKIs, exhibit pH-dependent solubility and require an acidic environment for optimal absorption. However, osimertinib, a third-generation EGFR-TKI, is less affected by pH-dependent solubility (7). Therefore, the bioavailability of gefitinib and erlotinib can be altered by commonly used gastrointestinal protectants, such as proton pump inhibitors (PPIs) or histamine 2 receptor antagonists (H2RAs), whereas osimertinib remains relatively stable under these conditions.
Previous meta-analyses have reported adverse effects when gastric acid suppressants (GASs), such as PPIs and H2RAs, were used concomitantly with EGFR-TKIs (8-13). However, some time has passed since the publication of these studies, and there have also been reports from individual studies suggesting no association (14-17). This discrepancy has led to the need for an updated meta-analysis that includes newly published studies.
Objective
The objective was to conduct an updated systematic review and meta-analysis to assess the impact of concurrent GAS and EGFR-TKI treatment on overall survival (OS) and progression-free survival (PFS) in patients with lung cancer. We present this article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-537/rc).
Methods
Ethical considerations and protocol registration
Given that this study involved a systematic analysis using previously published data, informed consent was not required. The protocol for this review was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY®) database (registration No. INPLASY202450108).
Search strategy and study selection
PubMed, Embase, Cochrane Library, Web of Science, Scopus, KoreaMed, as well as preprint repositories for Embase, Web of Science, and Scopus, were searched on 8 February 2024 by EJK, a medical librarian. Boolean operators, such as AND, OR, and NOT, were employed to refine and expand the search results. Our search was limited to studies published in English, specifically targeting research involving human participants, without regional, racial, or sample size restrictions. The detailed search strategies are outlined in Table S1. Abstracts, case reports, reviews, editorials, commentaries, and practice guidelines were excluded. Furthermore, we scrutinized all cited references as an adjunctive method to identify relevant literature that aligned with our criteria. The eligibility of titles and abstracts was independently evaluated by two reviewers (B.K.K. and C.Y.K.). Full-text articles were then assessed to determine their suitability for inclusion in the analysis. Any disagreements were resolved through consensus discussions.
Inclusion and exclusion criteria
Studies were included in the analysis if they met the following inclusion criteria: focused on patients with lung cancer, provided OS or PFS data, and did not restrict the types of EGFR-TKIs and GASs used. The exclusion criteria were as follows: irrelevant publication types to the study objectives, study results that did not align with the scope of our analysis, and studies involving EGFR-TKIs use in other cancer types.
Data extraction and quality assessment
The included studies were independently reviewed by two investigators (B.K.K. and C.Y.K.), and data were collected using a pre-defined data extraction form. Information including author, publication year, study design, country of study, duration of study, number of participants, sex, age, smoking status, Eastern Cooperative Oncology Group performance status, cancer type, clinical stage, type of EGFR mutation, frequency and type of GASs used, type of TKIs administered, overlap time of GASs use, sites of metastasis, and adverse events, were recorded for each study whenever available.
Study quality was assessed using the Newcastle-Ottawa Scale (NOS). Briefly, the NOS is utilized to assess the quality of observational or cohort studies. Scores are assigned based on selection (0–4 points), comparability (0–2 points), and outcome (0–3 points), and higher total scores indicate higher methodological quality. Methodological quality is categorized as low (scores 0–3), moderate (scores 4–6), or high (scores 7–9).
Statistical analysis
To combine the hazard ratios (HRs) and their 95% confidence intervals (CIs) from individual studies for both PPIs and H2RAs, we used the following approach. The variance for each study was calculated using the HR and the 95% CI with the formula: variance = [log(upper limit) – log(lower limit)]/(2 × 𝑧)2, where 𝑧 is the Z-value corresponding to the desired confidence level (typically 1.96 for a 95% CI). Weights for each study were calculated based on the inverse of the variance, giving higher weights to studies with lower variance. Heterogeneity among the studies was assessed using the I2 and Q statistic with their respective P values. An I2 value greater than 50% or a P value from the Q statistic less than 0.1 was considered indicative of significant heterogeneity, in which case a random-effects model was used. In the absence of significant heterogeneity, a fixed-effect model was employed.
Sensitivity analyses were conducted by sequentially excluding each study to evaluate its impact on overall heterogeneity. Publication bias was assessed using funnel plot analysis. Post-hoc analyses were performed to evaluate the impact of different types of GASs (PPIs versus H2RAs) on OS and PFS. Additionally, the influence of GAS overlap time on OS and PFS was assessed. All statistical analyses were conducted using Review Manager 5.2 (RevMan 5.2; Cochrane Collaboration, Oxford, UK) and R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). Significance was defined as a two-sided P value of <0.05, except in the heterogeneity test, where a one-sided P value of <0.1 was used for the Q statistic, which followed a chi-squared distribution.
Results
Study selection and analysis overview
A total of 3,501 studies were identified. After excluding 1,410 duplicate studies, the remaining 2,091 studies were screened for relevance according to their titles and abstracts. During this process, 2,073 studies were excluded based on suitability criteria, leaving 18 studies as final candidates. After thoroughly reviewing the full texts of these selected studies, seven that did not meet the inclusion criteria were subsequently excluded. Moreover, two additional relevant studies were included after a reference review of the 11 studies. Finally, 13 studies were included in the analysis. However, two individual studies included two cohorts each, resulting in 15 cohorts included in the analysis. The study selection flowchart is shown in Figure 1. Most studies exhibited NOS scores ranging from 5 to 9, indicating moderate or high methodological quality (Table S2).

Characteristics of the included studies
The 13 included studies (14-26) were all of retrospective design and involved a total of 10,814 patients. Among them, 3,741 (34.6%) patients used GASs. Of the 13 studies, 8 were conducted in Asian populations, and 60.4% (6,537/10,814) of the patients were female. Information on smoking status was available in 10 studies, and 71.6% of the patients were non-smokers (6,344/8,866). Tissue examination results were available in seven studies, and adenocarcinoma accounted for 71.2% (1,303/1,829) of all diagnoses, consistent with the typical characteristics of lung cancer with common EGFR mutations. All studies included older individuals aged ≥60 years, and almost all (2,243/2,249, 99.7%) expressed common EGFR mutations (exon 19 deletion or L858R mutation). Both first-generation EGFR-TKIs (erlotinib and gefitinib) and second-generation EGFR-TKIs (afatinib and dacomitinib) were used, although most studies used first-generation agents. The overlap time between PPIs and TKIs varied widely. Common side effects, such as skin rash, diarrhea, and liver dysfunction, were frequently observed (Tables 1,2).
Table 1
| First author | Publication year | Design | Country/region | Study periods | Participants (GAS, %) | Females (%) | Age (years) | Smoking (never, %) |
|---|---|---|---|---|---|---|---|---|
| Chen | 2016 | Retrospective | Taiwan | Dec 2010–Dec 2013 | 269 (57, 21.2%) | 156 (58.0%) | 65.1±12.3 | 182 (67.7%) |
| Chu | 2015 | Retrospective | Canada | Jan 2007–Dec 2012 | 507 (124, 24.5%) | 272 (53.6%) | 64 [28–86] | N/A |
| Fang | 2019 | Retrospective | Taiwan | Jun 2011–Jun 2013 | 1,278 (309, 24.2%) | 827 (64.7%) | >65 (42.1%) | N/A |
| Hilton | 2013 | Retrospective | Global | N/A | 485 (190, 39.2%) | 172 (35.5%) | ≥60 (57.5%) | 104 (21.4%) |
| Ho | 2022 | Retrospective | Taiwan | 2014–2019 | 918 (330, 35.9%) | 536 (58.4%) | >65 (41.8%) | 783 (85.3%) |
| Kumarakulasinghe | 2016 | Retrospective | Singapore | Jan 2008–Dec 2013 | 157 (55, 35.0%) | 82 (52.2%) | GAS: 61.7±9.8; non-GAS: 62.0±10.8 |
104 (66.2%) |
| Kwok | 2020 | Retrospective | China | Jan 2010–Dec 2018 | 193 (61, 31.6%) | 138 (71.5%) | PPI: 71.9 [40–88]; H2RA: 73.3 [57–88]; non-GAS: 66.3 [35–92] |
156 (80.8%) |
| Lee, gefitinib cohort |
2022 | Retrospective | Taiwan | Jan 2010–Dec 2018 | 4,340 (1,498, 34.5%) | 2,875 (66.2%) | PPI: 68.7±12.4; H2RA: 68.9±12.5; non-GAS: 66.3±12.9 |
3,324 (76.6%) |
| Lee, erlotinib cohort |
2022 | Retrospective | Taiwan | Jan 2010–Dec 2018 | 1,635 (719, 44.0%) | 902 (55.2%) |
PPI: 68.1±10.8; H2RA: 66.7±11.5; non-GAS: 63.9±12.3 |
1,143 (69.9%) |
| Li, dacomitinib cohort | 2021 | Retrospective | Global | N/A | 235 (83, 35.3%) | 148 (63.0%) | Non-GAS: 60 [28–83]; PPI: 66 [36–87]; extensive PPI: 67 [37–81] |
150 (63.8%) |
| Li, gefitinib cohort |
2021 | Retrospective | Global | N/A | 229 (70, 30.6%) | 132 (57.6%) | Non-GAS: 60 [33–83]; PPI: 64 [35–86]; extensive PPI: 68 [35–79] |
149 (65.1%) |
| Saito | 2021 | Retrospective | Japan | Mar 2005–Dec 2014 | 87 (31. 35.6%) | 48 (55.2%) | Non-GAS: 64 [51–82]; H2RA: 61 [37–87] |
41 (47.1%) |
| Sedano | 2018 | Retrospective | Spain | Jan 2012–Dec 2015 | 163 (118, 72.4%) | 58 (35.6%) | 70 [39–89] | N/A |
| Zenke | 2016 | Retrospective | Japan | Jan 2008–Dec 2011 | 130 (47, 36.2%) | 82 (63.1%) | 64 [36–87] | 84 (64.6%) |
| Guo | 2020 | Retrospective | China | Jan 2016–Dec 2018 | 188 (49, 26.1%) | 109 (58.0%) | 61 [50–74] | 124 (66.0%) |
Categorical variables are presented as number and percentages, while continuous variables are described as mean and standard deviation, median and interquartile range, or medians and ranges. GAS, gastric acid suppressant; N/A, not applicable; PPI, proton pump inhibitor; H2RA, histamine 2 receptor antagonist.
Table 2
| First author | GAS used | GAS overlap time (%) | Pathology (ADC, %) | Clinical stage | EGFR status (common*, %) | EGFR-TKI used | ECOG PS (≥2, %) |
Side effects |
|---|---|---|---|---|---|---|---|---|
| Chen | PPI (18, 31.6%); H2RA (39, 68.4%) | >80% (56.1%); 51–80% (15.8%); 31–50% (28.1%) |
247 (91.8%) | N/A | 242, 90.0% | First generation | 52 (19.3%) | Skin rash (116, 43.1%); diarrhoea (33, 12.3%) |
| Chu | PPI (115, 92.7%); H2RA (9, 7.3%) | 100% (80.6%); 80–99% (4.0%); 60–79% (3.2%); 40–59% (2.4%); 20–39% (9.7%) |
318 (62.7%) | IIIB (89, 18%); IV (418, 82%) |
N/A | Erlotinib | 333 (65.7%) | Skin rash (301, 59.4%); diarrhoea (103. 20.3%) |
| Fang | PPI (309, 100%) | >20% (46.9%); ≤20% (53.1%) | N/A | N/A | N/A | Gefitinib | N/A | N/A |
| Hilton | N/A | N/A | 244 (50.3%) | N/A | N/A | Erlotinib | 167 (34.4%) | Skin rash (96, 19.8%); diarrhoea (53, 10.9%) |
| Ho | PPI (330, 100%) | N/A | N/A | N/A | 918, 100% | Afatinib | N/A | N/A |
| Kumarakulasinghe | N/A | ≥30% (100%) | N/A | N/A | N/A | Gefitinib, erlotinib | Karnofsky <90 (55, 35.0%); 90–100 (102, 65.0%) | N/A |
| Kwok | PPI (27, 44.3%); H2RA (34, 55.7%) | ≥75% (100%) | N/A | N/A | 193, 100% | Gefitinib | N/A | N/A |
| Lee, gefitinib cohort | PPI (604, 40.3%); H2RA (894, 59.7%) | 80–100% (34.3%); 50–79% (23.0%); 20–49% (42.7%) |
N/A | IIIB (178, 4.1%); IV (4,162, 95.9%) | N/A | Gefitinib | 1,058 (24.4%) | N/A |
| Lee, erlotinib cohort | PPI (293, 40.8%); H2RA (426, 59.2%) | 80–100% (32.3%); 50–79% (23.8%); 20–49% (43.9%) |
N/A | IIIB (55, 3.4%); IV (1,580, 96.6%) |
N/A | Erlotinib | 339 (20.7%) | N/A |
| Li, dacomitinib cohort | PPI (83, 100%) | 50–100% (62.7%); 0–49% (37.3%) |
N/A | N/A | 235, 100% | Dacomitinib | None | N/A |
| Li, gefitinib cohort | PPI (70, 100%) | 50–100% (54.3%); 0–49% (45.7%) |
N/A | N/A | 229, 100% | Gefitinib | None | N/A |
| Saito | H2RA (31, 100%) | N/A | 84 (96.6%) | N/A | 86, 98.9% | Gefitinib | 14 (16.1%) | Skin toxicity (64, 73.6%); diarrhoea (28, 32.2%); liver dysfunction (31, 35.6%) |
| Sedano | PPI (85, 72.0%); H2RA (33, 28.0%) | ≥20% (100%) | 97 (59.5%) | IIIA (8, 4.9%); IIIB (10, 6.1%); IV (145, 89.0%) |
42, 25.8%# | Gefitinib, erlotinib | 37 (22.7%) | TKI intolerance (13, 8.0%) |
| Zenke | PPI (27, 57.4%); H2RA (20, 42.6%) | N/A | 127 (97.7%) | IIIB (2, 1.5%); IV (107, 82.3%), others (21, 16.2%) | 124, 95.4% | Gefitinib, erlotinib | 20 (15.4%) | Skin rash (111, 85.4%); diarrhoea (40, 30.8%), liver dysfunction (22, 16.9%) |
| Guo | PPI (46, 93.9%); H2RA (3, 4.1%) | 76–100% (26.5%); 51–75% (36.7%); 26–50% (24.5%); 0–25% (12.2%) |
186 (98.9%) | IIIB (7, 3.7%); IV (181, 96.3%) |
174, 92.6% | Gefitinib | 9 (4.8%) | Skin rash (25, 13.3%); diarrhoea (11, 5.9%), liver dysfunction (17, 9.0%) |
Categorical variables are presented as numbers and percentages. *, common mutations refer to exon 19 deletions and exon 21 L858R mutations. #, the specific type of EGFR mutation could not be determined. “EGFR mutation-positive” indicates the number and proportion of cases with EGFR mutations. GAS, gastric acid suppressant; ADC, adenocarcinoma; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PPI, proton pump inhibitor; H2RA, histamine 2 receptor antagonist; N/A, not applicable.
OS and PFS
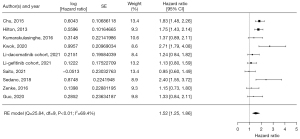
Data on OS were available in 11 studies comprising 15 cohorts and 10,463 patients. Among these patients, 3,574 (34.2%) and 6,889 (65.8%) did and did not receive concurrent GAS treatment, respectively. Concurrent use of EGFR-TKI and GAS significantly affected OS, with an HR of 1.34 (95% CI: 1.26–1.42) based on the fixed-effect model (I2=26.6%, P=0.18, Figure 2). Analysis of the funnel plot indicated a low likelihood of publication bias (Figure S1). Data on PFS were available in nine studies comprising 10 cohorts and 2,374 patients. In total, 828 (34.9%) and 1,546 patients (65.1%) did and did not receive concurrent GAS treatment, respectively. The meta-analysis showed a significant difference in PFS when EGFR-TKIs and GASs were administered concurrently, with an HR of 1.52 (95% CI: 1.25–1.86) (I2=69.4%, random effect model; P<0.01, Figure 3). Visual inspection of the funnel plot for PFS indicated asymmetry, suggesting potential publication bias. This implied that studies with non-significant or unfavorable results may be underrepresented, necessitating caution in interpreting the results (Figure S2).

Sensitivity analysis
To assess the robustness of our findings, we conducted sensitivity analyses by sequentially excluding each study to evaluate its impact on overall heterogeneity and the summary estimates of both OS and PFS. The results remained consistent, with the HR indicating that the exclusion of any single study did not significantly alter the overall effect estimate. This suggested that the observed association between the concurrent use of EGFR-TKIs and GASs and reduced OS and PFS was robust and not driven by any single study (Figures S3,S4).
Subgroup analysis
We conducted a subgroup analysis to examine the effect of PPIs and H2RAs concurrently used with EGFR-TKIs on OS and PFS. For OS, the PPI subgroup included four studies (five cohorts), and the H2RAs subgroup included three studies (four cohorts). The HR for OS was higher in the PPI subgroup than that in the H2RA subgroup [1.64 (95% CI: 1.51–1.79) vs. 1.11 (95% CI: 0.95–1.31)], indicating a more negative impact of PPIs on the effectiveness of EGFR-TKIs (I2=87.1%, random effect model; P<0.01, Figure S5). For PFS, the HRs were 1.66 (95% CI: 0.84–3.28) in the PPI subgroup and 1.48 (95% CI: 0.63–3.48) in the H2RA subgroup. Despite a trend towards a more negative impact in the PPI subgroup, these results were not statistically significant (I2=85.1%, random effect model; P<0.01, Figure S6).
Additionally, we performed a subgroup analysis to investigate the effect of the overlap time of GASs on OS. Studies with overlap information were categorized as follows: ≥0% overlap (three studies, three cohorts), ≥20% overlap (three studies, three cohorts), ≥30% overlap (two studies), and ≥75% overlap (one study). The results indicated an increasing HR trend with longer overlap times: 1.34 (95% CI: 1.13–1.60) for ≥0% overlap, 1.29 (95% CI: 1.19–1.40) for ≥20% overlap, 1.58 (95% CI: 1.12–2.24) for ≥30% overlap, and 2.00 (95% CI: 1.26–3.16) for ≥75% overlap (I2=10.8%, fixed effect model; P=0.34, Figure S7). However, for PFS, there was no significant correlation with overlap time (Figure S8).
Discussion
Key findings
There is no recent evidence on the adverse effects of concurrent GAS treatment on the efficacy of EGFR-TKIs. Based on our analysis of a total of 13 studies comprising 15 cohorts and involving 10,814 patients (3,741 and 7,073 patients who did and did not receive concurrent GASs, respectively), concurrent use of GASs and EGFR-TKIs was significantly associated with decreased OS and PFS in patients with lung cancer. These results underscore the importance of investigating drug interactions to optimize lung cancer treatment strategies. Further, they highlight the urgent need to identify the optimal timing for minimizing the risk of poor survival associated with concurrent use of GASs during EGFR-TKI treatment.
Strengths and limitations
Our study has several strengths. It includes multiple studies of moderate or high methodological quality. Further, considering the characteristics of the included populations, the study was conducted under conditions similar to real-world scenarios where EGFR-TKIs are used. Therefore, the results likely accurately reflect real-world data. However, there are also some limitations that need to be considered. First, all the studies included in the meta-analysis are based on observational study data, which poses potential confounding factors. Second, a significant portion of the research was conducted in Asian populations, necessitating caution when generalizing the results. However, given that EGFR mutations are common in lung cancer in Asia, this may be an unavoidable situation. Third, one study accounted for over 50% of the weight, which could mask the OS results. This indicates that the results of this particular study could disproportionately influence the meta-analysis outcome. Fourth, excluding studies in languages other than English might limit the generalizability of the meta-analysis results to non-English-speaking or low-income countries. Fifth, additional consideration of various confounding variables is necessary. For example, there is a possibility that patients using GASs may have more severe cancer pathology, which could negatively affect survival outcomes. Lastly, some of the included studies used early data on EGFR-TKIs, mostly first-generation EGFR-TKIs, potentially not reflecting the impact of the latest treatment methods or new EGFR-TKIs.
Comparison with similar research
Previous meta-analyses have also shown the adverse effects of GASs on the efficacy of EGFR-TKIs in various cancers. Indini et al. (8) conducted a meta-analysis of 16 retrospective studies with 372,418 patients and found that GAS use was linked to poorer OS (HR =1.31; 95% CI: 1.20–1.43) and PFS (HR =1.30; 95% CI: 1.07–1.57) in solid tumors treated with oral anti-cancer drugs, including EGFR-TKIs. Song et al. (9) evaluated 45,626 patients from seven randomized controlled trials and 18 observational studies. Their results showed that GAS use was associated with worse OS (HR =1.13; 95% CI: 1.05–1.21) and PFS (HR =1.64; 95% CI: 1.14–2.37) in patients with lung cancer on EGFR-TKIs, but not in patients with esophageal/gastric, colorectal, or kidney cancer. Du et al. (10) reviewed 12 studies (nine cohort and three case-control) on patients with NSCLC taking EGFR-TKIs and concluded that GAS use resulted in shorter OS (HR =1.50; 95% CI: 1.31–1.72) and PFS (HR =1.66; 95% CI: 1.40–1.98). Specifically, PPIs were linked to shorter OS (HR =1.56; 95% CI: 1.21–2.02).
Wei et al. (11) analyzed 14 retrospective studies involving 13,709 patients with advanced NSCLC and observed that PPIs were associated with worse OS (HR =1.35; 95% CI: 1.21–1.51) and PFS (HR =1.50; 95% CI: 1.25–1.80) in those receiving targeted therapy and immunotherapy. Xia et al. (12) evaluated 13 retrospective studies with 12,259 patients with NSCLC and showed that GAS use was associated with worse OS (HR =1.38; 95% CI: 1.19–1.61) and PFS (HR =1.57; 95% CI: 1.31–1.89). Sim et al. (13) analyzed 14 studies with 4,010 patients and found that EGFR-TKI users without GASs had better OS (HR =1.46; 95% CI: 1.27–1.72) and PFS (HR =1.63; 95% CI: 1.35–1.98). PPI use worsened survival outcomes, while H2RA use exerted no significant effect.
Overall, all six studies support the evidence of a negative impact of concomitant GAS therapy on survival outcomes in patients with cancer, particularly in those receiving EGFR-TKIs. However, there are some limitations to consider. Indini et al. (8) and Song et al. (9) included patients with various cancer types, introducing potential heterogeneity in treatment responses. Additionally, the studies by Du et al. (10) and Xia et al. (12) may have missed recent data, which could impact the overall conclusions and may affect the comprehensiveness of their analysis. Wei et al. (11) evaluated patients with lung cancer, but included treatments beyond EGFR-TKIs, such as programmed death-ligand 1 inhibitor, potentially confounding the results. Furthermore, in the study by Sim et al. (13), in addition to the lack of recent data, two Japanese-language articles without clear reference citations were included, introducing uncertainty regarding the quality and relevance of those studies to the overall analysis.
The current study analyzed 13 studies comprising 15 cohorts and 10,814 patients, and the studies had moderate quality at the least. Regarding the patient characteristics, the population was predominantly Asian females, and a majority were non-smokers and had common EGFR mutations associated with lung adenocarcinoma. This demographic profile reflects conditions similar to those in which EGFR-TKIs are typically used. Therefore, it can be argued that the adverse impact of concurrent use of EGFR-TKIs and GASs on the OS and PFS of patients with lung cancer, as observed in our study, holds particular significance. Additionally, subgroup analysis showed that compared to H2RAs, PPIs had a more negative impact on OS in EGFR-TKI-treated patients with lung cancer. Although the results were not significant, there was a trend towards a more negative impact on PFS with PPIs.
Despite the limited number of included studies, longer overlap times of GASs with EGFR-TKIs were associated with worse trends in OS outcomes, while no significant correlation was observed for PFS. These findings present important implications for prescribing EGFR-TKIs. PPIs are known to more effectively reduce gastric acidity than do H2RAs, and the efficacy of medication generally increases with longer treatment durations. Thus, it may be beneficial to consider using H2RAs instead of PPIs when GASs are necessary for patients on EGFR-TKIs. Moreover, prescribing GASs for the shortest duration possible could help minimize their negative effect. Further research is needed to confirm these observations and to develop optimized treatment strategies.
Explanation of findings
EGFR is a receptor protein that regulates cell growth and survival, and abnormal EGFR signaling is linked to cancers, especially NSCLC. Mutations in EGFR increase the sensitivity of NSCLC to EGFR-TKIs, improving treatment outcomes (27-29). EGFR-TKIs, such as erlotinib and gefitinib, require an acidic gastric environment for optimal absorption in preclinical settings (7). Recent human studies have demonstrated that both dacomitinib and erlotinib show significantly reduced absorption in the presence of PPIs (30,31). Therefore, the impact of GASs on the pharmacokinetics and pharmacodynamics of specific type of EGFR-TKIs in patients with lung cancer is a significant concern. Notably, the administration of ranitidine, an H2RA, to maintain an intragastric pH of >5.0 substantially reduced the area under the plasma concentration-time curve of gefitinib by 44%. This was further accompanied by a corresponding 70% decrease in maximum observed plasma concentration (32). These findings suggest that GASs can significantly impair the bioavailability of EGFR-TKIs, potentially leading to subtherapeutic drug levels and reduced clinical efficacy, which can negatively affect the treatment outcomes for lung cancer.
In the oral administration of TKIs, new drug-drug interactions related to gastrointestinal absorption have become evident when they are co-administered with PPIs, antacids, and other TKIs. In concurrent PPI and TKI treatment, the most common pharmacokinetic interactions involve absorption and metabolism by cytochrome P450 enzymes (33). The gastrointestinal absorption of a drug depends on its inherent characteristics, particularly solubility, but it can also be influenced by drug-drug interactions. These interactions predominantly occur with TKIs that have incomplete absorption. Key factors affecting the absorption of TKIs include changes in stomach pH due to coadministration of H2 antagonists, PPIs, or antacids and the inhibition of P-glycoprotein and intestinal CYP3A4 enzymes in enterocytes (34).
Although Afatinib is generally not known to interact with GASs (35), the observation of increased HR when Afatinib is used in combination with GASs, as seen in the study by Ho et al. (21), is notable. One possibility is the broader pharmacokinetic and pharmacodynamic interactions that are not yet fully understood. Additionally, patient-specific factors, such as comorbidities or concurrent medications, might have influenced the results. These findings suggest that while the primary mechanism of interaction for EGFR-TKIs, such as erlotinib and gefitinib is their pH-dependent solubility, other factors may also play a significant role in influencing treatment outcomes with Afatinib when GASs are involved. Further research is needed to clarify these potential interactions and their clinical implications.
Other mechanistic insights on drug interaction
In addition to the pH-dependent solubility changes, other mechanisms may contribute to the reduced efficacy of EGFR-TKIs when co-administered with GASs. A notable mechanism involves the interactions between the metabolites of PPIs and H2Ras, and EGFR-TKs. The metabolites can form disulfide bonds with cysteine residues at the ATP-binding site of EGFR-TKs, potentially inhibiting the binding of TKIs to these receptors, thus reducing their tumor-suppressive effects. Furthermore, prolonged use of GASs has been associated with alterations in gut microbiota, which may affect the metabolism and systemic absorption of TKIs. These microbiota changes can potentially lead to an accelerated metabolism of EGFR-TKIs, thus reducing drug concentration and effectiveness. Moreover, the chronic use of PPIs and H2RAs is linked to an increased risk of infections, such as Clostridioides difficile and aspiration pneumonia, which can complicate the overall clinical management of patients with NSCLC. These factors highlight the importance of careful management of GAS use in patients undergoing EGFR-TKI therapy to optimize therapeutic outcomes (36).
Differential impact of H2RAs and PPIs on EGFR-TKI therapy
Our study found that patients using H2RAs during EGFR-TKI therapy had a higher risk of death than those using PPIs. Uryu et al. (36) suggest several mechanisms for this difference. PPIs irreversibly inhibit the H+/K+ ATPase, leading to a sustained increase in gastric pH that reduces the solubility and absorption of EGFR-TKIs, such as erlotinib and gefitinib. In contrast, H2RAs result in more variable pH changes, causing inconsistent drug absorption and potentially reduced efficacy. PPIs also inhibit cytochrome P450 enzymes, such as CYP2C19 and CYP3A4, stabilizing drug plasma levels and reducing resistance. H2RAs allow more fluctuations in drug levels, which may promote resistance. The study indicates that patients using H2RAs might have different baseline characteristics or more severe comorbidities, contributing to higher mortality risk. Additionally, PPIs can alter gut microbiota, increasing infection risks, such as C. difficile, complicating treatment outcomes. These findings highlight the need to consider pharmacological mechanisms and patient-specific factors in assessing the impact of GASs on EGFR-TKI therapy, emphasizing further research to optimize treatment strategies.
Implications and actions needed
The oral absorption of TKIs is significantly altered by the concomitant use of acid-suppressive treatments. Ideally, the combination of these TKIs with H2 antagonists, PPIs, or antacids should be avoided. However, if such combinations are unavoidable, it is necessary to administer acid-suppressive drugs with sufficient time separation from TKIs to minimize interactions (37,38). In cases of suspected interactions, and when pharmacokinetic data are unavailable, physicians should weigh the available evidence, extrapolate pharmacokinetic data for the individual patient if possible, and closely monitor the patient response. Additionally, the second-generation and third-generation EGFR-TKIs, afatinib and osimertinib, are known to be less sensitive to changes in gastric pH. As such, they are less affected in absorption and efficacy when co-administered with acid-suppressive agents, making them effective treatment options for patients requiring acid suppression (35,39).
Conclusions
The concurrent use of GASs with EGFR-TKIs in patients with advanced NSCLC is associated with poorer OS and PFS. Given the negative impact on survival outcomes, GASs should be carefully prescribed to patients on EGFR-TKI treatment. If the use of GASs is unavoidable, measures such as adjusting the timing of administration, minimizing the duration of overlap, or opting for H2RAs over PPIs are suggested to mitigate the negative effects on EGFR-TKI efficacy. Further research is necessary to establish optimal strategies for managing acid suppression therapy in these patients, specifically addressing the duration of overlap time, to improve their overall treatment outcomes.
Acknowledgments
We extend our heartfelt appreciation to Eun-Ji Kang for her invaluable assistance in formulating the search strategy and conducting the systematic analysis for this meta-analysis. Additionally, we would like to thank Editage (www.editage.co.kr) for their support with English language editing.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-537/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-537/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-537/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Nan X, Xie C, Yu X, et al. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017;8:75712-26. [Crossref] [PubMed]
- Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol 2018;29:i3-9. [Crossref] [PubMed]
- Yang X, Yang K, Kuang K. The efficacy and safety of EGFR inhibitor monotherapy in non-small cell lung cancer: a systematic review. Curr Oncol Rep 2014;16:390. [Crossref] [PubMed]
- Zhao P, Zhen H, Zhao H, et al. Efficacy and safety of adjuvant EGFR-TKIs for resected non-small cell lung cancer: a systematic review and meta-analysis based on randomized control trials. BMC Cancer 2022;22:328. [Crossref] [PubMed]
- Riessk J. Shifting paradigms in non-small cell lung cancer: an evolving therapeutic landscape. Am J Manag Care 2013;19:s390-7. [PubMed]
- Yasumuro O, Uchida S, Kashiwagura Y, et al. Changes in gefitinib, erlotinib and osimertinib pharmacokinetics under various gastric pH levels following oral administration of omeprazole and vonoprazan in rats. Xenobiotica 2018;48:1106-12. [Crossref] [PubMed]
- Indini A, Petrelli F, Tomasello G, et al. Impact of Use of Gastric-Acid Suppressants and Oral Anti-Cancer Agents on Survival Outcomes: A Systematic Review and Meta-Analysis. Cancers (Basel) 2020;12:998. [Crossref] [PubMed]
- Song HJ, Rhew K, Lee YJ, et al. Acid-suppressive agents and survival outcomes in patients with cancer: a systematic review and meta-analysis. Int J Clin Oncol 2021;26:34-50. [Crossref] [PubMed]
- Du X, Liu W, Chen K, et al. Impact of the Gastric Acid Suppressant Use on the Safety and Effectiveness of EGFR-TKIs: A Systematic Review and Meta-Analysis. Front Pharmacol 2022;13:796538. [Crossref] [PubMed]
- Wei N, Zheng B, Que W, et al. The association between proton pump inhibitor use and systemic anti-tumour therapy on survival outcomes in patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. Br J Clin Pharmacol 2022;88:3052-63. [Crossref] [PubMed]
- Xia J, Zhu J, Li L, et al. Concomitant Gastric Acid Suppressants on the Survival of Patients with Non-Small-Cell Lung Cancer Treated with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Meta-Analysis. Int J Clin Pract 2022;2022:3102641. [Crossref] [PubMed]
- Sim W, Jain SR, Lim WH, et al. Interactions between epidermal growth factor receptor tyrosine kinase inhibitors and proton-pump inhibitors/histamine type-2 receptor antagonists in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2021;10:3567-81. [Crossref] [PubMed]
- Hilton JF, Tu D, Seymour L, et al. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer 2013;82:136-42. [Crossref] [PubMed]
- Kumarakulasinghe NB, Syn N, Soon YY, et al. EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: a retrospective database analysis of potential drug interaction. Oncotarget 2016;7:85542-50. [Crossref] [PubMed]
- Saito Y, Takekuma Y, Kobayashi M, et al. Impact of histamine type-2 receptor antagonists on the anticancer efficacy of gefitinib in patients with non-small cell lung cancer. Eur J Clin Pharmacol 2021;77:381-8. [Crossref] [PubMed]
- Zenke Y, Yoh K, Matsumoto S, et al. Clinical Impact of Gastric Acid-Suppressing Medication Use on the Efficacy of Erlotinib and Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer Harboring EGFR Mutations. Clin Lung Cancer 2016;17:412-8. [Crossref] [PubMed]
- Chen YM, Lai CH, Chang HC, et al. Antacid Use and De Novo Brain Metastases in Patients with Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Were Treated Using First-Line First-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PLoS One 2016;11:e0149722. [Crossref] [PubMed]
- Chu MP, Ghosh S, Chambers CR, et al. Gastric Acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clin Lung Cancer 2015;16:33-9. [Crossref] [PubMed]
- Fang YH, Yang YH, Hsieh MJ, et al. Concurrent proton-pump inhibitors increase risk of death for lung cancer patients receiving 1st-line gefitinib treatment - a nationwide population-based study. Cancer Manag Res 2019;11:8539-46. [Crossref] [PubMed]
- Ho MC, Chung YS, Lin YC, et al. Combination Use of First-Line Afatinib and Proton-Pump Inhibitors Reduces Overall Survival Among Patients with EGFFR Mutant Lung Cancer. Onco Targets Ther 2022;15:1573-82. [Crossref] [PubMed]
- Kwok CW, Man Ho CJ, Leung Lam CD, et al. Clinical Impact of Gastric Acid Suppressants Use on the Efficacy of Gefitinib in Patients with Advanced Adenocarcinoma of the Lung Harboring Common EGFR Mutations. Clinical Cancer Drugs 2020;7:57-61. [Crossref]
- Lee CH, Shen MC, Tsai MJ, et al. Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib. Sci Rep 2022;12:7002. [Crossref] [PubMed]
- Li J, Nickens D, Wilner K, et al. Evaluation of the Effect of Proton Pump Inhibitors on the Efficacy of Dacomitinib and Gefitinib in Patients with Advanced Non-Small Cell Lung Cancer and EGFR-Activating Mutations. Oncol Ther 2021;9:525-39. [Crossref] [PubMed]
- Nieves Sedano M, Manuel Caro Teller J, García Muñoz C, et al. Clinical impact of gastric acid suppressing medication on the effectiveness of tyrosine kinase inhibitors in lung cancer patients. J BUON 2018;23:647-53. [PubMed]
- Guo Z, Qiong D, Xuan Y, et al. Concomitant administration of gastric acid suppression might attenuates the clinical efficacy of gefitinib: a single cancer center retrospective study. Journal of Chinese Pharmaceutical Sciences 2020;29:192-8. [Crossref]
- Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006;366:2-16. [Crossref] [PubMed]
- Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol 2018;12:3-20. [Crossref] [PubMed]
- Bethune G, Bethune D, Ridgway N, et al. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis 2010;2:48-51. [PubMed]
- Ruiz-Garcia A, Masters JC, Mendes da Costa L, et al. Effect of food or proton pump inhibitor treatment on the bioavailability of dacomitinib in healthy volunteers. J Clin Pharmacol 2016;56:223-30. [Crossref] [PubMed]
- Veerman GDM, Hussaarts KGAM, Peric R, et al. Influence of Cow's Milk and Esomeprazole on the Absorption of Erlotinib: A Randomized, Crossover Pharmacokinetic Study in Lung Cancer Patients. Clin Pharmacokinet 2021;60:69-77. [Crossref] [PubMed]
- Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug-drug interactions. Cancer Treat Rev 2014;40:917-26. [Crossref] [PubMed]
- Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer 2006;6:546-58. [Crossref] [PubMed]
- van Leeuwen RW, van Gelder T, Mathijssen RH, et al. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 2014;15:e315-26. [Crossref] [PubMed]
- Veerman GDM, Hurkmans DP, Paats MS, et al. Influence of esomeprazole on the bioavailability of afatinib: A pharmacokinetic cross-over study in patients with non-small cell lung cancer. Biomed Pharmacother 2022;155:113695. [Crossref] [PubMed]
- Uryu K, Imamura Y, Shimoyama R, et al. Prognostic impact of concomitant pH-regulating drugs in patients with non-small cell lung cancer receiving epidermal growth factor receptor tyrosine kinase inhibitors: the Tokushukai REAl-world Data project 01-S1. Cancer Chemother Pharmacol 2024;94:197-208. [Crossref] [PubMed]
- Budha NR, Frymoyer A, Smelick GS, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 2012;92:203-13. [Crossref] [PubMed]
- European Medicines Agency. (2013). European public assessment reports: Assessment history and product information. Retrieved May 28, 2024. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124
- Hussaarts KGAM, Veerman GDM, Jansman FGA, et al. Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol 2019;11:1758835918818347. [Crossref] [PubMed]


