State of the art of radiotherapy
Combined treatment versus radiotherapy alone
Potentially curative treatment of unresectable stage III necessitates adequate locoregional control as well as control of the micrometastatic disease that is likely to be present in most patients. In the 1980s, the standard of care for locally advanced disease was RT alone, led to a median survival time of less than 10 months and 3-year survival rates below 10%. In the early 1990s, a phase III trial conducted by the Cancer and Leukemia Group B (CALGB) group (1) showed a survival advantage using sequential therapy with CT and RT (Table 1). The trial randomized patients with unresectable stage III and medically inoperable stage II NSCLC to receive two cycles of cisplatin and vinblastine over 5 weeks followed by RT to 60 Gy versus RT alone. The response rate was 56% for patients receiving chemotherapy and radiation compared with 43% for patients receiving radiation therapy alone; median survival times were 13.7 versus 9.6 months, respectively (P=0.0066). More importantly, there was a 17% survival rate at 5 years in the combined-modality therapy arm versus a 7% rate in the radiation therapy alone arm, with few patients experiencing relapse after 2 to 3 years of follow-up. These results were duplicated in a separate phase III run by the US Intergroup study, reported by Sause et al. (2).
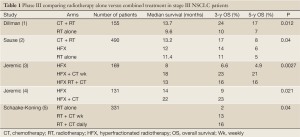
Full Table
Based on the radio sensitizer activity of most CT agent, particularly cisplatin and carboplatin, the concomitant administration of both modalities was also explored and compared with RT alone. Randomized data from Jeremic et al. (3,4) confirmed the clinical benefit of concurrent chemoradiotherapy compared with radiation alone by comparing hyperfractionated radiation therapy alone with hyperfractionated radiation therapy and different regimens of carboplatin/etoposide. The concurrent regimens significantly improved 3-year survival [23% vs. 6.6% (3), 23% vs. 9% (4)]. There was no significant reduction in the rate of distant metastasis with concurrent chemoradiation. Data reported by Shaake-Koning et al. (5) in a European Organization for Research and Treatment of Cancer (EORTC) three-arm trial evaluated 331 patients assigned to radiation alone (55 Gy), radiation with weekly cisplatin (30 mg/m2), or radiation with daily cisplatin (6 mg/m2). The addition of cisplatin to the thoracic radiation resulted in an improvement in overall survival compared with radiation alone. The 3-year survival rate for radiation alone was 2% compared with 13% for patients receiving radiation with weekly cisplatin and 16% for patients receiving radiation with daily cisplatin. In two-way comparisons, statistical significance was only achieved for the radiation and daily cisplatin arm (P=0.009). Although other trials have failed to show a survival advantage with concurrent chemoradiation therapy compared with radiation therapy alone, several meta-analyses, focused on adding platinum-containing chemotherapy either at systemic doses preceding or at low radiosensitizing dose concomitant with chest radiotherapy in patients with good performance and no significant weight loss, also showed a significantly improvement on the outcomes as compared with single modality chest radiotherapy with traditional dose and fractionation schedules (1.8-2.0 Gy per fraction per day to 60-70 Gy in 6-7 weeks). The first meta-analysis was published in 1995 (6). The patients treated with chemotherapy and radical radiotherapy experienced a 13% reduction in risk of death with an absolute survival benefit of 4% at 2 years. Studies in which radiotherapy and chemotherapy were given concurrently were specifically excluded from this analysis, so this benefit was observed for sequential chemoradiotherapy. In 2004, an individual patient data metaanalysis (7) was published comparing radiotherapy alone with concurrent chemoradiotherapy based on nine trials and 1,764 patients. It showed an absolute survival advantage of 4% at 2 years when combining radiation therapy with chemotherapy (HR 0.89; 95% CI, 0.81-0.98; P=0.02). Finally, the Cochrane review of 2004 (8) concluded that the addition of concurrent chemotherapy to radical radiation therapy reduced the risk of death at 2 years by 7% [relative risk (RR), 0.93; 95% CI, 0.88-0.98; P=0.01]. The risk of acute esophagitis (grade ≥3) is greater with concurrent treatment (RR, 1.58; 95% CI, 1.19-2.09; P=0.001), but there was no significant difference in the risk of acute pneumonitis. Thus, sequential and concomitant combined-modality strategies were each established to be superior to radiotherapy alone offering a 4% increase in absolute survival at 2 years and confirmed combined modality as the standard of care in the management of locally advanced inoperable NSCLC.
Sequential versus concomitant chemoradiation
The next generation of clinical trials investigated concurrent chemoradiation versus sequential approach, showing in two of three phase III studies favorable results to concomitant therapy (Table 2). The West Japan Lung Cancer Group conducted the first published trial about this topic (9). They randomized 320 patients to receive thoracic radiation (56 Gy, split-course) either after or concurrent with cisplatin (80 mg/m2), vindesine (3 mg/m2), and mitomycin (8 mg/m2) chemotherapy. Patients receiving concurrent therapy had a median survival time of 16.5 months compared with 13.3 months for the sequentially treated patients (P=0.03). The 5-year survival rate was also superior for the concurrently treated patients (15.8%) compared with patients receiving sequential therapy (8.9%). However, the study NPC 95-01 run by the Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie (10) did not achieve a statistical significance difference on survival between concomitant and sequential approaches (P=0.24). In this trial, 205 patients were randomized to receive either 3 cycles of cisplatin (120 mg/m2) and vinorelbine (30 mg/m2 weekly) followed by thoracic radiation at a dose of 66 Gy or concurrent therapy consisting of cisplatin (20 mg/m2) and etoposide (50 mg/m2) for two cycles along with thoracic radiation. Patients in the concurrent arm received 2 further cycles of consolidation chemotherapy that consisted of cisplatin (80 mg/m2) and vinorelbine (30 mg/m2 weekly) to match the total cisplatin dose given in the other arm. In spite of similar findings to the West Japan Lung Cancer Group trial, with numerical advantage for the concurrent therapy in median survival time (14.5 versus 16.3 months) and in the 2- and 4-year survival rates (26.5% and 14.2%, versus 39.3% and 20.7%, respectively), the trend toward prolonged survival with concomitant therapy did not achieve statistical significance. One possible explanation is the high number of toxic deaths in the concurrent arm comparing to the sequential one (10 versus 6). Finally, the third and largest randomized phase III trial comes from the Radiation Therapy Oncology Group (RTOG) (11). In RTOG 94-1012, 610 patients were randomly assigned to the following three treatment arms: once-daily radiation (60 Gy) after induction cisplatin (100 mg/m2) and vinblastine (5 mg/m2) chemotherapy; once-daily radiation [60] concurrent with the same chemotherapy; or hyperfractionated radiation (69.6 Gy) with concurrent cisplatin (50 mg/m2) and oral etoposide (50 mg twice daily). The median survival time was superior for patients receiving concurrent therapy with daily radiation (17.0 months) compared with patients receiving sequential treatment (14.6 months); this result was statistically significant (P=0.038). The overall 4-year survival rate was also better for patients on the concurrent arm compared with the sequential arm (21% vs. 12%, respectively). Several phase II studies and meta-analysis also supported the benefit of concomitant over sequential therapy. A systematic review and individual patient data meta-analysis including 1,205 patients conducted by the NSCLC Collaborative Group (12) confirmed a significant benefit of concomitant therapy on overall survival (HR, 0.84; 95% CI, 0.74 to 0.95; P=0.004), with an absolute benefit of 5.7% (from 18.1% to 23.8%) at 3 years and 4.5% at 5 years. Notably, although rates of distant failures were equivalent (HR, 1.04; 95% CI, 0.86 to 1.25; P=0.69), concomitant treatment decreased locoregional progression (HR, 0.77; 95% CI, 0.62 to 0.95; P=0.01). Concomitant therapy also increased acute esophageal toxicity (grade 3-4) from 4% to 18% with a relative risk of 4.9 (95% CI, 3.1 to 7.8; P=0.001) but not acute pulmonary toxicity. Authors concluded that concomitant therapy improved survival of patients with locally advanced NSCLC, primarily because of a better locoregional control, but at the cost of manageable increased acute esophageal toxicity.

Full Table
Chemoradiation plus induction or consolidation strategies
In spite of the advantage in survival demonstrated by the use of concomitant CT and RT, both locoregional and distant failure remain a problem. Following treatment with chemoradiotherapy, 70% to 75% of patients develop recurrent or progressive disease; roughly one third of patients fail in the radiation field (local failure), one third of patients fail outside the irradiated field (distal failure) and one third of patients fail both locally and distally. Based on this, several trials were focused in adding more CT as induction or consolidation strategies to concomitant therapy.
The Cancer and Leukemia Group B (CALGB) conducted several studies focused on the induction strategy. They published in 2002 (13) the results of a randomized phase II trial comparing efficacy and toxicities of three regimens in which patients were randomized to receive one of these three agents (paclitaxel, gemcitabine, or vinorelbine) in combination with cisplatin for two cycles as induction chemotherapy followed by two additional cycles of these drugs with concurrent standard chest radiotherapy. They postulated that given the encouraging activity of these agents, in the stage III setting might lead to further prolongation of survival times. In addition, all three agents have been demonstrated to act as radiation sensitizers in preclinical models. The primary end points were response to both induction and concomitant chemoradiotherapy. One hundred eighty seven patients were accrued. Total response rates to induction chemotherapy on the three study arms were 40%, 33%, and 44% (gemcitabine, paclitaxel, and vinorelbine) and best overall response rates were 74%, 67%, and 73% with overlapping 95% CIs. The most common toxicities to induction chemotherapy were grade 3 or 4 granulocytopenia on all three arms (observed in approximately 50% of patients) and 25% grade 3 or 4 thrombocytopenia on the gemcitabine arm. However, there were notable differences among the three study arms in the toxicities during concomitant chemoradiotherapy. Grade 3 or 4 granulocytopenia was seen in 51% of patients treated with gemcitabine and 53% of patients treated with paclitaxel, which contrasts with 27% of patients treated with vinorelbine. In addition, thrombocytopenia was seen in 56% of patients on the gemcitabine arm. Grade 3 or 4 esophagitis was most pronounced on the gemcitabine arm (35% of patients grade 3 and 17% of patients grade 4) whereas these numbers were 35% and 4% for paclitaxel and 13% and 12% for vinorelbine. Overall median survival time for all patients was 17 months. For the three study arms, median survival times and 3-year survival rates were 18.3, 14.8 and 17.7 months and 28%, 19% and 23% for gemcitabine, paclitaxel and vinorelbine, respectively. Based on its widespread acceptance by oncologists and general good tolerance they chose carboplatin and paclitaxel as a chemotherapy regimen for the subsequent phase III trial, the CALGB 39-801 (14) study. The primary endpoint was to detect a 40% increase in median survival, from 13 to 18.2 months, with the addition of induction chemotherapy. Three hundred sixty-six patients were randomly assigned to immediate concurrent chemoradiotherapy with weekly carboplatin and paclitaxel during 66 Gy of chest radiotherapy, or induction CT with two cycles of carboplatin and paclitaxel administered every 21 days followed by identical chemoradiotherapy. The study was negative because survival differences were not statistically significant, with a median survival on concomitant arm of 12 versus 14 months on induction CT arm and a 2-year survival of 29% and 31% respectively. However, the toxicity, mainly of neutropenia grade 3 or 4 was superior in the induction CT arm (18% and 20%, respectively). Remarkably, the survival times were at the lower range of reported values for patients with stage III disease treated with concomitant chemoradiotherapy even after adjusting for prognostic factors such as the weight loss. Possible explanations could be the selection of a weekly regimen of CT during RT treatment and/or the use of a carboplatin-based regimen instead of a cisplatin-based one. In any case, this study demonstrated the absence of value to adding induction CT with currently established agents.
Testing the hypothesis of consolidation CT the Southwest Oncology Group (SWOG) run two consecutive phase II studies. In the first one (SWOG-9019) (15), published in 2002, all patients received cisplatin, 50 mg/m2/d on days 1, 8, 29, and 36; etoposide, 50 mg/m2/d on days 1 to 5 and 29 to 33; and RT, 1.8 Gy per day, 5 days a week, starting within 24 hours of the first day of chemotherapy followed by two cycles of the same CT regimen. Fifty eligible patients were accrued. Grade 4 neutropenia was the most common toxicity (32%). Grade 3/4 esophagitis occurred in 12% and 8%. Median follow-up was 52 months, and overall median survival was 15 months and 3- and 5-year survivals were 17% and 15%. The second phase II study, S9504 (16), was designed to test the concept of taxane sequencing in combined-modality therapy and patients were selected using identical eligibility, staging criteria, and treatment, excepting docetaxel consolidation that those of the predecessor study (S9019). The primary objective was to estimate, within the limitations of a historical comparison, whether substitution of docetaxel for continued PE during the consolidation phase of treatment would improve survival compared with the predecessor trial S9019, and whether toxicities were acceptable. A sample size of 80 eligible patients with stage IIIB disease confirmed on central review was required to demonstrate a 6 months increase in median survival compared with that observed in S9019. Concurrent chemoradiotherapy was generally well tolerated, but two patients died from probable radiation-associated pneumonitis. The esophagitis rate was 17% (20% in S9019). Neutropenia during consolidation docetaxel was common (57% with grade 4). At a median follow-up of 71 months, the median progression free survival was 16 months and the median survival 26 months. Overall survival at 3, 4, and 5 years was 37%, 29%, and 29%, respectively. Although the survival results were provocative, particularly the long-term results updated in a second paper (17), confirmation and validation using a phase III design was necessary. Regrettably, when that study was run18, the results did not confirm the survival advantage of using three cycles of docetaxel after concomitant CT/RT versus concomitant CT/RT alone. The Hoosier Oncology Group (18) randomized patients to receive three cycles of docetaxel versus observation after finishing without progression concomitant chemoradiation with the same regimen than previous SWOG phase II studies. The primary objective was overall survival. Based on a data and safety monitoring board recommendation, the trial was closed after an analysis of the initial 203 patients. The grade 3-5 toxicities were clearly superior in the docetaxel arm (febrile neutropenia 10.9%, pneumonitis 9.6%, 5.5% died) as well as the percentage of patients hospitalized (28.8% during docetaxel versus 8.1% in observation arm). Although the MST for all patients was extremely good (21.7 months), no statically differences were found between docetaxel and observation arms (21.2 and 23.2 months, respectively). An update in survival was published (19), adding a retrospective analysis of efficacy and toxicity in older patients included in the trial. The 3-, 4-, and 5-year survival rates for the overall study were 30.7%, 18.0%, and 13.9%, respectively, without differences between docetaxel and observation. Older patients had similar MST but higher rates of grade 3/4 toxicity and hospitalization during induction.
Direct comparison between induction and consolidation strategies
Four randomized phase II study and an early closed phase III trials were focused on directly compared combined chemoradiation plus full doses of CT previously or at the end of concomitant therapy (Table 3). Belani and cols (20) published in 2005 a phase II randomized noncomparative trial conducted to determine the optimal sequencing and integration of paclitaxel/carboplatin with radiotherapy in stage III NSCLC patients. Survival data were compared with historical standard sequential chemoradiotherapy data from the RTOG. Patients received two cycles of induction paclitaxel/carboplatin every 21 days followed by RT (arm 1, sequential) or two cycles of induction paclitaxel/carboplatin followed by weekly paclitaxel/carboplatin with concurrent RT (arm 2, induction/concurrent), or weekly paclitaxel/carboplatin/RT followed by two cycles of full doses of paclitaxel/carboplatin (arm 3, concurrent/consolidation). The primary objective was survival. For analyses, each arm was compared with a historical control using the sequential chemoradiotherapy arm of the RTOG 88-08 trial, for which the available reported median survival time was 13.7 months. The final number of patients enrolled was 276. According to the paper, when the accrual to the phase II study reached the projected number of patients, an interim statistical analysis using the triangle test was applied to all three arms. Arm 2 was closed to accrual due to the low likelihood of benefit compared with historical control. Sample sizes in arms 1 and 3 were expanded to accommodate a phase III design. Subsequently, when data from the RTOG 9410 study became available and confirmed the benefit of concurrent therapy, accrual decreased and the study was permanently closed to accrual. Although the study was not designed to directly compassion among arms, the final results were favorable to consolidation arm with median overall survival of 13.0, 12.7, and 16.3 months for arms 1, 2, and 3, respectively. The most frequent grade 3-4 toxicity during induction chemotherapy was granulocytopenia (32% and 38% of patients on study arms 1 and 2, respectively) and the most common locoregional grade 3/4 toxicity during and after RT was esophagitis, as expected more pronounced with concomitant therapy (arms 2 and 3).
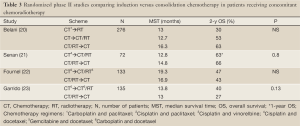
Full Table
Three European studies have been also designed to compare face-to-face induction and consolidation strategies but using a phase II approach. The Pulmon Art (21) was a multicenter trial conducted in 15 centers from 8 European countries designed to examine the safety and toxicity profile of two sequences cisplatin-docetaxel, either as induction before or consolidation after concurrent CT-RT with radiosensitizing doses of the same doublet in order to identify the most feasible regimen for further studies. The primary end point was the incidence of grade ≥3 esophagitis in the two treatment sequences. They estimated that the maximal rate considered acceptable by clinicians was 25%. Seventy-two patients (36 patients each arm in the intent to treatment design) were randomly allocated but 5 patients were switched from consolidation to induction arm due to higher V20 than permitted. The safety population consisted of 41 patients treated in the induction arm and 29 in the consolidation one. Adverse events that were grade ≥3 were reported for 63% of patients and 72%, respectively. The incidence of grade ≥3 esophagitis was not significantly different from the allowable incidence of 25% (Grade 3-4 in 32% and 2% in the induction arm and 21% and 3%, in the consolidation arm). A total of 18 patients developed grade ≥2 radiation pneumonitis but no significant correlation was observed between V20 and incidence of grades 2-5 pneumonitis. The authors did not find differences in overall response rate, overall survival (with a median OS of all eligible patients was 28.0 months) or progression free survival between arms. In spite of the selection of patients, 26% discontinued treatment prematurely and only 55-57% received the planned RT dose of 66 Gy.
A French multicenter phase II study included 133 patients in 35 centers (22). It compared 3 cycles of cisplatin every 21 days and reduced vinorelbine doses on day 1 and 8 concomitant with RT plus two cycles of cisplatin and paclitaxel as induction or consolidation. The primary objective was response rate at the end of treatment. Toxicities and response rates are similar in both arms, but induction followed by CT/RT appears to provide a better therapeutic outcome with median survival time of 19.3 versus 16.9 months and 2-year survival rates of 47% versus 43% (induction and consolidation, respectively).
Finally, the SLCG 0008 study conducted by the Spanish Lung Cancer Group (23) initially compared three arms (sequential CT followed by thoracic radiation; concurrent CT/TRT followed by consolidation CT and induction CT followed by concurrent CT/RT). However, based on the preliminary results of the RTOG 9410 trial published at that time, the sequential arm was closed with only 19 patients enrolled. The study continued comparing concomitant arms plus induction or consolidation CT and results of 135 patients from 16 Spanish university hospitals were available. The full dose regimen selected was a non-platinum schema (docetaxel and gemcitabine) based on an expected better tolerability profile. Weekly docetaxel and carboplatin was chosen to receive in combination with RT (60 Gy). The primary endpoint was response rate, with no statistically differences founded between the two arms (56% consolidation and 57% induction). Hematological toxicity was mild but significantly superior with consolidation CT; the esophagitis rate was similar in both arms (16% and 15%). With a median follow-up of 57 months, no statistically significant differences were found between consolidation and induction arm in median survival (13 and 13.8 months) or long-term survival (2-y OS 27% vs. 40%, 5-y OS 16% vs. 22%). Based on the modest results founded in median survival time and similar toxicities to other platinum regimen, authors concluded that this regimen cannot be recommended as an alternative to platinum-based CT/TRT. A phase III study was designed to directly compare both strategies using a triplet combination of CT with cisplatin, gemcitabine and vinorelbine but it was prematurely closed for poor accrual due to administrative problems (24). To clarify this question a meta-analysis of the pooled data of the phase II studies should be addressed since they are very similar in design and patient selection.
Studies comparing second and third generation chemotherapy agents
Clinical research efforts have focused on incorporating newer chemotherapeutic agents, either singly or in combination with a platinum compound, into concurrent chemoradiation regimens for locally advanced NSCLC. A large number of pilot studies have been reported, many of which have shown encouraging results. However, for many newer chemotherapeutic agents, dose-limiting toxicities require that lower doses be given during the concurrent phase. Two Japanese phase III studies addressed this topic, being both published in 2010. The Okayama Lung Cancer Study Group run a phase III (OLCSG 0007) (25) comparing the West Japan Lung Cancer Group regimen (cisplatin, vindesine, and mitomycin) with docetaxel and cisplatin administered on a day 1 and 8 regimen for two cycles plus RT, which was not administered in split course in any arms. The primary endpoint was the survival time at 2 years considering on the basis of previous report of an approximately 35% 2-year survival rate for the MVP arm and 55% for the DP arm. Based on this analysis, 96 patients in each arm were required. According to the results, the study was negative because the difference on survival at 2 years did not reached statistical significance (P=0.059) although was numerically superior (78.8% versus 70.3%). Similarly, although the response rate, median survival time, and progression free survival rates tended to be greater in the DP arm than in the MVP arm, the differences were not statistically significant (P>0.05). Authors remarked unpredictably better survival in the MVP arm, possibly related to a better selection of patients as well as the use of a non split course of RT. Based on this, the sample size was small to detect survival differences.
The West Japan Oncology Group conducted other phase III trial (WJTOG0105) with 3 arms (26). Treatment was composed of concurrent chemoradiotherapy and subsequent consolidation chemotherapy. Patients enrolled on arm A received 4 cycles of the MVP regimen. On day 2 of chemotherapy, RT was begun at the dose of 2 Gy/fraction given in 15 fractions over 3 weeks, followed by a rest period of 1 week. Subsequently, radiation was again resumed at the dose of 2 Gy/fraction given in 15 fractions over 3 weeks. The total dose of radiation administered was 60 Gy. In arms B patients received weekly irinotecan and carboplatin during RT followed by two cycles of full dose of both agents. RT was initiated on day 1. The total dose of 60 Gy was given in 30 fractions over a 6-week period. Finally, patients in the arm C were allocated the same schema but irinotecan was substituted by paclitaxel. The primary end point was comparison of the overall survival between the control group (arm A, with an estimated median OS time of 16.5 months) and each of the treatment groups (arm B or C, that would show an increase in the median OS to 20.5 months). A total of 456 patients were registered in a period of 4 years [2001-2005]. Regarding the toxicity, the incidences of grade 3 or worse severe hematologic toxicity, infection, febrile neutropenia, and gastrointestinal toxicity were significantly higher in arm A than in arm B or C. There were no statistically significant differences in the incidences of esophagitis, dyspnea, or pneumonitis. Similarly to the previous study, the differences in survival were not statically significant (arm A vs. B, P=0.392; arm A vs. C, P =0.876) with median survival time and 3- and 5-year survival rates of 20.5 months, 35.3%, and 17.5% in arm A, 19.8 months, 24.2%, and 17.8% in arm B, and 22.0 months, 26.4%, and 19.5% in arm C. The authors emphasized the more favorable profile of arm C (paclitaxel/carboplatin) to justify their conclusion about that regimen should be considered standard.
Finally, a small trial bi-centric phase II trial designed to assess the activity and safety of weekly paclitaxel-carboplatin versus cisplatin-etoposide (PE) and RT has been recently published (27). Consolidation treatment was delivered as per local protocol considering either platinum-based doublet chemotherapy regimen or single agent chemotherapy regimen both acceptable. The primary endpoint of this trial was 3-year overall survival but only 35 patients in each arm were considerer needed based on an assumption of differences in 3-year survival between 35% for PE regimen and 18% for the weekly paclitaxel/carboplatin schema. The results in terms of median survival time were favorable to PE (20.2 versus 13.5 months) in the PC arm. The 3-year survival rates were 33.1% and 13%, respectively. By contrary, the incidence of Grade 3-4 neutropenia was higher in the PE arm than that in the PC arm (78.1% vs. 51.5%, P=0.049). Once again, the total failure, locoregional relapses, and distant metastases were high in both arms (57.6%, 33.3%, and 33.3% in the PE arm and 78.1%, 46.9%, and 40.7% in the PC arm), highlighting the need to explore new strategies.
New chemotherapy agents
Pemetrexed, a multitargeted antifolate active in advanced non-squamous NSCLC patients have been also tested in stage III. Several phase I studies (28-30) founded that it was feasible to combine pemetrexed/carboplatin or cisplatin at full dose with RT. In addition, phase II (31,32) results showed promising results compared with historical studies, but these results needed to be confirmed in larger trials. Regrettably, the phase III trial comparing the combination of pemetrexed, cisplatin with cisplatin, etoposide concomitant with RT in patients with nonsquamous NSCLC stopped the accrual on September 2012 because the experimental arm has crossed the futility boundary and it is unlike to attend the HR of 0.74 in favor of the pemetrexed arm.
Molecular targeted agents
In non-selected population different types of agents have been evaluated in stage III NSCLC. The SWOG investigated the use of gefitinib as maintenance after maximum cytoreduction with chemoradiotherapy in the phase II study S0023 (33). All patients received cisplatin, etoposide concomitant with radiotherapy followed by 3 cycles of docetaxel. Patients whose disease did not progress were randomly assigned to gefitinib 250 mg/d or placebo until disease progression, intolerable toxicity, or the end of 5 years. The planned sample size was 672 patients to confer power of 0.89 to detect a 33% increase over the expected median survival time of 21 months. However, an unplanned interim analysis rejected the alternative hypothesis of improved survival at the P=0.0015 level for 243 randomly assigned patients and the study was closed. The median survival time was 23 months for gefitinib and 35 months for placebo (P=0.013). Although the reasons for this result remain unclear, it was established that routine use of maintenance EGFR-TKIs in stage III disease outside of a clinical trial should be avoided.
Cetuximab has been also tested in several studies in the stage III setting (34). Blumenschein and colleagues reported a median survival of 22.7 months and 50% 2-year survival in RTOG 0324 (35), adding weekly cetuximab to low-dose weekly paclitaxel-carboplatin with RT, followed by consolidation cetuximab-paclitaxel-carboplatin. On the basis of these results, an intergroup phase III trial (RTOG 0617) was designed to test radiation with carboplatin and paclitaxel, with or without cetuximab. However, on June 2011, two of the four arms in the protocol were closed to accrual when a planned interim analysis showed that the higher radiation dose being tested, 74 Gy, could not produce an overall survival benefit compared with the lower, standard dose of 60 Gy (36). Although data are immature, treatment-related toxicities were not significantly different (37), and differences in local versus distant disease failure have not been reported. The 60 Gy control and cetuximab arms of the study are currently ongoing.
Antiangiogenic therapy has been also tested in stage III. Unfortunately, to date, phase II trials of bevacizumab combined with platinum-based chemoradiotherapy were closed early because of an excess risk of hemorrhage and tracheoesophageal fistulas (38).
Limited data with molecular targeted agents are available in selected population such as the EGFR mutated patients but several phase II studies are ongoing (39-42) (Table 4).

Full Table
Future directions
Unfortunately, despite much effort during the last 20 years, we have witnessed little progress in treating unresectable stage III NSCLC. Treatment failures continue to occur both locoregionally and/or distantly, although radiographic evidence of locoregional failures only account for approximately one third of recurrences, suggesting the urgent need for more adequate systemic control. Similarly, novel approaches to improve radiation therapy delivery are needed. Strategies such as intensity-modulated radiotherapy, which enhances the radiation oncologist’s ability to contour radiation doses around a tumor with selective sparing of adjacent structures, and proton therapy are being investigated but caution should be used when interpreting the results of the trials exploring these modalities due to selection biases inherent in phase II studies and the lack of level 1 evidence. Therefore, outside the context of clinical trials, these techniques cannot be recommended as a standard alternative. Finally, it should be pointed out that therapeutic advances will likely come from a greater understanding of tumor biology and optimal patient selection. Improving our understanding of molecular subtypes will hopefully lead to rational drug design and more precise clinical trial questions. Only through active partnerships between patients and their healthcare providers to enroll patients in appropriate clinical trials will we see significant improvements in outcomes in our patients in a near future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210-5. [PubMed]
- Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [PubMed]
- Jeremic B, Shibamoto Y, Acimovic L, et al. Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non-small-cell lung cancer. J Clin Oncol 1995;13:452-8. [PubMed]
- Jeremic B, Shibamoto Y, Acimovic L, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small-cell lung cancer: a randomized study. J Clin Oncol 1996;14:1065-70. [PubMed]
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 1992;326:524-30. [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [PubMed]
- Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473-83. [PubMed]
- Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004;CD002140. [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [PubMed]
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7. [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- Vokes EE, Herndon JE, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol 2002;20:4191-8. [PubMed]
- Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007;25:1698-704. [PubMed]
- Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 2002;20:3454-60. [PubMed]
- Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol 2003;21:2004-10. [PubMed]
- Gandara DR, Chansky K, Albain KS, et al. Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small-cell lung cancer: a phase II Southwest Oncology Group Study (S9504). Clin Lung Cancer 2006;8:116-21. [PubMed]
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26:5755-60. [PubMed]
- Jalal SI, Riggs HD, Melnyk A, et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol 2012;23:1730-8. [PubMed]
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005;23:5883-91. [PubMed]
- Senan S, Cardenal F, Vansteenkiste J, et al. A randomized phase II study comparing induction or consolidation chemotherapy with cisplatin-docetaxel, plus radical concurrent chemoradiotherapy with cisplatin-docetaxel, in patients with unresectable locally advanced non-small-cell lung cancer. Ann Oncol 2011;22:553-8. [PubMed]
- Fournel P, Vergnenégre A, Robinet G, et al. Induction (ICT) or consolidation chemotherapy (CT) with cisplatin (C) and paclitaxel (P) plus concurrent chemo-radiation (CT/TRT) with cisplatin and vinorelbine (V) for unresectable non-small cell lung cancer (NSCLC) patients (pts): Randomized phase II trial GFPC-GLOT-IFCT 02-01. J Clin Oncol 2006;24:abstr 7048.
- Garrido P, Rosell R, Massutí B, et al. Predictors of long-term survival in patients with lung cancer included in the randomized Spanish Lung Cancer Group 0008 phase II trial using concomitant chemoradiation with docetaxel and carboplatin plus induction or consolidation chemotherapy. Clin Lung Cancer 2009;10:180-6. [PubMed]
- Berghmans T, Van Houtte P, Paesmans M, et al. A phase III randomised study comparing concomitant radiochemotherapy as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2009;64:187-93. [PubMed]
- Segawa Y, Kiura K, Takigawa N, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol 2010;28:3299-306. [PubMed]
- Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol 2010;28:3739-45. [PubMed]
- Wang L, Wu S, Ou G, et al. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 2012;77:89-96. [PubMed]
- Brade A, Bezjak A, MacRae R, et al. Phase I trial of radiation with concurrent and consolidation pemetrexed and cisplatin in patients with unresectable stage IIIA/B non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1395-401. [PubMed]
- Cardenal F, Arnaiz MD, Morán T, et al. Phase I study of concurrent chemoradiation with pemetrexed and cisplatin followed by consolidation pemetrexed for patients with unresectable stage III non-small cell lung cancer. Lung Cancer 2011;74:69-74. [PubMed]
- Li BS, Gong HY, Huang W, et al. Phase I study of pemetrexed, cisplatin, and concurrent radiotherapy in patients with locally advanced non-small cell lung cancer. Am J Clin Oncol 2012;35:115-9. [PubMed]
- Gadgeel SM, Ruckdeschel JC, Patel BB, et al. Phase II study of pemetrexed and cisplatin, with chest radiotherapy followed by docetaxel in patients with stage III non-small cell lung cancer. J Thorac Oncol 2011;6:927-33. [PubMed]
- Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011;29:3120-5. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Nieder C, Pawinski A, Dalhaug A, et al. A review of clinical trials of cetuximab combined with radiotherapy for non-small cell lung cancer. Radiat Oncol 2012;7:3. [PubMed]
- Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8. [PubMed]
- Bradley J, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy +/- cetuximab for stage IIIa/IIIb non-small cell lung cancer: Preliminary findings on radiation dose in RTOG 0617. The 53rd Annual Meeting of the American Society of Radiation Oncology, October 2-6, 2011, Miami, FL.
- Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys 2012;82:1042-4. [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [PubMed]
- Raben D, Bunn PA. Biologically targeted therapies plus chemotherapy plus radiotherapy in stage III non-small-cell lung cancer: a case of the Icarus syndrome? J Clin Oncol 2012;30:3909-12. [PubMed]
- Rengan R, Maity AM, Stevenson JP, et al. New strategies in non-small cell lung cancer: improving outcomes in chemoradiotherapy for locally advanced disease. Clin Cancer Res 2011;17:4192-9. [PubMed]
- Stinchcombe TE, Bogart JA. Novel approaches of chemoradiotherapy in unresectable stage IIIA and stage IIIB non-small cell lung cancer. Oncologist 2012;17:682-93. [PubMed]
- Ausborn NL, Le QT, Bradley JD, et al. Molecular profiling to optimize treatment in non-small cell lung cancer: a review of potential molecular targets for radiation therapy by the translational research program of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 2012;83:e453-64. [PubMed]


