Are immune checkpoint blockade monoclonal antibodies active against CNS metastases from NSCLC?—current evidence and future perspectives
Introduction
Lung cancer remains the leading cause of cancer mortality in men and women worldwide (1). Non-small cell lung cancer (NSCLC) is the most common subtype accounting for approximately 85% of all lung cancers (2). The 5-year survival in unselected NSCLC at all stages of diagnosis remains less than 20% and for stage IV disease is less than 5% (3,4). In advanced NSCLC, testing for distinct molecular genotypes has led to a personalized approach to treatment, which has improved outcomes when compared to standard platinum chemotherapy (5-13). Maintenance chemotherapy and other targeted agents have had a modest impact on survival (14-16). Immune checkpoint inhibitors (ICIs) are negative regulators of T cells and include anti cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibodies and anti-programmed cell death-1 (anti-PD-1)/programmed cell death receptor ligand-1 (PD-L1) antibodies. These drugs have demonstrated efficacy in NSCLC, melanoma and renal cell cancer, three cancer types with a predilection to brain metastases. Approximately 30–50% of patients with NSCLC can expect to develop CNS disease at some point (17,18). The number of patients with brain metastases is rising and can be explained by the more frequent use of sensitive imaging techniques such as magnetic resonance incidence (MRI) and by the improved survival seen in patients owing to new systemic treatments (19,20). Patients with molecular subtypes such as epidermal growth factor receptor positive (EGFR+) and anaplastic lymphoma kinase positive (ALK+) lung cancers may have an increased risk of CNS disease at diagnosis compared with EGFR/ALK wild-type (WT) NSCLC however this risk may also be explained by a potential lag in diagnosis in this patient population (21-23). The overall survival (OS) in patients with brain metastases is variable and ranges from 3 to 15 months (24). Prognostic factors such as number of lesions, performance status and extra-cranial control are important determinants (24). In the EGFR+ and ALK+ subgroups a superior survival of 34 and 38 months respectively has been reported (25).
Historically standard treatments for brain metastases in NSCLC focused on achieving local control with mixed results. Dependent on size, number, symptoms, site and histology of lesions, patients may have been offered surgery and or whole brain radiation (WBRT). WBRT is associated with cognitive decline and inferior quality of life (26-28). While stereotactic radiosurgery (SRS) has the advantage of less cognitive impairment and shorter treatment times, the number of metastases is thought to limit SRS (28). Systemic treatment has inferior CNS disease control due to variable penetration across the blood brain barrier (BBB) (29). Platinum regimens have however demonstrated response rates between 23–50%, which approximated extra-cranial responses (30). Guidelines have suggested that chemotherapy could have a role in patients with asymptomatic disease where local therapies are not possible (31). Bevacizumab in combination with carboplatin/paclitaxel has demonstrated efficacy and early results of a phase II study of 67 patients with non-squamous histology and brain metastases, revealed a 61.2% overall response rate (ORR) in intracranial lesions and a 6-month progression-free survival (PFS) of 56.5% (32). Oral EGFR-tyrosine kinase inhibitors (TKIs) and ALK inhibitors can gain access to the CNS and response rates, especially in ALK+ NSCLC are promising (33-38).
ICIs in NSCLC
The evasion of immune destruction is now recognized as a hallmark of cancer (39). Immune checkpoints are crucial to this and under normal physiological conditions control immune homeostasis and prevent autoimmunity (40). Immune checkpoints belong to a large diverse family of receptors that can negatively impact the efferent immune response by impairing T cell clonal expansion, repressing function and activation and by preventing immune attack against tumor antigens (41). The PD-1/PD-L1 and CTLA-4 axes are the most common checkpoints studied with monoclonal antibodies that can inhibit ligand binding. CTLA-4 is expressed on T cells and appears to primarily inhibit the early activation of effector T cells within lymphoid organs and can enhance the immunosuppressive FOXP3+ regulatory T (Treg) cell population (42). PD-1 counterattacks the T cell response foremost at the tumor or inflammatory site and is upregulated on activated T-cells and other immune cells within the tumor microenvironment. Binding of PD-1 to its ligands (PD-L1 and PD-L2) promotes tumor immune escape by initiating a signaling cascade that inhibits T cell proliferation and limits cytotoxic function (41,43). PD-L1 can be found on a spectrum of cells including endothelial and epithelial cells together with T and B cells, mast and dendritic cells and the high expression of PD-L1 in NSCLC may correlate with inferior prognosis (44). Nivolumab and pembrolizumab are IgG4 monoclonal antibodies targeting PD-1 with early efficacy data presented in phase I studies (45,46). Three large randomized trials have recently confirmed the activity and improved survival of PD-1 inhibitors after failure of first line platinum chemotherapy in unselected NSCLC as well as those selected by tumor PD-L1 expression (47-49). Durable responses across trials are reported in approximately 20% of patients, 30% of those with PD-L1 tumor expression (45,48-50). PD-1 inhibitors now represent a standard option in NSCLC patients with metastatic disease. The efficacy of PD-L1 inhibitors post platinum doublet chemotherapy (POPLAR) and the combination of CTLA-4 inhibitors and PD-L1 inhibitors has also been established (51,52). Trials comparing ICIs to chemotherapy in the first-line setting are expected to report in 2016, with ongoing trials of combination ICI plus chemotherapy regimens versus standard first-line chemotherapy (53,54). The only biomarker known to predict response to PD-1 axis inhibitors in NSCLC is the percentage of PD-L1 positive tumor cells. In KEYNOTE-010, untreated patients who had a tumor proportion score ≥50% (membranous PD-L1 expression in at least 50% of tumor cells) demonstrated higher response rates of 50% (47). This is however far from an ideal biomarker and the lack of PD-L1 expression does not preclude a response (48,49,53,55,56). There has been a growing interest in mutation load as a predictive marker for immune checkpoint inhibition; determining this however, may be costly and impractical on a global scale (57,58). Most of the published studies of ICIs in NSCLC required local CNS control and stability prior to study entry, thus the value of ICIs in patients with brain metastases is understudied.
The immunogenicity of the CNS
Until recently the brain was considered an immune-privileged organ, a term first coined by Billingham and Boswell in the 1950s (59,60). The limited regenerative capacity of neural cells means that strict control must be in place to prevent autoimmunity. Over the past century foreign tissues and pathogens have been shown to evade the immune system when transplanted into brain parenchyma (61-63). Anatomical barriers such as the BBB and an absent lymphatic system were thought responsible for poor CNS immunogenicity. The latter has now been refuted since the discovery of an intact CNS lymphatic system, which questions our traditional understanding of CSF flow and explains how peripheral immune responses can be generated (64,65). CNS-specific immune cells have also been shown to traverse the cribriform plate in order to reach deep cervical nodes (66). Although the BBB restricts access and flow of peripheral innate and adaptive immune cells, other interfaces such as the CSF and choroid plexus can provide mechanisms of entry (67).
The various compartments of the CNS are complex and heterogeneous in immune cell composition. Microglia are the only immune cells within brain parenchyma and are considered poor antigen presenting cells (68). However within the ventricles, leptomeninges and perivascular spaces are cells of the innate immune system, predominantly macrophages, as well as of the adaptive immune system with a relatively high density of CD4+ memory T cells (67,69). These resident cells are important for ongoing immunesurveillance. Once the CNS becomes inflamed or tumourigenesis initiates, the BBB becomes more permeable and the production of cytokines and chemokines may perpetuate immune cell infiltration (60). Despite this theory, primary CNS tumors do not appear to have a high density of tumor infiltrating lymphocytes (TILs) whereas renal cell carcinomas and melanomas have a higher TIL burden in the microenvironment in CNS metastases (70,71). Similar to systemic disease, the reasons for immune cell heterogeneity within the tumor environment have not been fully explained.
A number of studies have evaluated the prognostic impact of TILs in systemic cancers (72). Within the CNS, the association of TILs with survival has been conflicting. Harter et al. investigated a large cohort of patients with CNS tumors including NSCLC metastasis (n=62) and could not find a correlation between TIL burden and patient survival. This group also reported low TIL levels in lung cancer brain metastases, with highest density of TILs in RCC and melanoma (73). Similarly Berghoff reported increased TILs in RCC and melanoma brain metastases but also reported high density in NSCLC samples (n=57), and correlated survival with density of TILs and the ‘immunoscore’ (71). Both studies were retrospective and the latter only included patients with a single brain metastasis. The median number of lesions in the study by Harter et al. was also one. Lung cancer genotype was not available in either study.
An analysis of PD-L1 and TIL densities in NSCLC primary tumor and matched brain metastases revealed higher PD-L1 expression in brain metastases (52% vs. 32%) but denser TILs in primary tumors (74). The density of TILs in tumor may be a predictive marker for immune checkpoint inhibition. Given that the non-synonymous mutational burden may represent a predictive marker in NSCLC, the differences in mutational load in systemic disease versus brain metastases may be a contributing factor in TIL differences but this theory has not been explored (57).
Immunotherapy in NSCLC CNS disease—clinical evidence
Clinical evidence to support the efficacy of ICIs in CNS disease is limited. Early data from a phase II study has been reported by Goldberg et al. and represents the first report of PD-1 inhibitors in untreated or progressive NSCLC brain metastases (75). This single institution study enrolled 18 patients with melanoma and 18 patients with NSCLC including one EGFR+ and one ALK+ lung cancer patient. Patients were required to have asymptomatic intracranial disease with at least one brain metastasis measuring between 5 and 20 mm that was untreated. Primary NSCLC tumors had to have at least 1% PD-L1 staining. In the lung group, 10/18 patients had received previous local therapy for brain metastases but evidence of progressive disease. All patients received pembrolizumab 10 mg/kg every 2 weeks until disease progression. Among the patients with NSCLC, 33% of patients (n=6) had a response (four with complete response, one each with confirmed and unconfirmed partial response) with a median response duration of more than 6 months. The numbers of CNS responders in both cohorts correlated with patients achieving a systemic response. Responses in the CNS lasted from 3 to 7 months. It is unknown if responders included specific molecular subtypes. Another third (n=6) of NSCLC patients had confirmed progressive disease intra-cranially and an additional four (22%) could not be evaluated due to rapid systemic progression. The median OS in the NSCLC cohort was 7.7 months but had not been reached in the melanoma group. Neurological toxicities were predominantly grade 1–2, such as seizures, headache and dizziness, and did not result in treatment cessation. Cognitive dysfunction and stroke were less common although a melanoma patient experienced a transient but severe episode of cognitive dysfunction.
In a phase II study (CheckMate 063) of nivolumab, lung cancer patients with squamous cell cancer who had received at least two lines of systemic treatment were treated with nivolumab. Of two patients with evaluable CNS disease, both had a response (55). Neurotoxicity was again uncommon. A further retrospective review of five patients with NSCLC and new or progressing brain metastases not requiring corticosteroids were treated with nivolumab. Two patients had an intracranial response, including one partial response and one complete response both sustained for over 24 weeks (76). A number of early phase immunotherapy trials are now including patients with untreated asymptomatic CNS disease; however as yet there are no phase III studies that allow enrolment of patients with untreated brain metastases from NSCLC (Table 1).
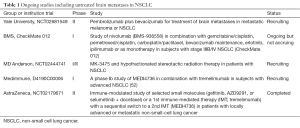
Full table
In patients with brain metastases from melanoma, the role of ICIs has been more extensively investigated. Ipilimumab, a CTLA-4 inhibitor, was evaluated in both patients with asymptomatic brain metastases and those with symptomatic disease requiring steroids. The response rates were 18% and 5% respectively (77). It should be noted that 76% of patients with asymptomatic disease had progressive brain metastases at 12 weeks, likely requiring local interventions (78). A retrospective study of ipilimumab reported similar responses (79).
Updated analysis from a phase II study of ipilimumab and fotemustine in metastatic melanoma (NIBIT-M1) has confirmed that 7 of 20 patients enrolled with brain metastases were alive over 2 years from study entry (80). The NIBIT 3 phase III study includes a cohort of patients with untreated asymptomatic brain metastases (81).
Nivolumab has also demonstrated activity in hypermutated glioblastoma and may have a role in primary neurodegenerative disorders such as Alzheimer’s disease which reinforces the potential application of ICIs in select populations with intracranial pathology (58,82).
While limited data suggest that intracranial response rates to ICIs are similar to response rates with platinum doublet therapy, ICI therapy has the distinct advantage of producing durable responses in select patients. As yet there is no definitive biomarker to enrich this population. The role of ICIs in EGFR+ and ALK+ NSCLC has been controversial, with subgroup analyses of phase III trials suggesting no significant survival advantage over second-line chemotherapy (47,48). Gettinger et al. on the other hand did report responses in EGFR+ patients and a recent study has shown that EGFR/ALK+ lung cancer may upregulate PD-L1 expression through activation of PI3K-AKT and MEK-ERK signaling pathways (53,83). In these molecular subgroups where the incidence of brain metastases is high, further clarification of response to ICIs will be important. When brain metastases develop, the cost of patient care rises significantly (84). It is unlikely that use of ICIs without better patient selection will be cost effective in treating an overall poor prognostic cohort of patients.
Future prospects
A number of studies are now investigating the role of ICIs in patients with untreated brain metastases and it is likely that this will expand following the recent report of Goldberg and colleagues. For example, CheckMate 012, a phase I study of combination nivolumab and ipilimumab in NSCLC, includes an arm of patients with asymptomatic brain metastases (Table 1). The role of combination radiation and immunotherapy is a rapidly evolving field. Specifically in the brain metastases population, combinations of ipilimumab/SRS and nivolumab/SRS have demonstrated safety and feasibility in retrospective analyses of melanoma patients (85-87). Kniesley reported a series of melanoma patients with brain metastases and found an improvement in median survival of 21.3 vs. 4.9 months when ipilimumab was added to SRS. Radiation necrosis is however, thought to occur with a higher frequency when immunotherapy is used (88). Also the potential for an abscopal effect in malignancy is a subject of great interest, with case reports in NSCLC (89,90). Radiation is thought to repair aberrant vasculature and attract tumor specific T cells into the tumor microenvironment therefore enhancing the immune response (91). Recently it has been shown in mouse models that there is a persistent influx of bone marrow-derived immune cells into the CNS after radiation, suggesting that the physiologic effects of radiation may unleash restraints on the regulation of immune homeostasis (92). The diagnosis of pseudoprogression can be a challenge and case reports of surgical resections have revealed necrotic tissue with inflammatory cells and only scattered tumor cells (93,94).
Given that patients with small asymptomatic brain lesions seem to respond best to ICIs, and that brain metastases have a lower TIL infiltrate compared to primary lung tumors, immunotherapy in the adjuvant setting may be more efficacious in delaying time to development of CNS disease. The adjuvant studies of immunotherapy versus placebo post resection or radical chemoradiation in stage III disease (NCT02273375, NCT02595944, NCT02125461) will help address this question.
Conclusions
A select group of patients with brain metastases from NSCLC may have durable responses to immune checkpoint blockade. More data are needed for better patient selection but this cohort is likely to reflect extra-cranial responders. Combination treatments including radiotherapy may enhance outcomes. In a historically poor prognostic patient population, ICIs offer a promising systemic approach to intracranial disease without major toxicity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Howlader NN, Krapcho M, Garshell J, et al. editors. SEER Cancer Statistics Review, 1975–2011. Available online: http://seer.cancer.gov/archive/csr/1975_2012/
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763-74. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer 1954;7:682-9. [Crossref] [PubMed]
- Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol 2009;92:275-82. [Crossref] [PubMed]
- Bernardo G, Cuzzoni Q, Strada MR, et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest 2002;20:293-302. [Crossref] [PubMed]
- Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312-22. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Stanic K, Zwitter M, Hitij NT, et al. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol 2014;48:173-83. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Pitz MW, Desai A, Grossman SA, et al. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol 2011;104:629-38. [Crossref] [PubMed]
- Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 2014;40:716-22. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27-39. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- Hata A, Katakami N. Afatinib for Erlotinib Refractory Brain Metastases in a Patient with EGFR-Mutant Non-Small-Cell Lung Cancer: Can High-Affinity TKI Substitute for High-Dose TKI? J Thorac Oncol 2015;10:e65-6. [Crossref] [PubMed]
- Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol 2013;36:110-5. [Crossref] [PubMed]
- Gainor JF, Chi AS, Logan J, et al. Alectinib Dose Escalation Reinduces Central Nervous System Responses in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer Relapsing on Standard Dose Alectinib. J Thorac Oncol 2016;11:256-60. [Crossref] [PubMed]
- Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 2015;10:232-6. [Crossref] [PubMed]
- Rosell R, Gettinger SN, Bazhenova LA, et al. 1330: Brigatinib efficacy and safety in patients (Pts) with anaplastic lymphoma kinase (ALK)-positive (ALK+) non-small cell lung cancer (NSCLC) in a phase 1/2 trial. J Thorac Oncol 2016;11:S114. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271-5. [Crossref] [PubMed]
- Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009;10:1185-92. [Crossref] [PubMed]
- Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:203-8. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Brahmer J. Nivolumab in Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2016;374:493-4. [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015;10:985-9. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Bouffet E, Larouche V, Campbell BB, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 2016;34:2206-11. [Crossref] [PubMed]
- Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci 1953;141:392-406. [Crossref] [PubMed]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol 2007;28:12-8. [Crossref] [PubMed]
- Murphy JB, Sturm E. Conditions Determining the Transplantability of Tissues in the Brain. J Exp Med 1923;38:183-97. [Crossref] [PubMed]
- Medawar PB. Immunity to homologous grafted skin; the relationship between the antigens of blood and skin. Br J Exp Pathol 1946;27:15-24. [PubMed]
- Stevenson PG, Hawke S, Sloan DJ, et al. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol 1997;71:145-51. [PubMed]
- Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-41. [Crossref] [PubMed]
- Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991-9. [Crossref] [PubMed]
- Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol 2006;80:797-801. [Crossref] [PubMed]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012;12:623-35. [Crossref] [PubMed]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol 2009;9:429-39. [Crossref] [PubMed]
- Kivisäkk P, Tucky B, Wei T, et al. Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy. BMC Immunol 2006;7:14. [Crossref] [PubMed]
- Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 2015;17:1064-75. [Crossref] [PubMed]
- Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 2015;5:e1057388. [Crossref] [PubMed]
- Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Harter PN, Bernatz S, Scholz A, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 2015;6:40836-49. [PubMed]
- Berghoff AS, Inan C, Ricken G, et al. Tumor-infiltrating lymphocytes (TILS) and PD-L1 expression in non-small cell lung cancer brain metastases (BM) and matched primary tumors (PT). Annals of Oncology 2014;25:iv465-6. [Crossref]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114-7. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Nieder C. Ipilimumab in patients with melanoma and brain metastases. Lancet Oncol 2012;13:e277; author reply e277-8.
- Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 2014;118:109-16. [PubMed]
- Di Giacomo AM, Ascierto PA, Queirolo P, et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann Oncol 2015;26:798-803. [Crossref] [PubMed]
- Di Giacomo AM, Margolin K. Immune checkpoint blockade in patients with melanoma metastatic to the brain. Semin Oncol 2015;42:459-65. [Crossref] [PubMed]
- Baruch K, Deczkowska A, Rosenzweig N, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat Med 2016;22:135-7. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Guérin A, Sasane M, Dea K, et al. The economic burden of brain metastasis among lung cancer patients in the United States. J Med Econ 2016;19:526-36. [Crossref] [PubMed]
- Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899-906. [Crossref] [PubMed]
- Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012;117:227-33. [Crossref] [PubMed]
- Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434-41. [Crossref] [PubMed]
- Cohen JV, Kluger HM. Systemic Immunotherapy for the Treatment of Brain Metastases. Frontiers in oncology 2016;6:49. [Crossref] [PubMed]
- Siva S, Callahan J, MacManus MP, et al. Abscopal [corrected] effects after conventional and stereotactic lung irradiation of non-small-cell lung cancer. J Thorac Oncol 2013;8:e71-2. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. [Crossref] [PubMed]
- Moravan MJ, Olschowka JA, Williams JP, et al. Brain radiation injury leads to a dose- and time-dependent recruitment of peripheral myeloid cells that depends on CCR2 signaling. J Neuroinflammation 2016;13:30. [Crossref] [PubMed]
- Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma Brain Metastasis Pseudoprogression after Pembrolizumab Treatment. Cancer Immunol Res 2016;4:179-82. [Crossref] [PubMed]
- Doherty MK, Jao K, Shepherd FA, et al. Central Nervous System Pseudoprogression in a Patient Treated with PD-1 Checkpoint Inhibitor. J Thorac Oncol 2015;10:e100-1. [Crossref] [PubMed]


