Anti-angiogenetic therapies for central nervous system metastases from non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths in worldwide (1). The majority of patients diagnosed with non-small cell lung cancer (NSCLC) have locally advanced or metastatic disease at baseline with a 5-year survival <5% (2). In the last years, advances in the understanding of NSCLC biology have identified two molecularly defined subset of patients: those with epidermal growth factor receptor (EGFR) activating mutations treated with EGFR tyrosine kinase inhibitors (TKIs) and those with echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) translocations responding to crizotinib (3,4). The development of brain metastases (BM) is a common complication in lung cancer and affects largely morbidity and mortality of NSCLC patients, determining a poor clinical outcome despite active treatment. In patients with EGFR mutations, treatment with an EGFR TKIs might result in an intracranial objective response approaching 80% and encouraging overall survival (OS) (5). Unfortunately, in patients with ALK translocation, crizotinib does not appear to work well on intracranial disease despite an important effect on extracranial disease (6). BM are detected in 10–20% of NSCLC patients at diagnosis and occur in about 40% of patients during the course of the disease (7). Common symptoms of BM include headache, localizing weakness, seizures, altered mental status and ataxia. Whole brain radiotherapy (WBRT) and steroids are the standard treatment for most of the patients with a reduction in symptoms in 75–80% of the cases (8). Local approaches, as surgery and stereotactic radiosurgery (SRS), are indicated in solitary or oligometastatic disease. The median OS of untreated patients with BM is 1–2 months (4,8). However, earlier diagnosis, more sensitive radiological imaging and therapeutic options (SRS, surgery and WBRT) can prolong survival to 4–6 months. The role of chemotherapy in the treatment of BM remains unclear. Some intracranial responses have been reported with vinorelbine plus gemcitabine/carboplatin (9) and cisplatin/carboplatin plus gemcitabine (10,11). Indeed, the role of the blood-brain barrier (BBB) in reducing drug access to BM has always been a concern. Tumor angiogenesis plays a central role in cancer development, invasion, progression and metastatic dissemination (12). The inhibition of tumor-related angiogenesis is without any doubt an attractive target for anticancer therapy. However, a frequent complication of this therapy is hemorrhage at tumor site or at distant site. It is known that central nervous system (CNS) bleeding in patients with BM is an important complication. The rate of the CNS bleeding is different across the type of tumor: 1–7% in lung cancer and 70% in renal cancer (13). On the basis of these observations patients with BM are frequently not candidate to clinical studies with anti-vascular endothelial growth factor (VEGF) therapy. This review will focus on the role of anti-angiogenetic drugs in the treatment of BM in patients with NCSLC, in particular, we will discuss monoclonal antibodies that block VEGF-VEGF receptor (VEGFR) binding and small molecule TKIs, that inhibit the downstream VEGFR mediated signalling.
Rational for targeting angiogenetic pathways in CNS metastases from NSCLC
Irrespectively of the origin and the site of metastases, growth and survival of tumor cells depend on the establishment of an adequate blood supply (14,15), mainly supported by neo-angiogenesis. Angiogenesis is regulated by several pro- and anti-angiogenetic factors. Among pro-angiogenetic factors, VEGF is the most extensively studied and stimulates angiogenesis primarily through activation of VEGFR-2 (16), which are both commonly expressed in NSCLC (17).
Immunohistochemical and morphometric analyses in human lung cancer BM demonstrated that the density of blood vessels within BM is lower than the adjacent tumor-free brain parenchyma. However, BM blood vessels are dilated and contain many dividing endothelial cells (15).
Real-time imaging with multiphoton laser scanning microscopy in a BM mouse model, reveals that early angiogenesis is a mandatory step for successful macrometastases formation (18).
According to the diffusion coefficient of oxygen within tissue of about 150 µm, Fidler et al. demonstrated that tumor cells within autochthonous and experimentally induced BM from lung cancer located less than 100 µm from a blood vessel are viable, whereas more distant tumor cells undergo programmed cell death (15). The same authors also provided support to the role of VEGF pathway in the growth of BM by transfecting human lung cancer cells with an antisense-VEGF165 gene, which decreased the frequency of BM in nude mice. Conversely, transfection of human lung cancer cells with sense-VEGF-121 or sense-VEGF165 either increase or inhibited the formation of BM. These data suggest that VEGF expression is necessary, but not sufficient for the development of BM and that VEGF represent one among other targets for therapeutic intervention.
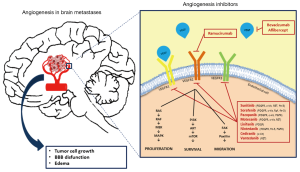
The primary goal for using anti-angiogenetic therapies is to block the development of malignant neovasculature, in order to reduce oxygen availability in the tumor and to decrease its growth.
Using a mouse model where single metastasizing cancer cells were tracked by intravital microscopy, it was demonstrated that chronic anti-angiogenetic treatment with bevacizumab can prevent an early angiogenic switch that is mandatory for brain outgrowth of non-squamous NSCLC cells (18). In a rat model of human lung cancer BM it was also demonstrated that, compared to controls, bevacizumab slowed the rate of tumor growth (P=0.003), measured through magnetic resonance imaging biomarkers (19).
Moreover, treatment with bevacizumab inhibited BM formation in a mouse model of haematogenous non-squamous NSCLC metastases. In this study, a total of 112 BM events (defined as single cells, micrometastases and macrometastases), were observed in the eight control animals, while only two brain metastatic events occurred in the ten bevacizumab-treated mice (P<0.001) (20).
A secondary goal for using anti-angiogenetic therapies in BM is to reduce edema, which impacts patients quality of life (QoL) inducing neurological deficits and headache. There is clinical evidence in glioblastoma that bevacizumab is able to induce significant antiedemigenous effect by restoring the integrity of the BBB (21). Tumor neoangiogenesis in BM, in fact, leads to the development of blood vessel that lack the physiological function of the BBB, the structure that regulates the flow of ions, nutrients, drugs and cells into the brain (15). The BBB is intact in and around experimental BM derived from human lung cancer smaller than 0.25 mm, while it is leaky in larger metastases (15).
In a rat model of lung cancer BM bevacizumab partially restored the normal low permeability characteristics of the BBB, measured through magnetic resonance imaging before and one day after treatment (19), preventing edema development.
A third rationale for the use of bevacizumab in BM is its role in vascular normalization. It has been suggested that blocking VEGF signalling means to normalize tumor blood vessels, which may result in a more efficient chemotherapy delivery and in a higher efficacy of chemo- and radiotherapy (22).
A final rational for the use of anti-VEGF therapies in BM is that, contrarily to most chemotherapeutic agents, anti-angiogenetic therapies exert their activity by inhibiting the endothelial cell, which does not require to cross the BBB.
All these above mentioned data (Figure 1) suggest that VEGF could represent an important target in the treatment of BM from NSCLC (23).
Efficacy and safety of antibody targeting VEGF for CNS metastases from NSCLC
Bevacizumab
Bevacizumab is a recombinant, humanized monoclonal antibody, that selectively binds VEGF and prevents interaction with its receptor. It is approved for the first-line treatment of advanced, metastatic or recurrent NSCLC with non-squamous cell histology, in combination with platinum-based chemotherapy (24,25). Bevacizumab significantly improved OS and progression free survival (PFS) when combined to first-line carboplatin and paclitaxel for treatment of NSCLC compared with chemotherapy alone in the Eastern Cooperative Oncology Group (ECOG) phase II trial E4599 (25) and prolonged PFS in combination to first-line cisplatin and gemcitabine in the phase III AVAiL study (26).
Patients with BM were initially excluded from trials with bevacizumab due to concerns about a potentially greater risk of cerebral haemorrhage, following a single case in 1997 of a patient with hepatocellular carcinoma, who experienced a fatal cerebral haemorrhage from a previously undiagnosticated brain metastasis, in a phase I study of bevacizumab (27).
More recently, several clinical trials have been conducted to investigate the safety and efficacy of bevacizumab in advanced NSCLC patients, including those with BM. Bevacizumab safety was first investigated in patients with asymptomatic treated BM. Five prospective trials, PASSPORT, ATLAS, BeTa, ERACLE and PRONOUNCE trials included patients with treated BM and recorded a low rate of CNS haemorrhage (28-32).
PASSPORT (28) is an open-label single arm phase II trial of bevacizumab in combination with first- or second-line therapy in patients with treated BM from non-squamous NSCLC. First- and second-line therapy consisted of either chemotherapy or erlotinib with bevacizumab, according to institutional standards. Patients with BM were allowed to enter the trial after previous treatment with whole CNS radiation therapy, radiosurgery and/or neurosurgery. Median treatment duration was 85 (range, 1–379) days. Among the 106 safety-evaluable patients no grade ≥2 CNS haemorrhage were reported, demonstrating a minimal risk of intracerebral haemorrhage after the use of bevacizumab in this setting of patients. Twenty-six patients (24.5%) discontinued study treatment as a result of an adverse event, and 37 (34.9%) discontinued due to disease progression.
In the phase III ATLAS trial (29) 1,145 patients with untreated stage IIIB, stage IV or recurrent NSCLC underwent four cycles of chemotherapy plus bevacizumab and 743 of these patients, without disease progression after induction, were subsequently randomized (1:1 ratio) to receive bevacizumab in combination with erlotinib or placebo. In this trial patients with BM were eligible when treated and not requiring treatment with steroids. Among 743 patients included in the final analyses, 29 presented BM at baseline. The addition of erlotinib to bevacizumab significantly improved PFS [median PFS: 3.7 vs. 4.8 months; hazard ratio (HR) =0.71; 95% CI: 0.58–0.86], but not OS (median OS: 13.3 vs. 14.4 months; HR =0.92; 95% CI: 0.70–1.21).
A pooled analyses (33) of the 131 patients with treated BM receiving bevacizumab in either PASSPORT (28) or ATLAS (29) reported no symptomatic grade >2 brain haemorrhage during the main treatment phases of study. In the ATLAS trial one grade 2 CNS haemorrhage occurred during treatment after CNS progression (29).
The double-blind, placebo controlled, phase III BeTa trial (30) aimed to assess the efficacy and safety of bevacizumab in combination to erlotinib versus erlotinib alone in advanced NSCLC after failure of standard first-line chemotherapy. In this study, patients with a history of BM, who were treated with a minimum of WBRT and with no ongoing steroids requirement were included. Among the 319 patients treated with bevacizumab 37 had treated BM at baseline. In patients treated with bevacizumab and erlotinib one grade 3–4 CNS haemorrhage was reported (<1%). OS did not differ between the patients in the bevacizumab group and controls (median OS: 9.2 vs. 9.3 months, HR =0.97; 95% CI: 0.80–1.18; P=0.7583). No specific data about the outcome of patients with CNS metastases were reported.
Both ERACLE and PRONOUNCE randomized phase III trials aimed to compare cisplatin and pemetrexed followed by maintenance pemetrexed and carboplatin with paclitaxel and bevacizumab followed by maintenance bevacizumab, standard first-line treatment for advanced nonsquamous NSCLC. Primary endopoint was the difference in QoL between the two treatment arms after 12 weeks of maintenance in the ERACLE trial and PFS without grade 4 toxicity in the PRONOUNCE trial. In both trial stable, previously treated CNS metastases were allowed. In the ERACLE trial six patients had BM at baseline (four in the cisplatin and pemetrexed arm and two in the carboplatin, paclitaxel and bevacizumab arm), none intracranial haemorrhage occurred and no statistically significant difference in the QoL between the two regimens was described in the entire population. Among the 179 patients treated with bevacizumab in the PRONOUNCE trial 32 (17.9%) had treated CNS metastases at baseline and none developed any grade intracranial bleeding. The primary end point was not reached.
Two large cohort studies focused on bevacizumab safety: SAiL (34) and ARIES (35).
SAiL was a phase IV trial (34), evaluating the safety of bevacizumab in 2,212 patients treated with bevacizumab in combination with first line standard chemotherapy for a maximum of six cycles, followed by bevacizumab alone until disease progression. Although evidence of BM was an exclusion criterion in SAiL, some patients with asymptomatic BM have been potentially included, since brain imaging was not mandated before enrolment. Among the 281 patients who were assessed as having BM during the course of the study, 5 (2%) reported CNS bleeding.
The ARIES trial (35) was conducted on 1,967 patients with NSCLC, treated with first-line chemotherapy in combination with bevacizumab. Eight percent of patients had BM at baseline and 3 patients (0.2%) had grade 3 to 5 CNS haemorrhage.
Besse et al. conducted a non-randomized, phase II trial to investigate bevacizumab-based regimens in both first and second line setting in patients with NSCLC and asymptomatic, untreated BM (BRAIN trial) (36). Sixty-seven patients were treated with first line bevacizumab in combination with carboplatin and paclitaxel and 24 patients received second line bevacizumab in combination with erlotinib. In the first-line cohort 6-month PFS was equal to 56.5%, with a median PFS of 6.7 months (95% CI: 5.7–7.1) and median OS was 16.0 months. Overall response rate (ORR) was 61.2% in intracranial lesions and 64.2% in extracranial lesions. In the second-line cohort (n=24), 6-month PFS was 57.2%, median PFS was 6.3 months (95% CI: 3.0–8.4), median OS was 12.0 months and ORR was 12.5%. In the ECOG 4599 phase III randomized controlled trial, which tested the same treatment scheme in patients without BM, median OS was 12.3 months and a median PFS was 6.2 months, compared to 16.0 and 6.7 months, respectively, in the BRAIN first-line cohort. The favourable outcomes in BRAIN compared to E4599, should be related to differences in baseline characteristics, including the better performance status of patients evaluated in BRAIN. The BRAIN trial registered a rate of CNS haemorrhage comparable to what observed in other studies: only one grade I intracranial haemorrhage occurred and resolved, without sequelae in the first-line arm. In the BRAIN trial patients were enrolled and treated before they became symptomatic and required steroids and this make this population in any case different from what observed in daily clinical practice.
Few data are available in terms of safety and efficacy of bevacizumab in patients with active (treatment naive, progressive or symptomatic) BM.
A small retrospective study evaluated the safety and efficacy of bevacizumab in six NSCLC patients with active (treatment naive or progressive) CNS metastases: bevacizumab was administered alone (n=1) or in combination with different cytotoxic chemotherapies (n=5). No grade ≥2 CNS haemorrhage occurred, neither in patients with a prior history of such haemorrhage. Best CNS response, was partial in two, stable disease in three, and progression in one patient. Improvement in symptoms and reduction in corticosteroid requirements was reported (37).
A small prospective study investigated the safety and efficacy of bevacizumab-based therapy in patients with symptomatic, clinical or radiographic progressive BM from NSCLC. None of the 13 patients enrolled in the study developed CNS haemorrhage. Median PFS was 9.1 months and median OS was 9.6 months. The authors also reported a considerable improvement in the QoL of patients with relief from neurological symptoms and reduction of dexamethasone administration (38).
Khasraw et al. conducted a retrospective analysis to investigate the association between treatment with bevacizumab and intracranial haemorrhage in various types of tumors. In the NSCLC population, the incidence rates of intracranial bleeding were 3.6% (28/789) in patients with BM treated without bevacizumab and 3.9% (3/77) in patients with BM treated with bevacizumab (39). Similarly, an evidence-based review on the risk of CNS haemorrhage in patients with BM from NSCLC concluded that there was no significantly increased risk of intracranial bleeding associated with anti-VEGF therapy (40).
Two other retrospective exploratory analyses of randomized controlled trials reported similar results, indicating that bevacizumab should be considered as a therapeutic opportunity, even in patients with active BM (33,41).
Nevertheless, several recent phase II and III trials (JO19907, JO25567 and BEYOND), with bevacizumab in advanced NSCLC, still excluded patients with BM (42-44).
Finally, bevacizumab, has been proposed in a retrospective study, as the treatment for radiation necrosis of BM post SRS, with the aim to reach a symptomatic relief, the reduction in steroid requirement and a radiographic response (decrease in enhancement and edema at magnetic resonance imaging scans) (45).
The international guidelines permit the use of bevacizumab in patients with advanced, metastatic or recurrent NSCLC with non-squamous histology and performance status 0–1, even in the presence of asymptomatic BM (2).
Table 1 summarises the results of the prospective trials evaluating the anti-VEGF antibody, in which patients with CNS metastases from NSCLC have been included.
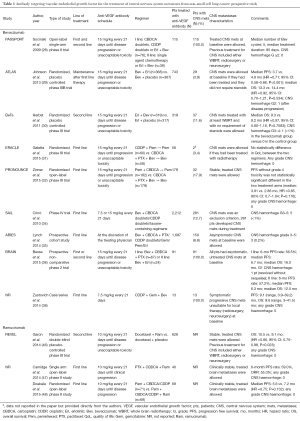
Full table
Ramucirumab
Ramucirumab is a fully human IgG1 monoclonal antibody that targets the VEGFR-2 extracellular domain with high affinity, preventing binding of all VEGF ligands and receptor activation (49).
Currently, ramucirumab is approved for the second-line treatment of metastatic NSCLC in combination with docetaxel (50).
The phase III REVEL trial (46) randomized 1,253 patients with squamous or non-squamous NSCLC, who had progressed during or after a first-line platinum-based chemotherapy regimen to receive docetaxel plus either ramucirumab (n=628) or placebo (n=625) until progression or unacceptable toxicity. This study demonstrated that ramucirumab added to docetaxel improved OS (median OS: 10.5 vs. 9.1 months, HR =0.86, 95% CI: 0.75–0.98, P=0.023), PFS (median PFS: 4.5 vs. 3.0 months, HR =0.76, 95% CI: 0.68–0.86, P<0.0001) and ORR (23% vs. 14%, P<0.001) compared to docetaxel alone.
Patients with treated BM were eligible if they were clinically stable with regard to neurologic function, after cranial irradiation (whole brain radiation therapy, focal radiation therapy, and SRS) or surgery resection. The patient may have had no evidence of grade ≥1 CNS haemorrhage. The number of patients with BM at baseline is not reported, however none grade 1–4 CNS haemorrhage were observed.
To date, ramucirumab has been studied as first line treatment option in patients with advanced or metastatic NSCLC in 2 open-label phase II studies (47,48). In the first study 40 patients received, ramucirumab in combination with paclitaxel and carboplatin, obtaining a 6-month PFS rate of 59.0% and an ORR of 55.0%. In the latter phase II trial, 140 non-squamous NSCLC patients were randomized to receive pemetrexed and carboplatin (n=71) or ramucirumab plus pemetrexed and carboplatin (n=69). The primary endpoint of significant prolongation of PFS was not met (median PFS: 5.6 vs. 7.2 months, HR =0.75; P=0.132). In both studies treated CNS were allowed but the number of patients with CNS at baseline were not reported. None CNS haemorrhage occurred.
Aflibercept
Aflibercept is a recombinant fusion protein, consisting of human VEGFR-1 extracellular domain 2 and VEGFR-2 extracellular domain 3, fused to the hinge region of the human IgG1 Fc domain. Aflibercept has high affinity VEGF binding and the ability to bind VEGF-B, as well as placental growth factor (PLGF)-1 and -2 (51). In a single-arm clinical trial assessing the safety and efficacy of single-agent aflibercept in patients with erlotinib- and platinum-resistant advanced lung adenocarcinomas, aflibercept was well tolerated but had little single-agent activity; the ORR was 2.0% (95% CI: 0.2–7.2%), median PFS 2.7 months, and OS 6.2 months (52).
In the second-line setting, the phase III VITAL trial randomized patients with non-squamous NSCLC to aflibercept or placebo in combination with docetaxel and failed to meet its primary endpoint of improvement in OS (median OS: 10.1 vs. 10.4 months, HR =1.01, P=0.90) (53). Both trials excluded patients with BM at baseline. Therefore, no data are actually available about the safety and efficacy of aflibercept as treatment for BM from NSCLC, but in any case to date there is no indication for this drug in the lung cancer therapeutic approach.
Efficacy and safety of multi targeted anti-angiogenetic agents for CNS from NSCLC
TKIs with anti-angiogenic activity are small molecules that bind to the ATP-binding catalytic site of the tyrosine kinase domain of VEGFRs, resulting in a blockade of intracellular signalling. Many TKIs have been studied in a variety of combinations and lines of therapy for patients with lung cancer. A number of these drugs are effective as single agents in other advanced cancers, such as renal cell carcinoma and soft tissue sarcomas. Unfortunately, the development of anti-angiogenic TKIs has failed to yield an indication for use in lung cancer due to lack of efficacy or increased cumulative toxicity when combined with chemotherapy. Data are summarised in Table 2.
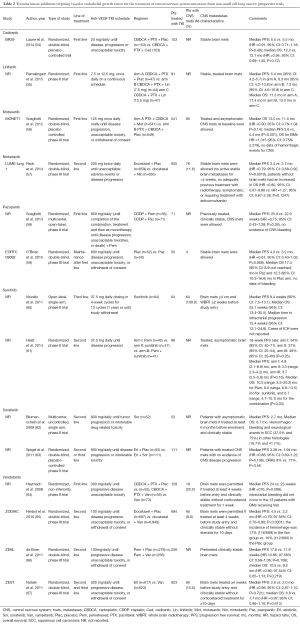
Full table
Sunitinib
Sunitinib is an oral, multitargeted inhibitor of VEGFRs 1, 2, 3, platelet derived growth factor receptors (PDGFRs α and β), stem-cell factor receptor (Kit), FMS-like tyrosine kinase 3 (FLT3), colony-stimulating factor 1 receptor (CSF-1R) and glial cell line-derived neurotrophic factor receptor (RET) (68). Sunitinib is approved for the treatment of metastatic renal cell carcinoma, imatinib resistant/intolerant gastrointestinal stromal tumors (GISTs) and advanced pancreatic neuroendocrine tumors (69,70). A phase II, open-label, single-arm study evaluated the efficacy and safety of sunitinib in patients with NSCLC and irradiated BMs (60). The primary endpoint was PFS and secondary endpoints included OS, patient reported outcomes and safety, with particular attention to risk of intracranial hemorrhage (ICH) associated with focal neurological deficit. Patients with radiologically confirmed BM ≤4 cm and WBRT ≥2 weeks before study entry were included. Sixty-four patients were enrolled and received sunitinib 37.5 mg on a continuous daily dosing schedule, in 4-week cycles for 13 cycles (1 year) or until study withdrawal. Most of patients (98%) received WBRT. Median PFS was 9.4 weeks (90% CI: 7.5–13.1), median OS was 25.1 weeks (95% CI: 13.4–35.5). With regard to intracranial antitumor activity, median time to intracranial progression was 15.4 weeks (95% CI: 12.1–24.8). Overall, sunitinib was well tolerated and cases of ICH were not reported. A randomized phase II study assessed the efficacy of pemetrexed alone versus sunitinib alone versus pemetrexed with sunitinib, as second line in 130 advanced NSCLC (61). Patients with treated, asymptomatic BM were eligible. Primary endpoint was 18-week PFS rate and secondary endpoints included response, OS and toxicity. No specific evaluation was planned for outcomes in patients with BM. The 18-week PFS rate in the pemetrexed, sunitinib and combination arms was 54% (95% CI: 40–71), 37% (95% CI: 25–54) and 48% (95% CI: 35–66) (P=0.25), respectively. Median PFS in the pemetrexed, sunitinib and combination arms was 4.9 (range, 2.1–8.8), 3.3 (range, 2.3–4.2) and 3.7 (range, 2.5–5.8) months, respectively (P=0.18). Median OS was 10.5 (range, 8.3–20.2) months for pemetrexed, 8.0 (range, 6.8–13.5) months for sunitinib and 6.7 (range, 4.1–10.1) months for the combination (P=0.03). In terms of adverse events hemorrhagic episodes were clustered in the sunitinib arms and episodes of ICH bleeding were not observed.
Sorafenib
Sorafenib is a multikinase inhibitor targeting VEGFRs 2, 3, PDGFR-β, c-Kit, FLT3 and RET (71). It is approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma (72). Sorafenib has shown activity in preclinical models and phase I–II studies in patients with NSCLC (71). A phase II single arm trial evaluated sorafenib in patients with relapsed or refractory advanced NSCLC (62). Patients with asymptomatic, stable BM and squamous cell carcinoma (SCC) histology were eligible. The primary endpoint was response rate and secondary endpoints were PFS, OS and toxicity. Fifty-four patients were enrolled. Median PFS of 2.7 months and median OS of 6.7 months were reported in 51 patients. Stable disease was found in 59% of 51 evaluable patients. Drug-related bleeding was observed in four patients and hemorrhagic/bleeding and neurological events were more common in patients with SCC (37.5% and 75%, respectively) compared to other histology. A phase II trial evaluated the efficacy of sorafenib plus erlotinib vs. placebo plus erlotinib in patients with advanced NSCLC, pretreated with one or two regimens (63). Patients with treated CNS metastases with no evidence of CNS disease progression were eligible. The co-primary endpoints were ORR and PFS. One hundred and sixty-eight patients were randomized to sorafenib plus erlotinib or placebo plus erlotinib. The combination of sorafenib/placebo did not statistically improve ORR and PFS (median PFS was 3.38 months for sorafenib/erlotinib vs. 1.94 months for placebo/erlotinib, HR =0.86; 95% CI: 0.60–1.22; P=0.196). No specific evaluation was planned for outcomes for patients with BMs and no episodes of bleeding were reported.
Motesanib
Motesanib is a selective, oral inhibitor of VEGFRs 1, 2, 3, PDGFR and c-Kit (73). Motesanib has demonstrated antitumor activity when administered as monotherapy in advanced solid tumors (74) or combined with chemotherapy in metastatic NSCLC (75). A randomized, double-blind, placebo controlled phase III trial (MONET1) compared motesanib plus chemotherapy (carboplatin/paclitaxel) vs. placebo plus chemotherapy in patients with stage IIIB/IV or recurrent advanced non-squamous NSCLC (56). Primary endpoint was OS and secondary endpoints were PFS, ORR and safety. Patients with symptomatic or untreated BM were not eligible. A total of 1,090 patients were randomized and 80 presented stable BM at randomization. Treatment with motesanib did not significantly improve OS among patients with non-squamous histology (median OS was 13.0 months in the experimental arm vs. 11.0 months in the placebo arm, HR =0.90; 95% CI: 0.78–1.04; P=0.14). A significant improvement in PFS (median PFS was 5.6 vs. 5.4 months, P<0.001) was described. Prespecified subgroup analyses suggested that patients with BM receiving motesanib did not have longer survival (HR =1.315; 95% CI: 0.758–2.279) compared to those receiving chemotherapy. In terms of safety, the hemorrhagic events were more in the experimental arm than in the placebo arm (3% vs. 1%), in particular: gastrointestinal hemorrhage (n=1 vs. n=0), pulmonary hemorrhage (n=2 vs. n=1), hemoptysis (n=3 vs. n=1). Episodes of CNS bleeding were not observed in the study.
Pazopanib
Pazopanib is an oral, selective inhibitor of VEGFRs 1, 2, 3, PDGFRs α and β and c-Kit (76). It is approved for the treatment of metastatic renal cell carcinoma and advanced soft-tissue sarcoma who underwent prior chemotherapy (77). An open-label, multicenter, randomized, phase II study compared pazopanib in combination with pemetrexed vs. cisplatin/pemetrexed in patients with chemonaive, advanced, non-squamous NSCLC (58). Patients with previously treated, clinically stable BM were eligible. The primary endpoint was PFS and secondary endpoints were OS, safety and tolerability. One hundred-six patients were randomized. PFS was not statistically significant different between the two arms (median PFS was 25.0 vs. 22.9 weeks, HR =0.75; 95% CI: 0.43–1.28; P=0.26). No subanalysis for patients with BM was planned and/or performed. In terms of safety, there was no evidence of severe hemorrhagic events (grade ≥3). O’Brien et al. evaluated pazopanib vs. placebo as maintenance therapy in advance NSCLC patients without progression disease after first line platinum-based chemotherapy (59). The primary endpoint was OS and secondary endpoints were PFS and safety. One hundred-two patients were randomized and four patients had BM at randomization. The trial was prematurely stopped following an interim analysis. The median OS was 17.4 for pazopanib vs. 12.3 months for placebo (HR =0.72, 95% CI: 0.40–1.28; P=0.257). The median PFS was 4.3 months in pazopanib vs. 3.2 months in placebo arm (HR =0.67; 95% CI: 0.43–1.03; P=0.068). No severe hemorrhagic events (grade ≥3) were reported.
Linifanib
Linifanib is an oral, selective inhibitor of VEGFRs 1, 2, 3, PDGFRs α and β and FLT3 and has demonstrated activity in preclinical studies (78). A recent randomized, phase II study evaluated two doses of linifanib (7.5 and 12.5 mg) in combination with carboplatin/paclitaxel vs. placebo plus chemotherapy (55) in patients with advanced non-squamous NSCLC. Patients with untreated CNS metastases were not eligible. The primary endpoint was PFS and secondary endpoints included OS and ORR. One hundred thirty-eight patients were randomized. Addition of linifanib 7.5 mg to chemotherapy was associated with a significantly improved PFS compared to placebo (8.3 vs. 5.4 months, P=0.022) and the addition of linifanib 12.5 mg showed a non-significant increase in OS compared to placebo (13.0 vs. 11.3 months, P=0.65). No subgroup analysis for patients with BM was performed. In terms of safety, both dose of linifanib were associated with increased toxicity (anemia, hypertension and diarrhea). No episode of CNS bleeding was registered.
Cediranib
Cediranib is an oral, potent inhibitor of VEGFRs 1, 2, 3, PDGFR-β and c-Kit (79). Preclinical data showed that cediranib prevents angiogenesis and inhibits the growth of tumor xenografts when administered chronically. Up to now, there are limited data available on cediranib and BMs in NSCLC (80). A randomized, double-blind, placebo-controlled trial evaluated the addition of cediranib to standard carboplatin/paclitaxel chemotherapy in advanced NSCLC (54). The primary endpoint was OS and secondary endpoints were PFS, ORR and AEs. Patients with untreated, symptomatic, cavitating or haemorrhagic BMs were not eligible. This trial was halted at an interim analysis due to significantly higher rates grade 3 or greater hypertension, anorexia, and diarrhea without statistically significant increases in PFS or OS. No data are available specifically for patients with BM. Bleeding was not otherwise different between the two arms and episodes of bleeding of CNS were not reported.
Vandetanib
Vandetanib is an oral, multikinase inhibitor of VEGFRs 2, 3, RET, EGFR and has shown antitumor activity in advanced NSCLC patients (81). There are limited data available on vandetanib in the treatment of BMs. A phase II non-inferiority trial evaluated vandetanib alone or with chemotherapy carboplatin/paclitaxel for untreated NSCLC (64). Patients with BM were allowed if treated and clinically stable without corticosteroid treatment. Primary endpoint was PFS and secondary endpoint was OS. One hundred and eighty-one patients were randomized and 6 patients (8%), 7 (12%) and 6 (12%) had BM in vandetanib, vandetanib plus chemotherapy and vandetanib plus placebo, respectively. Vandetanib combined with chemotherapy was not inferior to chemotherapy alone. In details, median PFS was 24 weeks in the vandetanib group vs. 23 weeks in chemotherapy group. Patients with BM were not evaluated in a sub-analysis. In terms of AEs, intracranial bleeding did not occur in the 13 patients with BM receiving vandetanib. Two phase III trials evaluated vandetanib in combination with second-line chemotherapy. The ZODIAC study evaluated vandetanib in combination with docetaxel vs. docetaxel plus placebo in NSCLC patients progressing after platinum-based first-line chemotherapy (65). Patients with BM were allowed if treated and clinically stable without steroids. Primary endpoint was PFS and secondary endpoints were OS, ORR, disease control rate, safety and time to deterioration of disease-related symptoms. 1,391 patients were randomized, 65 patients (9%) in experimental arm and 80 (11%) in controlled arm had BMs, respectively. Median PFS was 4.0 months in the vandetanib arm vs. 3.2 months in the control arm (HR =0.97, 95% CI: 0.70–0.90; P<0.0001). In term of safety, the incidence of hemorrhage was 17% (116/689) in the vandetanib group vs. 16% (112/690) in the placebo group (no data about CNS bleeding). The randomized, double blind phase III trial (ZEAL) evaluated vandetanib plus pemetrexed as second line therapy in advanced NSCLC (66). Patients with pretreated clinically stable BM were eligible. Primary endpoint was PFS and secondary endpoints were OS, ORR, disease control rate, time to deterioration of symptoms and safety. Five hundred and thirty-four patients were randomized. The study did not meet its primary endpoint (median PFS 17.6 weeks for vandetanib vs. 11.9 weeks for placebo, HR =0.86; 97.58% CI: 0.69–1.06; P=0.108). There was no significant difference in OS (median OS was 10.5 for vandetanib vs. 9.2 months for placebo, HR =0.86; 97.54% CI: 0.65–1.13; P=0.219). The incidence of hemorrhagic events (hemoptysis, epistaxis, GI bleeding, hematuria, metrorrhagia or CNS hemorrhage) was similar in both treatment arms. One patient in each arm had non-fatal cerebral hemorrhage, neither of whom had known BM. The randomized, double-blind, phase III trial (ZEST) assessed the efficacy of vandetanib vs. erlotinib in unselected patients with advanced NSCLC after treatment with one or two prior lines of chemotherapy (67). Patients with BM were eligible if treated and if clinically stable without corticosteroid. Primary endpoint was PFS and secondary endpoints were OS, ORR, safety and time to deterioration of symptoms. One thousand two hundred and forty patients were randomized and 60 patients (10%) in the experimental arm and 70 patients (11%) in the controlled arm had BM. Significant improvement in PFS was not evidenced (median PFS was 2.6 months for vandetanib vs. 2.0 months for erlotinib HR =0.98, 95% CI: 0.87–1.10; P=0.721) and OS was similar in the two arms. In terms of safety, fewer hemorrhagic events (hemoptysis, GI bleeding, epistaxis, hematuria, metrorrhagia and CNS hemorrhage) occurred in vandetanib arm compared with the erlotinib arm (11% vs. 17%, respectively).
Nintedanib
Nintedanib is an oral, triple angiokinase inhibitor of VEGFRs 1, 2, 3, FGFRs 1, 2, 3 and PDGFRs α and β (82). The randomized, double-blind phase III trial (LUME-lung 1) evaluated nintedanib in combination with docetaxel vs. placebo plus docetaxel in patients with advanced NSCLC progressing after first line chemotherapy (57). Primary endpoint was PFS and the key secondary endpoint was OS, analyzed in a prespecified stepwise order: first in patients with adenocarcinoma who progressed within 9 months after start of first-line therapy, then in all patients with adenocarcinoma, then in all patients. Patients with active BM (defined as stable for <4 weeks, no adequate previous treatment with radiotherapy, symptomatic, or requiring treatment with anticonvulsants) were not allowed. One thousand three hundred and fourteen patients were randomized and 38 patients (5.8%) had stable BM in the experimental arm and in the control arm, respectively. PFS was significantly improved in the experimental arm (median PFS was 3.4 vs. 2.7 months; HR =0.79; 95% CI: 0.68–0.92, P=0.0019). OS was significantly improved for patients with adenocarcinoma who progressed within 9 months after start of first-line treatment in the docetaxel plus nintedanib group compared with those in the controlled group (median OS was 10.9 vs. 7.9 months, HR =0.75; 95% CI: 0.60–0.92, P=0.0073). This study showed that patients without BM, who received nintedanib in combination with docetaxel, had an increased in OS (HR =0.80, 95% CI: 0.67–0.96 vs. HR =1.27, 95% CI: 0.67–2.38; P=0.1247). In terms of safety, bleeding grade ≥3 was 2.3% in nintedanib group vs. 1.8% in docetaxel group.
Conclusions
Currently the only anti-angiogenetic agents approved for the treatment of NSCLC are bevacizumab in combination with first-line platinum-based chemotherapy, ramucirumab in combination with second-line docetaxel and nintedanib in combination with second-line docetaxel. An increasing body of evidence indicates that the use of bevacizumab-based therapy seems to be feasible and safe in patients with CNS metastases from NSCLC (33). Moreover first-line bevacizumab in combination to standard chemotherapy demonstrated promising activity in patient with asymptomatic BM from NSCLC in the BRAIN trial (36). Even if treated CNS metastases were allowed in phase II (47,48) and III (46) with ramucirumab, no data are actually available about the efficacy of ramucirumab in this subgroup of patients.
Multitargeted angiogenesis inhibitors have been investigated for the treatment of advanced NSCLC patients with promising results in second line setting but an increased toxicity in first line setting in combination with chemotherapy without a meaningful improvement in OS. One exception is nintedanib, that showed an increased OS for patients with adenocarcinoma. Specific results of TKIs activity in the treatment of BM are still lacking and further investigational studies are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: Prof. Silvia Novello declared a role as speaker bureau for Roche, Boehringer Ingelheim, Eli Lilly, Astra Zeneca, MSD. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27-39. [Crossref] [PubMed]
- Rusch V, Klimstra D, Venkatraman E, et al. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 1997;3:515-22. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell Lung Cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012;18:4406-14. [Crossref] [PubMed]
- Chun SG, Choe KS, Iyengar P, et al. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther 2012;13:1376-83. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Bradley KA, Mehta MP. Management of brain metastases. Semin Oncol 2004;31:693-701. [Crossref] [PubMed]
- Bernardo G, Cuzzoni Q, Strada MR, et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest 2002;20:293-302. [Crossref] [PubMed]
- Edelman MJ, Belani CP, Socinski MA, et al. Outcomes associated with brain metastases in a three-arm phase III trial of gemcitabine-containing regimens versus paclitaxel plus carboplatin for advanced non-small cell Lung Cancer. J Thorac Oncol 2010;5:110-6. [Crossref] [PubMed]
- Crinò L, Scagliotti GV, Ricci S, et al. Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced non-small-cell lung cancer: A randomized phase III study of the Italian Lung Cancer Project. J Clin Oncol 1999;17:3522-30. [PubMed]
- Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med 1998;49:407-24. [Crossref] [PubMed]
- Schettino C, Bareschino MA, Rossi A, et al. Targeting angiogenesis for treatment of NSCLC brain metastases. Curr Cancer Drug Targets 2012;12:289-99. [Crossref] [PubMed]
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435-9. [Crossref] [PubMed]
- Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 2002;3:53-7. [Crossref] [PubMed]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353-64. [Crossref] [PubMed]
- Bonnesen B, Pappot H, Holmstav J, et al. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: relation to prognosis. Lung Cancer 2009;66:314-8. [Crossref] [PubMed]
- Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 2010;16:116-22. [Crossref] [PubMed]
- Pishko GL, Muldoon LL, Pagel MA, et al. Vascular endothelial growth factor blockade alters magnetic resonance imaging biomarkers of vascular function and decreases barrier permeability in a rat model of lung cancer brain metastasis. Fluids Barriers CNS 2015;12:5. [Crossref] [PubMed]
- Ilhan-Mutlu A, Osswald M, Liao Y, et al. Bevacizumab Prevents Brain Metastases Formation in Lung Adenocarcinoma. Mol Cancer Ther 2016;15:702-10. [Crossref] [PubMed]
- Chamberlain MC. Bevacizumab for the treatment of recurrent glioblastoma. Clin Med Insights Oncol 2011;5:117-29. [Crossref] [PubMed]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58-62. [Crossref] [PubMed]
- Hong S, Tan M, Wang S, et al. Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2015;141:909-21. [Crossref] [PubMed]
- Lauro S, Onesti CE, Righini R, et al. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res 2014;34:1537-45. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 2001;19:843-50. [PubMed]
- Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol 2009;27:5255-61. [Crossref] [PubMed]
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926-34. [Crossref] [PubMed]
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846-54. [Crossref] [PubMed]
- Galetta D, Cinieri S, Pisconti S, et al. Cisplatin/Pemetrexed Followed by Maintenance Pemetrexed Versus Carboplatin/Paclitaxel/Bevacizumab Followed by Maintenance Bevacizumab in Advanced Nonsquamous Lung Cancer: The GOIM (Gruppo Oncologico Italia Meridionale) ERACLE Phase III Randomized Trial. Clin Lung Cancer 2015;16:262-73. [Crossref] [PubMed]
- Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 2015;10:134-42. [Crossref] [PubMed]
- Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res 2010;16:269-78. [Crossref] [PubMed]
- Crinò L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010;11:733-40. [Crossref] [PubMed]
- Lynch TJ Jr, Spigel DR, Brahmer J, et al. Safety and effectiveness of bevacizumab-containing treatment for non-small-cell lung cancer: final results of the ARIES observational cohort study. J Thorac Oncol 2014;9:1332-9. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol 2010;100:443-7. [Crossref] [PubMed]
- Zustovich F, Ferro A, Lombardi G, et al. Bevacizumab-Based Therapy for Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Preliminary Results. Chemotherapy 2014;60:294-9. [Crossref] [PubMed]
- Khasraw M, Holodny A, Goldlust SA, et al. Intracranial hemorrhage in patients with cancer treated with bevacizumab: the Memorial Sloan-Kettering experience. Ann Oncol 2012;23:458-63. [Crossref] [PubMed]
- Sandler A, Hirsh V, Reck M, et al. An evidence-based review of the incidence of CNS bleeding with anti-VEGF therapy in non-small cell lung cancer patients with brain metastases. Lung Cancer 2012;78:1-7. [Crossref] [PubMed]
- Carden CP, Larkin JM, Rosenthal MA. What is the risk of intracranial bleeding during anti-VEGF therapy? Neuro Oncol 2008;10:624-30. [Crossref] [PubMed]
- Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012;76:362-7. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 2013;15:1257-63. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Camidge DR, Berge EM, Doebele RC, et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol 2014;9:1532-9. [Crossref] [PubMed]
- Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer 2015;121:883-92. [Crossref] [PubMed]
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7. [Crossref] [PubMed]
- Larkins E, Scepura B, Blumenthal GM, et al. U.S. Food and Drug Administration Approval Summary: Ramucirumab for the Treatment of Metastatic Non-Small Cell Lung Cancer Following Disease Progression On or After Platinum-Based Chemotherapy. Oncologist 2015;20:1320-5. [Crossref] [PubMed]
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 2014;17:471-94. [Crossref] [PubMed]
- Leighl NB, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 2010;5:1054-9. [Crossref] [PubMed]
- Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7. [Crossref] [PubMed]
- Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur J Cancer 2014;50:706-12. [Crossref] [PubMed]
- Ramalingam SS, Shtivelband M, Soo RA, et al. Randomized phase II study of carboplatin and paclitaxel with either linifanib or placebo for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2015;33:433-41. [Crossref] [PubMed]
- Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol 2012;30:2829-36. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Scagliotti GV, Felip E, Besse B, et al. An open-label, multicenter, randomized, phase II study of pazopanib in combination with pemetrexed in first-line treatment of patients with advanced-stage non-small-cell lung cancer. J Thorac Oncol 2013;8:1529-37. [Crossref] [PubMed]
- O'Brien ME, Gaafar R, Hasan B, et al. Maintenance pazopanib versus placebo in Non-Small Cell Lung Cancer patients non-progressive after first line chemotherapy: A double blind randomised phase III study of the lung cancer group, EORTC 08092 (EudraCT: 2010-018566-23, NCT01208064). Eur J Cancer 2015;51:1511-28. [Crossref] [PubMed]
- Novello S, Camps C, Grossi F, et al. Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J Thorac Oncol 2011;6:1260-6. [Crossref] [PubMed]
- Heist RS, Wang X, Hodgson L, et al. CALGB 30704 (Alliance): A randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:214-21. [Crossref] [PubMed]
- Blumenschein GR Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol 2009;27:4274-80. [Crossref] [PubMed]
- Spigel DR, Burris HA 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2582-9. [Crossref] [PubMed]
- Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2008;26:5407-15. [Crossref] [PubMed]
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619-26. [Crossref] [PubMed]
- de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011;29:1067-74. [Crossref] [PubMed]
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:1059-66. [Crossref] [PubMed]
- Abrams TJ, Lee LB, Murray LJ, et al. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003;2:471-8. [PubMed]
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516-24. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [Crossref] [PubMed]
- Iyer R, Fetterly G, Lugade A, et al. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother 2010;11:1943-55. [Crossref] [PubMed]
- Polverino A, Coxon A, Starnes C, et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res 2006;66:8715-21. [Crossref] [PubMed]
- Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007;25:2369-76. [Crossref] [PubMed]
- Blumenschein GR Jr, Kabbinavar F, Menon H, et al. A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol 2011;22:2057-67. [Crossref] [PubMed]
- Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep 2007;9:115-9. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [Crossref] [PubMed]
- Zhou J, Goh BC, Albert DH, et al. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: from bench to bedside. J Hematol Oncol 2009;2:33. [Crossref] [PubMed]
- Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 2005;65:4389-400. [Crossref] [PubMed]
- Williams KJ, Telfer BA, Shannon AM, et al. Inhibition of vascular endothelial growth factor signalling using cediranib (RECENTIN; AZD2171) enhances radiation response and causes substantial physiological changes in lung tumour xenografts. Br J Radiol 2008;81:S21-7. [Crossref] [PubMed]
- Whittles CE, Pocock TM, Wedge SR, et al. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation 2002;9:513-22. [Crossref] [PubMed]
- Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774-82. [Crossref] [PubMed]


