MUC16 in non-small cell lung cancer patients affected by familial lung cancer and indoor air pollution: clinical characteristics and cell behaviors
Introduction
Lung cancer is the leading cancer diagnosed worldwide (1.8 million/year) and has a mortality rate higher than the next three cancers combined (158,080 vs. 115,760 deaths) (1). Unfortunately, lung cancer survival remains poor, owing to diagnosis at advanced stage and resistance to standard chemotherapy (2). In order to study the complex elements in lung cancer development, we recruited lung cancer patients from China’s Yunnan Province, including Xuanwei/Fuyuan region, which reported some of the highest lung cancer rates in the world (3-6). Importantly, this subject population has special features: indoor air pollution caused by coal combustion and family aggregation of lung cancer (3-6).
Familial lung cancer (FLC) exhibits special characteristics when compared with its sporadic counterpart. Previous work revealed heterogeneity in different FLC populations. Many reported an increased lung cancer risk in FLC populations (7-12); some supported that FLC has a bigger effect in certain ethnic groups (7,8); others suggested female relatives have a higher risk than male relatives (9,10). Actually, lung cancer susceptibility could be inherited in complex patterns through generations, and there can be unique characteristics within each group or subpopulation.
MUC16 is a large transmembrane glycoprotein (20–25 mD) with 22,152 amino acid residues (13-15). MUC16 is overexpressed and associated with poor prognosis in various cancers, including lung cancer (14-17). Some studies showed that MUC16 could be potential therapy target for cancer patients (13,18,19). One study based on Cancer Genome Atlas reported that MUC16 was among the top mutated genes (TP53, USH2A, TTN, MUC16) in different cancers, including lung cancer (20). MUC16 has been shown to be associated with enhanced cancer cell growth, metastasis and chemoresistance (16,21-26), which are typical features of increased cancer aggressiveness.
Present work was designed to investigate the expression and clinical significance of MUC16 in non-small cell lung cancer (NSCLC) patients, affected by FLC and indoor air pollution caused by coal use, in China’s Yunnan Province; furthermore, to evaluate the role of MUC16 in the proliferation, migration, invasion and chemosensitivity of lung cancer cells.
Methods
Patients and tissue samples
Present study was designed to investigate the clinical significance of MUC16 in NSCLC patients affected by FLC and indoor air pollution in Yunnan, China. Patients were selected from those enrolled in Department of Thoracic Surgery I of Yunnan Cancer Hospital from Sep. 2015 to Jun. 2017. Subjects were selected based on the following criteria: (I) The case population was mainly composed of residents from Xuanwei/Fuyuan region of Yunnan Province, who primarily use coal for heating or cooking for more than 10 years; (II) the control subjects were patients from other areas in the same province, who reported no history of occupational or domestic coal use. In total, 185 cases and 92 controls were enrolled; (III) subjects with FLC were defined as individuals with three or more first-degree relatives affected by lung cancer. There were 51 patients classified as having FLC. All the information was based on self-report and confirmed by personal medical records.
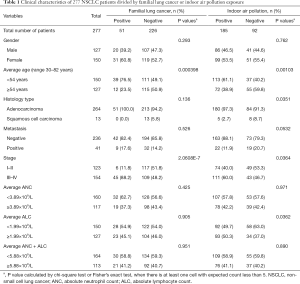
Clinicopathologic data were documented in hospital cooperated databank (https://www.linkdoc.com). The TNM stage was reviewed according to the 8th edition of The International Association for the Study of Lung Cancer (IASLC) staging system. Clinicopathologic data were shown in Table 1 and Table S1, majority of patients enrolled had adenocarcinoma (AD) and squamous cell carcinoma (SCC). The study was approved by the Ethical Committees of Yunnan Cancer Hospital (No. KY2019.57). All patients provided informed consent.
Full table
Full table
Tissue sample pairs including cancer and adjacent nonmalignant tissue of the same patient were stored in RNAlater (Sigma, St. Louis, MO, USA) immediately after surgery. A slide was cut from every sample for HE stain. Those containing >60% cancer tissue and <15% necrosis were used for study. Absolute neutrophil count (ANC) and absolute lymphocyte count (ALC) value were obtained before surgery or major treatment.
Quantitative real-time polymerase chain reaction (q-PCR)
Total RNA was extracted from tissue or cultured cells using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) Reverse transcription was performed using Promega reverse transcription kit (Promega, Madison, WI, USA). q-PCR was performed using SYBR Green Master Mix (Thermo Fisher, waltham, MA, USA)
Vectors construction for MUC16 gene knockout and overexpression
CRISPR-Cas9 vectors were constructed for MUC16 gene knockout as described in (27). In order to effectively knockout MUC16 gene, two sgRNA were combined to target the first exon of MUC16 (PX459-MUC16-sgRNA-1 and PX459-MUC16-sgRNA-2). Lenti-CRISPR-dCas9 system was used for MUC16 overexpression, three sgRNA were used simultaneously to increase activation efficiency, the vector construction and lentivirus packaging followed protocols in (28). PX459 and Lenti-CRISPR-dCas9 system were gift from Feng Zhang (Table S2). The sgRNA sequences were designed using CRISPRdirect (29) (http://crispr.dbcls.jp/) and listed in Tables S3,S4. More information is in the Supplementary File.

Full table

Full table

Full table
Cell culture, plasmid transfection and lentivirus infection
The cell lines used in this study were kindly provided by Cell Bank and Stem Cell Bank, Chinese Academy of Sciences. H23 and H838 lung cancer cells were cultured in RPMI medium supplemented with 10% FBS and antibiotics. Similarly, lentivirus packaging cell line 293T was cultured in DMEM medium with the above-mentioned supplements. The cells were incubated at 37 °C with 5% CO2.
MUC16-knockout vectors (PX459-MUC16-sgRNA-1; PX459-MUC16-sgRNA-2) were transfected into target cells using Lipofectamine 2000 (Thermo Fisher, waltham, MA, USA) according to the manufacturer’s instruction, empty vector was used as control. Lentivirus infection were carried out as mentioned in (28) with empty virus as control. Transfection and infection were performed freshly for each cell behavior experiment, MUC16 levels were monitored by q-PCR, cell populations with more than 60% MUC16 decrease and more than 3 times MUC16 increase were immediately used for the behavior experiments.
Immunoblot analysis
Cells were grown for 48 h after transfection or infection, then lysed using RIPA buffer (TIANGAN, Beijing, China), and the protein contents were measured using BCA Kit (TIANGAN). An amount of 60 µg protein from each sample was subjected to SDS-PAGE gel (5%) for electrophoresis, then transferred to PVDF membrane (Millipore, Bedford, MA, USA) and blocked in skim milk (5%) for 1 h. The membranes were incubated with primary antibody: mouse anti-MUC16 (Abcam, Cambridge, MA, USA) 1:500 in 1% BSA for 2 h at 37 °C; for loading control: mouse anti–β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1:1,000 in 1% BSA for 2 h at 37 °C. After washing, the membranes were incubated with secondary antibody: goat anti-mouse IgG peroxidase labeled (KPL, Gaithersburg, MD, USA). Proteins were detected by X-ray film (kodak) in a dark room using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Proliferation assay, cell migration and invasion assay
For growth kinetics analyses, cells were seeded in 96-well plates at a density of 100–500 cells/well, each group had 6 plates. MTT assay kit (TIANGAN) was used to reflect the number of viable cells present (metabolic activity growth). Cells in one plate were measured every 24 h for 6 days. The growth amount was determined as the relative absorbance.
For Migration assay, 1×106 cells were plated in the trans-well chamber (8 µm, Millipore) with serum-free medium, then inserted into 24-well plate containing 10% FBS in medium, and incubated for 15–20 hours. The inserts were fixed with methanol and HE stained, cells that did not migrate were removed. The insert membranes were scanned and analyzed using NIH image software (https://imagej.nih.gov/ij/), and the cell density is measured as pixel intensity.
For Invasion assay, the same trans-well chambers were first coated with Matrigel (BD Bioscience), other steps were the same as described in migration assay.
Cytotoxicity assay
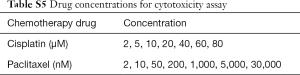
Cells were plated at 10,000–20,000 cells/well in 96-well plates, next day cells (confluence ~70%) were treated with increasing concentrations of cisplatin or paclitaxel (Sigma) for 72 h. The drug concentrations were listed in Table S5. Cell viability was measured by MTT assay kit (TIANGAN) following the manufacturer’s instruction. The percentage of cell survival was defined as the relative absorbance of treated versus untreated wells. All assays were performed in triplicate.
Full table
Data analysis
Statistical significance was evaluated by Student-t test, Chi-square test or Fischer’s exact test using SPSS 17.0 (SPSS Institute, Chicago, IL, USA). P<0.05 (two-sided P value) was considered to be significant.
Results
Lung cancer patients affected by family history and indoor air pollution: younger age and later stage at diagnosis
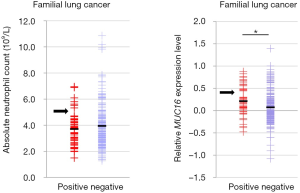
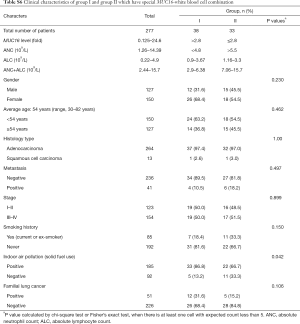
In total 277 subjects, 185 reported indoor air pollution from coal use, 92 were negative for coal burning exposure, and 51 were classified having FLC. The characteristics were shown in Table 1, Table S1 and Figure 1. FLC showed strong association with early-onset (P<0.01) (Figure 1A) and later stage (P<0.01). Indoor air pollution was associated with younger age (P<0.01) (Figure 1B), AD histology type (P<0.05) and later stage (P<0.05). FLC subjects had an average age of 50 (range, 36–70) and majority (45 cases, 88.2%) had stage III–IV disease, oppositely the average age of non-FLC subjects was 55 (range, 30–82), only 48.2% (109/226) of them were in stage III–IV. Divided by indoor air pollution exposure, positive group had an average age at 52 (range, 32–76), with 60.0% (111/185) patients in stage III–IV, comparatively negative group’s average age was at 57 (range, 30–82), with 46.7% (43/92) patients in stage III–IV.
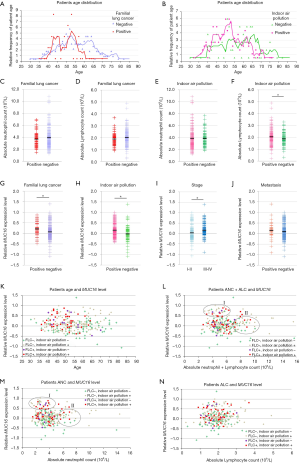
Interestingly, evaluated by relative ratio of patient age, FLC group showed double peaks (44 vs. 53 years), sporadic group also had two peaks (53 vs. 63 years), but both were much later than their FLC counterpart (Figure 1A). If divided by indoor air pollution exposure, positive group had a clear peak around age 52, while the negative group showed a much flatten curve (Figure 1B).
In addition, patients’ absolute neutrophil (ANC) and lymphocyte (ALC) count were also analyzed. Even not significant, FLC subjects tended to have slightly lower ANC (average: 3.70×109/L vs. 3.94×109/L) and ALC (average: 1.88×109/L vs. 2.01×109/L) than non-FLC subjects (Figure 1C,D). Indoor air pollution was associated with higher ALC value (P<0.05) (average: 2.05×109/L vs 1.87×109/L), but no obvious ANC value difference was found for indoor air pollution exposure (average: 3.90×109/L vs. 3.89×109/L) (Figure 1E,F).
Overexpression of MUC16 in lung carcinoma show association with FLC and indoor air pollution
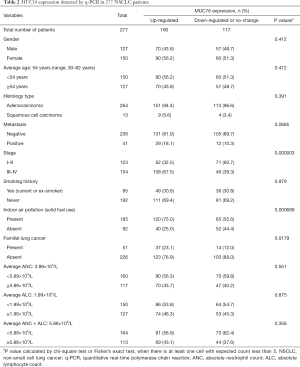
To investigate the significance of MUC16 in our subject population, we examined MUC16 expression in the above 277 cases by q-PCR. MUC16 overexpression was significantly associated with FLC (P<0.05) (Figure 1G) and indoor air pollution (P<0.01) (Figure 1H), and also later stage (P<0.01) (Figure 1I) (Table 2). FLC subjects had nearly doubled rate of MUC16 up-regulation (23.1% vs. 12.0%), and patients exposed to indoor air pollution were more likely to overexpress MUC16 (75.0% vs. 55.6%), stage III–IV patients showed much higher ratio of MUC16 overexpression (67.5% vs. 39.3%). Although not significant (P=0.0685), more metastasis events were observed in MUC16-upregulated group (18.1% vs. 10.3%) (Figure 1J). No apparent association was found for other parameters. However, one study suggested that MUC16 overexpression rate was higher in AD compared to SCC (26). It may be explained by the special characteristics and also the size of our subject population.
Full table
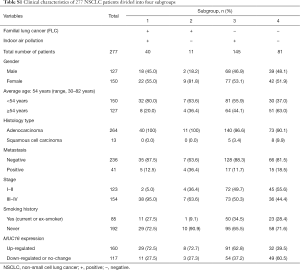
All patients were further divided into four subgroups (Table S1) (Figure 1K). (I) Subjects affected by both FLC and indoor air pollution developed lung cancer much earlier, together with more frequently up-regulated MUC16. (II) FLC + and indoor air pollution – group had only a few individuals, mainly younger with up-regulated MUC16. (III) FLC—but indoor air pollution + group also had relatively more young patients, some with clearly increased MUC16. (IV) Subjects negative for both FLC and indoor air pollution seemed to have more even age distribution, with slightly less MUC16 overexpression in young patients.
Previous reports indicated that MUC16 could suppress immune response (16,30), so the relationship between MUC16 level and patients’ absolute neutrophil (ANC)/lymphocyte (ALC) count was also analyzed. To better reflect immune reaction, the ANC, ALC values were analyzed separately and also combined (Figure 1L,M,N). In Figure 1L,M, two groups of subjects seemed drifting outside the main population, in MUC16-ALC (Figure 1N), subgroups positive for FLC or indoor air pollution had relatively higher MUC16 (already confirmed), but no obvious unbalance was found in ALC distribution. Therefore, threshold values were set to isolate those individuals in Figure 1L, M for further study (Table S6). Group I: MUC16 increase >2.8-fold (apparently elevated) and ANC <4.8×109/L (average-to-low). Group II: MUC16 level ≤2.8-fold increase (included those with less MUC16 increase, no-change and down-regulated) and ANC >5.5×109/L (higher-than-majority). The circles in Figure 1L,M covered major members to represent the group. Threshold standard is in the supplementary material (Figure S1).
Full table
Both groups were mostly composed of patients with either FLC or indoor air pollution or double positive. Group I included 38 individuals, with middle-to-low ANC + ALC value and clearly higher MUC16. Group II had 33 subjects, with higher-than-majority ANC + ALC value but lower MUC16. It indicated that patients with higher MUC16 overexpression seemed to have a lower number of white blood cells, for ANC alone (Figure 1M) and ANC + ALC (Figure 1L). Intriguingly, group I had nearly doubled rate for FLC (31.6% vs. 15.2%) and was also significantly higher for in indoor air pollution exposure (P<0.05), suggesting the two factors were not only associated with MUC16 up-regulation but also possibly suppressed immune reaction.
MUC16 gene knockout and overexpression in human lung cancer cell line
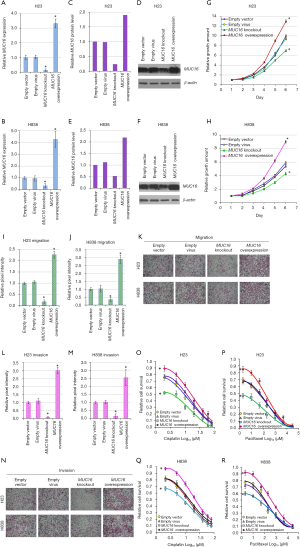
To examine the function of MUC16 in lung cancer, we performed gene knockout (two different sgRNA targets) and overexpression (three different sgRNA targets) of MUC16 in human lung cancer cell line H23 and H838. The MUC16 up/down regulation could be detected at both mRNA (Figure 2A,B) and protein level (Figure 2C,D,E,F). Since there was no further cell selection after vector transfection, protein down-regulation was around 25% (H23) and 50% (H838), but MUC16 up-regulation had apparent effect in both cell lines (~200%) (Figure 2C,D). Compared with ectopic overexpression of MUC16-Cter (the cytoplasmic tail region of MUC16) in several studies (21,22,24), the Lenti-CRISPR-dCas9 system can up-regulate MUC16 whole protein level by directly activating its transcription from promoter region (28), thus better for examining the influence of MUC16 overexpression.
High MUC16 level promote lung cancer cell proliferation and migration/invasion
In growth kinetics assay, MUC16 knockout cells had clearly decreased growth rate (P<0.05) compared to empty vector groups, on the other hand, MUC16 overexpressed cells had significantly higher growth rate compared to empty virus infected cells (P<0.05) (Figure 2G,H). The growth rate increase seemed more apparent in H838 than H23, possibly reflecting cell line variation. These results indicated that MUC16 could play positive role in lung cancer cell proliferation.
Migration assay showed that MUC16 knockout cells had decreased migratory capacity (P<0.05), in the opposite, MUC16 overexpressed cells had increased migratory capacity than empty virus infected cells (P<0.05) (Figure 2I,J,K). Compared to H23, the migratory capacity of H838 showed more increase and slightly less decrease. These results suggested that MUC16 could contribute to the migration of lung cancer cells.
Similar results were found in invasion assay. MUC16 knockout cells showed decreased invasion (P<0.05), while MUC16 overexpressed cells revealed increased invasion capacity (P<0.05) (Figure 2L,M,N). Cell line variations could still be observed: H23 seemed to have bigger changes in invasion capacity for both MUC16 up/down regulation. These results reflected that MUC16 might also boost the invasion capacity of lung cancer cells.
MUC16 overexpression is associated with chemoresistance in lung cancer cells
Overall, MUC16 knockout cells were more sensitive to cisplatin and paclitaxel (Figure 2O,P,Q,R). In addition, no significant change was observed between empty vector and empty virus treated cells. On the other hand, MUC16 overexpressed lung cancer cells were generally more resistant to the cytotoxic effects of cisplatin and paclitaxel. Taken together, these results indicated that MUC16 could contribute to chemoresistance in lung cancer cells.
The cell line variations were also detected: after MUC16 down-regulation, H23 showed bigger drop in both cisplatin and paclitaxel resistance (Figure 2O,P), while H838 had less resistance capacity decrease (Figure 2Q,R). In MUC16 up-regulation, H838 had bigger cisplatin-resistance increase than H23, the paclitaxel-resistance increase was also higher for H838, only visible in low concentrations for H23.
Discussion
MUC16 has been studied in different cancers across populations. Our subject population has its signature characters: FLC history, indoor air pollution caused by coal use, and also the highest lung cancer incidence in the world among never smokers (3-6). All make it unique to study the complex interaction between genetic and environmental factors in lung cancer etiology. FLC showed strong association with early-onset (P<0.01) and later stage (P<0.01), which was consistent with previous findings (8,9,12). Indoor air pollution was associated with younger age (P<0.01), later stage (P<0.05) and AD histology type (P<0.05). Both factors were considered as crucial elements in lung cancer development (9-11). Interestingly, the double age peaks of FLC and sporadic group (Figure 1A) suggested multiple major contributors to lung cancer in our subject population, besides FLC and indoor air pollution.
We found that MUC16 overexpression was associated with FLC (P<0.05), indoor air pollution (P<0.01), and later stage (P<0.01), furthermore, increased metastasis was observed in patients with up-regulated MUC16 (18.1% vs. 10.3%). Similarly, many studies supported high MUC16 was associated with increased metastasis and poor prognosis (17,22,24). Since MUC16 functions as molecular barrier on epithelial cells, it would be reasonable to predict that compositions in polluted air could stimulate MUC16 up-regulation as protective response. But in our study, no apparent correlation was found between high MUC16 and smoking, possibly suggesting MUC16 overexpression was a response to a wider spectrum of stimulants, and wasn’t specific to cigarette ingredients. Furthermore, lung cancers in non-smokers were also different from those in smokers (9,10,31). Importantly, the mechanism underlining the association between FLC and elevated MUC16 deserves further investigation. One study indicated that MUC16 mutation was associated with tumor mutation load (32), and FLC patients could possibly carry larger tumor mutation load, since the susceptible elements in FLC subjects made them more vulnerable to mutation-inducing carcinogens. The mutation rate could also be varied for different genes in one individual, and evidence suggested that certain genes were more frequently mutated in FLC population (31). As a result, elevated MUC16 might potentially be a unique feature to our subject population, like one molecular character of inherited lung cancer susceptibility in local residents.
To examine the function of MUC16 in lung cancer, we carried gene knockout and overexpression in human lung cancer cell line H23 and H838. We found that high MUC16 level promoted lung cancer cell proliferation, migration, invasion and also chemoresistance, additionally there were also variations among different cell lines. Our results were well supported by previous reports in different cancers (22-26). Some (22,23,26) found MUC16 mediated JAK2/STAT3/GR signal pathway, and promoted cancer cell growth/migration through TSPYL5. Moreover, MUC16 could induce resistance to chemotherapy drugs by up-regulating TSPYL5, which suppresses p53 activity.
Beside its positive roles in cancer cells, MUC16 also interferes with immune reaction. There were evidences that MUC16 could suppress human innate immune responses by regulating NK cells and macrophages (16,30). MUC16 can form aggregates with neutrophils, macrophages, and platelets, conferring protection to cancer cells during hematological dissemination (16). Intriguingly, we also found patients with more MUC16 up-regulation seemed to have a lower number of white blood cells, especially neutrophils. Oppositely some subjects showed less MUC16 could have much higher white blood cell count. It helped to explain that high MUC16 meant poor prognosis. On the contrary, presence of MUC16 neo-antigen-specific T cells in cancer patients suggested that MUC16 could serve as a potential target for cancer immunotherapy and radioimmunotherapy (16,18,19), which might possibly benefit our subject population.
Conclusions
MUC16 can play crucial roles in lung cancer pathogenesis, progression and chemoresistance. Interestingly, its association with FLC and indoor air pollution highlights the complexity of lung cancer etiology. Our findings provide useful information to study the intricate and dynamic interaction between environmental carcinogens and population genetic background.
Supplementary
Subject population background
Our subject population were recruited from China’s Yunnan Province, certain region here reported some of the highest lung cancer rates in the world, such as Xuanwei/Fuyuan (3-6). These areas have long been focus of lung cancer studies, including epidemiology, molecular or clinical research. Interestingly, the subject population has two characters: familial lung cancer (FLC) and indoor air pollution caused by coal combustion, because local residents use coal for cooking and heating for generations (3-6).
Present study was designed to investigate the clinical significance of MUC16 in NSCLC patients affected by familial lung cancer (FLC) and indoor air pollution caused by coal use in Yunnan, China. Subjects were selected by the following criteria: (I) the case population was mainly composed of residents from Xuanwei/Fuyuan region of Yunnan Province, who primarily use coal for heating or cooking for more than 10 years. (II) The control subjects were patients from other areas in the same province, who reported no history of occupational or domestic coal use. In total, 185 cases and 92 controls were enrolled. (III) Subjects with familial lung cancer were defined as individuals with three or more first-degree relatives affected by lung cancer. There were 51 patients classified as having familial lung cancer. All the information was based on self-report and confirmed by personal medical records.
The subject population can be further divided into 4 subgroups: FLC+, indoor air pollution+; FLC+, indoor air pollution−; FLC−, indoor air pollution+; FLC−, indoor air pollution−. Both characters, one genetic and one environmental were analyzed in our study. Clinicopathologic data were shown in Table 1 and Table S1.
Vectors construction for MUC16 gene knockout and overexpression
CRISPR-Cas9 vectors were constructed for MUC16 gene knockout as described in (27). In order to effectively knockout MUC16 gene, two sgRNA were combined to target the first exon of MUC16 (PX459-MUC16-sgRNA-1 and PX459-MUC16-sgRNA-2). Lenti-CRISPR-dCas9 system was used for MUC16 overexpression, three sgRNA were used simultaneously to increase activation efficiency. The vector construction and lentivirus packaging followed protocols in (28). PX459 and Lenti-CRISPR-dCas9 system were gift from Feng Zhang (Table S2, Addgene plasmid #62988; #61425, #61426, #61427). The sgRNA sequences were designed using CRISPRdirect (29) (http://crispr.dbcls.jp/) and listed in Tables S3,S4.
The sgRNA site on MUC16 genome
The MUC16 genome sequence showed here includes 350 bp upstream the transcription start site and part of the first exon. 200bp upstream is preferred for activation (28). Upstream sequence is in lowercase and the 1st exon is in uppercase. MUC16 knockout sgRNA is marked in yellow and MUC16 overexpression sgRNA is marked in green.
Oligo annealing and cloning into backbone vectors
MUC16 knockout
- Digest 1ug of pX459 with BbsI for 30 min at 37 °C
- Gel purify digested pX459 using Gel Extraction Kit (TIANGAN)
- Anneal each pair of oligos
- Ligation reaction
- Transformation into Stbl3 bacteria
1 µg pX459
1 µL FastDigest BbsI (Fermentas)
2 µL 10× FastDigest Buffer
X µL ddH2O
20 µL in total
1 µL oligo forward (100 mM)
1 µL oligo reverse (100 mM)
2 µL 5× annealing butter (TIANGAN)
6 µL ddH2O
10 µL in total
Anneal in a thermocycler using the following parameters
95 °C 5 min and then ramp down to 25 °C at 5 °C/min
X µL BbsI digested pX459 from step 2 (50 ng)
1 µL annealed oligo from step 3
1 µL 10× ligation Buffer
1 µL T4 Ligase (Fermentas)
X µL ddH2O
10 µL in total
Incubate reaction at 22 °C for 40 min
MUC16 overexpression
- Digest and 5 µg of lenti sgRNA zeo backbone with BsmBI for 60 min at 37 °C
- Gel purify digested plasmid using Gel Extraction Kit (TIANGAN)
- Anneal each pair of oligos
- Ligation reaction
- Transformation into Stbl3 bacteria
5 µg lenti sgRNA zeo backbone
3 µL FastDigest BsmBI (Fermentas)
6 µL 10× FastDigest Buffer
0.6 µL 100 mM DTT (freshly prepared)
X µL ddH2O
60 µL in total
1 µL oligo forward (100 mM)
1 µL oligo reverse (100 mM)
2 µL 5× annealing butter (TIANGAN)
6 µL ddH2O
10 µL in total
Anneal in a thermocycler using the following parameters:
95 °C 5 min and then ramp down to 25 °C at 5 °C/min
X µL digested sgRNA zeo backbone from step 2 (50 ng)
1 µL annealed oligo from step 3
1 µL 10× ligation Buffer
1 µL T4 Ligase (Fermentas)
X µL ddH2O
10 µL in total
Incubate reaction at 22 °C for 60 min
Plasmid transfection and lentivirus infection
MUC16-knockout vectors (PX459-MUC16-sgRNA-1; PX459-MUC16-sgRNA-2) were transfected into target cells using Lipofectamine 2000 (Thermo Fisher, waltham, MA, USA) according to the manufacturer’s instruction, empty vector was used as control. Lentivirus packing and infection were carried out as mentioned in (28) with empty virus as control.
Present work first studied the immediate effect of MUC16 change on a population of cancer cells, and no clone selection was carried to remove MUC16-unchanged cells. Since drug selection would purify subpopulations featured with up/down-regulated MUC16, but other genes level may also change during subpopulation selection, when compared with the original cell population. Therefore, transfection and infection were performed freshly for each cell behavior experiment, MUC16 levels were monitored by q-PCR, cell populations with more than 60% MUC16 decrease and more than 3 times MUC16 increase were immediately used for the behavior experiments. Furthermore, cancer cells show heterogeneity in patients’ tumor as well as cultured cells, if MUC16 change in a subpopulation could influence the behaviors of the whole cell population, it still provides meaningful information.
Cytotoxicity assay
We treated cells with increasing concentrations of cisplatin or paclitaxel for 72 h. The drug concentrations were listed in Table S5.
Results part
Threshold setting standard based on MUC16-ANC
Two groups of subjects seemed drifting outside the main population (Figure 1L,M) (Table S6), threshold values were set to isolate those individuals for further study (based on MUC16-ANC). Group I: MUC16 increase >2.8-fold (apparently elevated) and ANC <4.8×109/L (average-to-low). Group II: MUC16 level ≤2.8-fold increase (included those with less MUC16 increase, no-change and down-regulated) and ANC >5.5×109/L (higher-than-majority). The circles in Figure 1L,M covered major members to represent the group.
In FLC-ANC and FLC-MUC16 (Figure 1C,G), FLC+ subjects were clearly divided by a gap (black arrow), suggesting potential subgroups. We used the upper limit (2.8-fold) of MUC16 gap to separate group I and II as the first step (in Figure 1G, lg2.8=0.45), then we used the upper (5.5×109/L) and the lower (4.8×109/L) limit of ANC gap to separate group I and II further apart, so as to observe bigger difference.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No: 81702274); Yunnan Applied Basic Research Projects-Union Foundation [No: 2017FE468 (-159), 2015FB069, 2017FE467 (-0187), 2017FE468 (-214), 2017FA039]; Internal Organization Research Projects of Yunnan Cancer Hospital (No: 2017NS198, 2017NS199); Yunnan Health Training Project of High Level Talent (D-2017012,D-201641); Doctor Research Foundation of Yunnan Cancer Hospital (No: BSKY201705).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethical Committees of Yunnan Cancer Hospital (No. KY2019.57). All patients provided informed consent. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- AMC. Cancer Facts and Figures. Atlanta: American Cancer Society; 2014.
- Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest 2006;129:1088-97. [Crossref] [PubMed]
- Barone-Adesi F, Chapman RS, Silverman DT, et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ 2012;345:e5414. [Crossref] [PubMed]
- Chapman RS, Mumford JL, Harris DB, et al. The epidemiology of lung cancer in Xuan Wei, China: current progress, issues, and research strategies. Arch Environ Health 1988;43:180-5. [Crossref] [PubMed]
- Mumford JL, Chapman RS, Harris DB, et al. Indoor air exposure to coal and wood combustion emissions associated with a high lung cancer rate in Xuan Wei, China. Environment International 1989;15:315-20. [Crossref]
- Mumford JL, He XZ, Chapman RS, et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science 1987;235:217-20. [Crossref] [PubMed]
- Coté ML, Kardia SLR, Wenzlaff AS, et al. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA 2005;293:3036-42. [Crossref] [PubMed]
- Coté ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: A pooled analysis from the International Lung Cancer Consortium. Eur J Cancer 2012;48:1957-68. [Crossref] [PubMed]
- Lin KF, Wu HF, Huang WC, et al. Propensity score analysis of lung cancer risk in a population with high prevalence of non-smoking related lung cancer. BMC Pulm Med 2017;17:120. [Crossref] [PubMed]
- Lin H, Huang YS, Yan HH, et al. A family history of cancer and lung cancer risk in never-smokers: A clinic-based case-control study. Lung Cancer 2015;89:94-8. [Crossref] [PubMed]
- Karp I, Sylvestre MP, Abrahamowicz M, et al. Bridging the etiologic and prognostic outlooks in individualized assessment of absolute risk of an illness: Application in lung cancer. Eur J Epidemiol 2016;31:1091-9. [Crossref] [PubMed]
- Musolf AM, Simpson CL, de Andrade M, et al. Familial Lung Cancer: A Brief History from the Earliest Work to the Most Recent Studies. Genes (Basel) 2017;8. [Crossref] [PubMed]
- Das S, Batra SK. Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy. Cancer Res 2015;75:4669-74. [Crossref] [PubMed]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004;4:45-60. [Crossref] [PubMed]
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9:874-85. [Crossref] [PubMed]
- Bhatia R, Gautam SK, Cannon A, et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev 2019;38:223-36. [Crossref] [PubMed]
- Jonckheere N, van Seuningen I. Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20 signature is associated with poor survival in human carcinomas. J Transl Med 2018;16:259. [Crossref] [PubMed]
- Aithal A, Rauth S, Kshirsagar P, et al. MUC16 as a novel target for cancer therapy. Expert Opin Ther Targets 2018;22:675-86. [Crossref] [PubMed]
- Rao TD, Fernández-Tejada A, Axelrod A, et al. Antibodies Against Specific MUC16 Glycosylation Sites Inhibit Ovarian Cancer Growth. ACS Chem Biol 2017;12:2085-96. [Crossref] [PubMed]
- Kim N, Hong Y, Kwon D, et al. Somatic mutaome profile in human cancer tissues. Genomics Inform 2013;11:239-44. [Crossref] [PubMed]
- Akita K, Tanaka M, Tanida S, et al. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial cancer cells reduces cell-cell adhesion. Eur J Cell Biol 2013;92:257-63. [Crossref] [PubMed]
- Das S, Rachagani S, Torres-Gonzalez MP, et al. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015;6:5772-87. [Crossref] [PubMed]
- Lakshmanan I, Ponnusamy MP, Das S, et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene 2012;31:805-17. [Crossref] [PubMed]
- Thériault C, Pinard M, Comamala M, et al. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol 2011;121:434-43. [Crossref] [PubMed]
- Boivin M, Lane D, Piché A, et al. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol 2009;115:407-13. [Crossref] [PubMed]
- Lakshmanan I, Salfity S, Seshacharyulu P, et al. MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53. Clin Cancer Res 2017;23:3906-17. [Crossref] [PubMed]
- Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013;8:2281-308. [Crossref] [PubMed]
- Konermann S, Brigham MD, Trevino AE, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2015;517:583-8. [Crossref] [PubMed]
- Naito Y, Hino K, Bono H, et al. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015;31:1120-3. [Crossref] [PubMed]
- Felder M, Kapur A, Rakhmilevich AL, et al. MUC16 suppresses human and murine innate immune responses. Gynecol Oncol 2019;152:618-28. [Crossref] [PubMed]
- Gaughan EM, Cryer SK, Yeap BY, et al. Family history of lung cancer in never smokers with non-small-cell lung cancer and its association with tumors harboring EGFR mutations. Lung Cancer 2013;79:193-7. [Crossref] [PubMed]
- Li X, Pasche B, Zhang W, et al. Association of MUC16 mutation with tumor mutation load and outcomes in patients with gastric cancer. JAMA Oncol 2018;4:1691-8. [Crossref] [PubMed]