Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study
Introduction
According to the 2018 global status report on the worldwide burden of cancer, lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) (1). Specifically, in the United States, lung cancer is the second most common malignancy in both genders, and the estimated new cases were 116,990 in men and 105,510 in women in 2017, with lung cancer accounting for more than one-quarter of all cancer deaths (2), which is similar to the conclusions of the above status report.
The high mortality rate of lung cancer can be explained by the high incidence and the low detection rate. With the aggravation of environmental pollution (3), the increase of tobacco use (4), and the demographic trend of an aging society (5), the incidence of lung cancer is increasing year by year. Also, lung cancer is often asymptomatic at an early stage. Therefore, patients are likely to already have metastases at diagnosis (6,7). It was reported that bone metastases occupied 30–40% of patients with lung cancer (8) and that patients will suffer from unexpected skeletal complications, including pathological fractures, spinal cord compression, and severe bone pain in their short survival months (9).
The development of the systematic treatment of tumors, including the surgery of primary sites and distant metastasis sites, stereotactic body radiation therapy, chemotherapy with low toxicity and side effects drugs, especially targeted drugs like Pemetrexed and Gemcitabine, and immunotherapy represented by PD-1/PD-L1 immunological checkpoint inhibitors (ICI) significantly prolong the survival of cancer patients and improve the quality of patient life.
Although there have been some studies on LCBM (10,11), data in the epidemiology and signatures of lung cancer with bone metastasis (LCBM) are still unclear. In order to comprehensively demonstrate the epidemiological characteristics and analyze the possible prognostic factors in the survival of patients with lung cancer bone metastasis, and predict the survival time of individual patients, the Surveillance, Epidemiology and End Results (SEER) database was used in our present study. The prognostic factors in lung cancer including patient personal information, location, total number, pathological features of the tumor, and different treatments to lung cancer were selected to evaluate and predict prognosis for lung cancer patients with bone metastasis.
Methods
This study analyzed the de-identified data obtained from the SEER 18 registry. The SEER Program is one of the largest registry sources of cancer information supported by the National Cancer Institute of the United States. The 1975–2016 SEER Research Incidence data (November 2018 submission) became available on April 2019.
Analysis of the de-identified data from the SEER program was exempt from medical ethics review, and no informed consent was required. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study population selection
Selection criteria included the following: White and Black lung cancer patients diagnosed with bone metastasis from 2010 to 2016 of all ages. To identify the patients with metastatic lung cancer to bones, we selected cases with LCBM at first diagnosis for further research. Cases with brain, liver, and lung metastases from the lung cancer population were also selected in the same way to compare with bone metastases. Patients who were diagnosed via autopsy or death certificate, or whose detailed information was unknown or blank were excluded.
Data elements
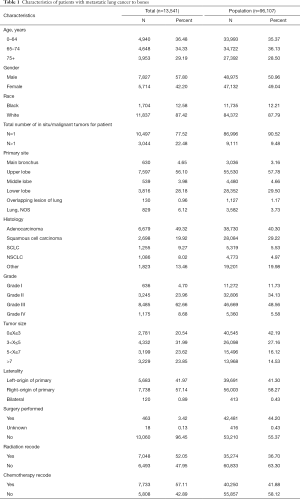
Data extracted for each case involved 12 prognostic variables. The detailed variables are shown in Table 1. Demographic variables included age at diagnosis, gender, race, and survival time (months). In addition, cancer characteristics including primary tumor sites, laterality, the total number of (in situ/malignant) tumor sites, tumor size, and histologic types, in addition to their treatment information, including surgery, chemotherapy, and radiation, were collected from the database. Among them, histological types were identified with the following IDO-O-3 codes: “8140. Adenocarcinoma”, “8070-8078. Squamous cell carcinoma”, “8041-8045. SCLC”, and “8046. NSCLC”. Tumor sizes were stratified according to the 8th edition of the TNM staging guideline released by the Union for International Cancer Control (UICC) in 2018. Patients with missing values or unspecified conditions on these items were excluded. In addition, diagnoses made by death certificate or autopsy were also excluded.
Full table
All the raw data in this study were downloaded from the SEER website (https://seer.cancer.gov/data/) via the SEER*Stat in client-server mode after we submitted a request for access and signed the SEER research data agreement.
Statistical analysis
The ages of patients were stratified by using the X-tile program (Yale University, New Haven, CT, USA), which was initially developed to determine the optimal cutoff values of variables for patients with breast cancer (12). The optimal cutoff value of age in terms of overall survival (OS) was identified as 65- and 75-year (Figure S1A,B), and survival curves were created using Kaplan-Meier methods for those age subgroups for OS (Figure S1C). In order to process the data conveniently, we divided the patients into 3 age groups (0–64, 65–74, and 75+ years).
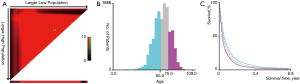
Using the SEER*Stat statistical software for Windows, we examined the incidence for cases diagnosed from 2010 to 2016 and its change over time. In addition, we also compared the incidence of different metastatic sites (bone, brain, liver, lung). One-, three-, six-, nine-month, and 1 year relative cancer survival rates for lung cancer with bone metastases were also examined for different metastases. Detailed differences between different age subgroups are also described. SEER describes relative survival as “a net survival measure representing cancer survival in the absence of other causes of death.” Relative survival is defined as the ratio of the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in a comparable set of cancer-free individuals. The formulation is based on the assumption of independent competing causes of death. Moreover, survival curves were created, using Kaplan-Meier methods for the race, histological type, total number of sites, primary sites, tumor size, grade, surgery performed, radiation performed, and chemotherapy performed for OS. The differences between the survival curves were compared using the log-rank test.
Prognostic nomogram for overall survival
We used Cox proportional hazards regression to analyze the association between OS and relevant prognostic factors, including age, gender, race, primary tumor sites, laterality, the total number of tumors, grade, tumor size, and histologic types, along with their treatment information based on the SEER database. We then developed a novel visual nomogram and tested it by using Heagerty’s concordance index (C-index) and calibration curve.
Statistically significant variables were analyzed using univariate and multivariable Cox proportional hazard regressions to identify hazard ratios (HRs) and their respective 95% confidence intervals (CI), and then to determine the relationship between prognostic factors and overall survival. All statistical analyses were completed in the SPSS software (version 18; IBM Corp., USA), STATA software (version 14.2; Stata Corp, College Station, TX, USA), and R software (version 3.3.0; http://www.r-project.org/). In all statistical analyses, a P value of <0.05 was considered significant.
Results
Study population characteristics
There were 96,107 lung cancer patients between 2010 and 2016 in the United States with complete data on our selected variables, and after excluding patients with missing follow-up or unknown data, a total of 13,541 people were included in the present study.
The demographic data of patients and the characteristics of tumors are shown in Table 1. According to the age distribution at diagnosis, patients were divided into several groups: 0–64 years (36.48%), 65–74 years (34.33%), and 75 years or older (29.19%). There was a slight male predominance (57.80%) versus female (42.20%) in sex distribution. Of those meeting all eligibility criteria, 11,837 (87.42%) were white patients, 1,704 (12.58%) were black patients, and other race patients were not included.
The tumor location was predominantly classified as upper lobe (56.10%). There was little lateralized specialization because the right lung accounted for 57.14% of all the study group, and the left accounted for 41.97%; 77.52% of the patients were affected with a single site, while 22.48% had multiple sites of the lung tumor. The distribution of each tumor size group appears to be uniform, with the 3–5 cm group accounting for 31.99% of the total. For treatment, 3.42% of patients chose to receive surgery, 52.05% chose radiation, and 57.11% chose chemotherapy.
Incidence
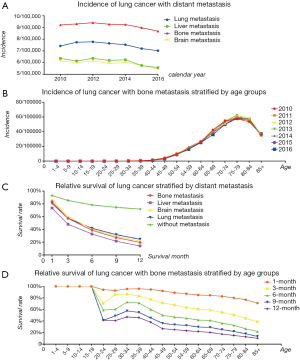
We compared the incidence of four common metastatic sites of lung cancer from 2010 to 2016, including bone, the brain, liver, and lung, and found that the highest incidence of metastasis was bone (Figure 1A). We then analyzed the annual incidence of patients in different age groups each year (Figure 1B). In the data from 2010 to 2016, as age increased, the incidence increased year by year, peaking at 75 to 79 years old, and then decreased.
Survival analysis
The OS of patients with lung cancer with/without different metastatic sites are shown. All patients in these four categories had a poor prognosis, and liver metastasis of lung cancer was the worst, followed by bone metastasis. Details and data are shown in Figure 1C. The survival rate of patients with LCBM is shown in Figure 1D, and it is obvious that the survival rate of adults continued to deteriorate with the increase of age.
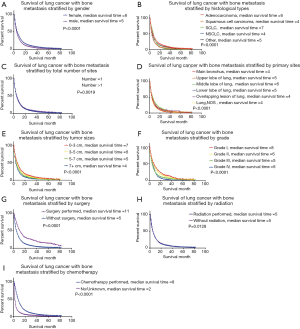
Kaplan-Meier survival curves also confirmed the effect of prognostic factors, including gender, histologic types, the total number of tumors, primary tumor sites, tumor size, and grade, along with their treatment information. The Log-Rank tests for all Kaplan-Meier survival curves are statistically significant (P<0.05), but the curves are crossed, meaning that there may be multiple factors of interference (Figure 2). Additionally, we found that the median survival time was similar and not optimistic.
Prognostic factors of OS
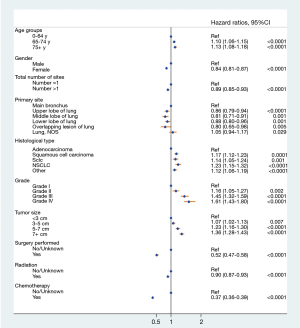
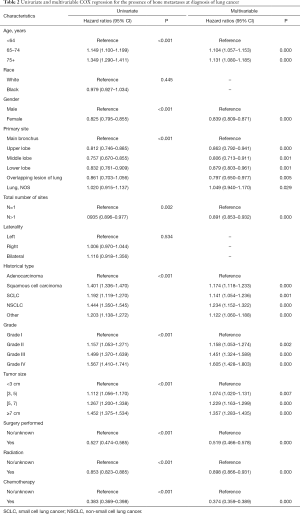
Prognostic factors of OS were calculated with univariate and multivariate Cox proportional hazard regression (Table 2), which revealed that age (P<0.001), gender (P<0.001), total number of sites (P=0.002), primary site location (P<0.001), histology type (P<0.001), grade (P<0.001) and treatment performed (P<0.001) were all independent prognostic factors. Race (P=0.445) and laterality (P=0.534) were not found to be significant factors. The forest plot makes the results more intuitive (Figure 3).
Full table
Nomogram
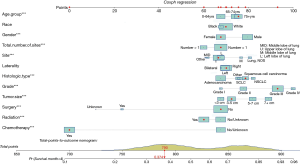
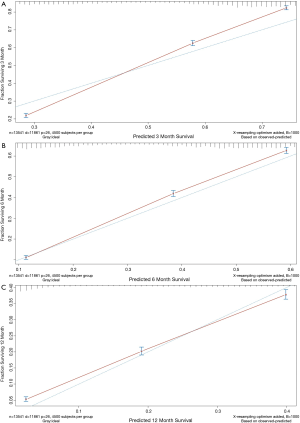
Finally, based on the Cox regressions results, we built and interpreted a nomogram (Figure 4) for predicting the survival rate. To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the total points axis, and a line is drawn downward to the survival axis to determine the likelihood of median survival time (4 months). In our new visualized nomogram, the blue boxes below the name and the yellow color block on the total points axis represent the sample size, which shows the demographic statistics of the LCBM and the population distribution of the prognosis. In the internal validation set, the c-index was 0.7188 [95% confidence interval (CI), 0.7154–0.7222]. The calibration curve (Figure S2) shows its concordance.
For example, a patient is more than 75 years old, is a white female, has a single primary site, a 4-cm tumor located in the upper right lobe, grade III squamous cell carcinoma, and she has undergone radiation and chemotherapy treatment but not surgery. Her total points are 790, and the probability of living longer than the median survival time (4 months) is 0.374.
Discussion
Our work unfolded in the following course. First, starting from epidemiology, we compared the incidence and survival rate of different metastases horizontally and compared the personalized differences in patients with bone metastasis in depth. Second, we selected a number of survival factors, including information of patients, the tumor itself, and treatment type, to carry out multivariate analysis to screen out some practical and common influencing factors. Third, we proposed a practical nomogram model in order to make an individual prediction after mastering certain case data.
Previous epidemiological studies on LCBM have shown that the prognosis is not optimistic (2,13,14), although there are some differences in survival rates due to differences in the study population inclusion criteria. For example, in Danish patients of lung cancer, Karynsa reported that the 1-year survival was 12.1% in patients with bone metastasis without skeletal-related events and 5.1% in patients with both bone metastasis and skeletal-related events (15). Yang reported that the mortality rates associated with bone metastasis were 73.2% in the patients with non-small cell lung cancer (NSCLC) (16). Thomas reported that bone is the second most frequent metastatic site of lung cancer observed during a study of Caucasians (28%), and its median overall survival is 10.1 months (17). In general, the prognosis of patients with LCBM is unsatisfactory. Therefore, we further screened and integrated more specific and suitable survival factors to make an effective and easy-to-use nomogram model.
The more that factors are being constantly discovered and incorporated into the prediction of lung cancer, the more stereoscopic and comprehensive the nomograms are. The study of lung cancer and its prognosis has always been a focus of research (18-20). Nowadays, with the development of basic research (21-23) and the emergence of new detection techniques (24) and indicators (25-27), the diagnosis and treatment of lung cancer are improving (28-30). Another item worth mentioning is the emergence of lung cancer screening programs. The use of PET-CT (31) or low-dose computed tomography (LDCT) (32) has led to the early discovery of cancer and metastases, and it can assist patients and their physicians when making decisions on admission to hospice and continued treatment. However, some of the latest research results are still in the laboratory stage and not sufficiently persuasive to be adopted by clinicians, let alone be used to find relevant data in the SEER database.
Combining the prognostic factors provided by the SEER database and the practical needs of clinical work, we have chosen the age at diagnosis, gender, race, primary tumor sites, laterality, the total number of tumor sites, tumor size, grade, and histologic types, along with their treatment information, for a nomogram, although some of these factors showed no significant difference in our Cox regression analysis.
Interestingly, radiotherapy does not play a significant role in our prediction model, which is both impressive and puzzling. We need to be more cautious in discussing this conclusion, and we speculate that the existing specific radiation doses and regimens need to be adjusted. As Audrey pointed out, most patients do receive high doses of radiotherapy, but the course of treatment is too long, resulting in no improvement in the final outcome, so the authors suggest that shorter courses of radiotherapy should be utilized (33).
The prognostic study of lung cancer will be of great help to physicians. Therefore, there are a considerable number of nomograms for various types of lung cancer, some specializing in treatment for lung cancer. For example, Wing-Keen developed a nomogram for radiotherapy for lung cancer with bone metastases (34), and others specializing in a type of lung cancer, such as Wang et al., developed a nomogram for small cell lung cancer (SCLC) (35). Some researchers have narrowed the scope of the study to improve accuracy. For instance, Mao developed a nomogram for NSCLC after surgery (36) and Hung innovated one for adenocarcinoma after surgery (37). However, the smaller the entry point, the smaller the scope of application. Therefore, we conducted the present study to cover as much of the population as possible, rather than just focusing on specific parts. For this reason, we have expanded the scope of inclusion in order to increase the number of patients included and to achieve the widest applicability under existing conditions.
Our study also has some shortcomings, such as the lack of some key indicators, especially specific types of surgery, radiotherapy dose options, and the choice of chemotherapeutic drugs. Secondly, because of a lack of data support from another database, our nomogram cannot be externally verified, and only internal verification is possible. Finally, the prognosis of patients with LCBM is unfortunately poor. The median survival time is about 4 months, and thus long-term predictions regrettably do not have much of a chance to demonstrate their value.
Conclusions
This study provides new insights into the epidemiological study of patients with lung cancer bone metastasis. Our user-friendly nomogram, a useful visual tool for risk assessment and survival prediction in cancer patients, can efficiently and accurately provide prognostic assessment for individual patients. Further prospective clinical trial studies are required to continue to supplement and revise our nomogram. We hope that through the quantitative analysis of survival predictors, our results can promote the progress of individualized treatment.
Acknowledgments
We are thankful for the contribution of the SEER database and the 18 registries supplying cancer research information.
Funding: This work was funded by the National Natural Science Foundation of China (81501933), Zhejiang Provincial Natural Science Foundation of China (LY14H060008), Zhejiang Provincial Medical and Health Technology Foundation of China (2018KY129), Wenzhou leading talent innovative project (RX2016004) and Wenzhou Municipal Science and Technology Bureau (Y20170389).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Analysis of the de-identified data from the SEER program was exempt from medical ethics review, and no informed consent was required. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Cardoso D, Painho M, Roquette R. A geographically weighted regression approach to investigate air pollution effect on lung cancer: A case study in Portugal. Geospat Health 2019;14. [Crossref] [PubMed]
- Warren GW, Ward KD. Integration of tobacco cessation services into multidisciplinary lung cancer care: rationale, state of the art, and future directions. Transl Lung Cancer Res 2015;4:339-52. [PubMed]
- PM dG, CC W, BW C, et al. The epidemiology of lung cancer. Translational lung cancer research 2018;7:220-33.
- Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 2019;19:9-31. [Crossref] [PubMed]
- Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther 2014;141:222-33. [Crossref] [PubMed]
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [Crossref] [PubMed]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. [Crossref] [PubMed]
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Lung India 2013;30:20-6. [Crossref] [PubMed]
- Sun JM, Ahn JS, Lee S, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer 2011;71:89-93. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Liu W, Wu J. Lung cancer with bone metastases in the United States: an analysis from the Surveillance, Epidemiologic, and End Results database. Clin Exp Metastasis 2018;35:753-61. [Crossref] [PubMed]
- DeSantis CE, Miller KD, Goding Sauer A, et al. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69:211-33. [Crossref] [PubMed]
- Cetin K, Christiansen CF, Jacobsen JB, et al. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer 2014;86:247-54. [Crossref] [PubMed]
- Yang J, Zhang Y, Sun X, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol 2018;144:1835-42. [Crossref] [PubMed]
- Klikovits T, Lohinai Z, Fabian K, et al. New insights into the impact of primary lung adenocarcinoma location on metastatic sites and sequence: A multicenter cohort study. Lung Cancer 2018;126:139-48. [Crossref] [PubMed]
- Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016;35:75-91. [Crossref] [PubMed]
- Fornetti J, Welm AL, Stewart SA. Understanding the Bone in Cancer Metastasis. J Bone Miner Res 2018;33:2099-113. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Liu Y, Zhang N, Wang Y, et al. Zinc finger E-box binding homeobox 1 promotes invasion and bone metastasis of small cell lung cancer in vitro and in vivo. Cancer Sci 2012;103:1420-8. [Crossref] [PubMed]
- Wang Q, Li Z, Sun L, et al. Platelets enhance the ability of bone-marrow mesenchymal stem cells to promote cancer metastasis. Onco Targets Ther 2018;11:8251-63. [Crossref] [PubMed]
- Ajona D, Zandueta C, Corrales L, et al. Blockade of the Complement C5a/C5aR1 Axis Impairs Lung Cancer Bone Metastasis by CXCL16-mediated Effects. Am J Respir Crit Care Med 2018;197:1164-76. [Crossref] [PubMed]
- Zhang X, Liu R, Yuan Q, et al. The Precise Diagnosis of Cancer Invasion/Metastasis via 2D Laser Ablation Mass Mapping of Metalloproteinase in Primary Cancer Tissue. ACS Nano 2018;12:11139-51. [Crossref] [PubMed]
- Zhang L, Liu M, Liu J, et al. 27-Hydroxycholesterol enhanced osteoclastogenesis in lung adenocarcinoma microenvironment. J Cell Physiol 2019;234:12692-700. [Crossref] [PubMed]
- Chen Y, Liu H, Wu W, et al. Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J Exp Clin Cancer Res 2013;32:45. [Crossref] [PubMed]
- Ganguly SS, Daft PG, Cao J, et al. Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions. Cancers (Basel) 2018;10. [Crossref] [PubMed]
- Zhou D, Bum-Erdene K, Xu D, et al. Small molecules inhibit ex vivo tumor growth in bone. Bioorg Med Chem 2018;26:6128-34. [Crossref] [PubMed]
- Sarkar D. Statins as Inhibitors of Lung Cancer Bone Metastasis. EBioMedicine 2017;19:6-7. [Crossref] [PubMed]
- Nakai Y, Okamoto K, Terashima A, et al. Efficacy of an orally active small-molecule inhibitor of RANKL in bone metastasis. Bone Res 2019;7:1. [Crossref] [PubMed]
- Gareen IF, Hillner BE, Hanna L, et al. Hospice Admission and Survival After (18)F-Fluoride PET Performed for Evaluation of Osseous Metastatic Disease in the National Oncologic PET Registry. J Nucl Med 2018;59:427-33. [Crossref] [PubMed]
- Lowenstein LM, Deyter GM, Nishi S, et al. Shared decision-making conversations and smoking cessation interventions: critical components of low-dose CT lung cancer screening programs. Transl Lung Cancer Res 2018;7:254-71. [Crossref] [PubMed]
- Wallace AS, Fiveash JB, Williams CP, et al. Choosing Wisely at the End of Life: Use of Shorter Courses of Palliative Radiation Therapy for Bone Metastasis. Int J Radiat Oncol Biol Phys 2018;102:320-4. [Crossref] [PubMed]
- Yap WK, Shih MC, Kuo C, et al. Development and Validation of a Nomogram for Assessing Survival in Patients With Metastatic Lung Cancer Referred for Radiotherapy for Bone Metastases. JAMA Netw Open 2018;1:e183242. [Crossref] [PubMed]
- Wang S, Yang L, Ci B, et al. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J Thorac Oncol 2018;13:1338-48. [Crossref] [PubMed]
- Mao Q, Xia W, Dong G, et al. A nomogram to predict the survival of stage IIIA-N2 non-small cell lung cancer after surgery. J Thorac Cardiovasc Surg 2018;155:1784-92.e3. [Crossref] [PubMed]
- Hung JJ, Yeh YC, Jeng WJ, et al. Prognostic Factors of Survival after Recurrence in Patients with Resected Lung Adenocarcinoma. J Thorac Oncol 2015;10:1328-36. [Crossref] [PubMed]