
OX40 and OX40L protein expression of tumor infiltrating lymphocytes in non-small cell lung cancer and its role in clinical outcome and relationships with other immune biomarkers
Introduction
Lung cancer was one of the major fatal cancer types, which lead to 835,550 deaths in The United States in 2018 (1). With highly malignancy and limited early detection methods, patients usually had poor prognosis and limited survival. The early detection of pathological and molecular features of lung cancer could be beneficial to subsequent treatments and patients’ prognosis. Non-small cell lung cancer (NSCLC) is the major pathology type in lung cancer, which take up to nearly 80% of total incidence. Driving mutations mainly take place in adenocarcinoma, such as KRAS, EGFR, BRAF, NF1 and ALK fusion, even with one fourth of none mutation detected (2). Combing with first-line chemotherapy and anti-vascular therapy, recent clinical trials had already demonstrated the efficacy of target therapy in prolonging the survival and effectuate better disease management than ever before (3-7). Although target therapy and (or) anti-vascular therapy showed promising results in overall survival (OS) and progression-free survival (PFS), the beneficiary was limited, due to the fact that relatively small amounts of patients harbor the driving mutation (8). Also, acquired drug resistance and second mutations also rendered existing target medication ineffective, posing a new challenge for updating neo-generation target medication against mutations (9-11).
Cancer immunotherapy (CIT) is a method of mobilizing immune system to actively kill cancer cells. The past few years had witnessed the unprecedented development of CIT in clinical use, especially in lung cancer (12-14). The core of immunotherapy focuses on modulating the biomarkers expressed on immune cell and tumor microenvironment (TME) that has close interaction with cancer cells and immune cells. To date, the immune checkpoint PD-1/PD-L1 inhibitors has become the standard first-line medicine for advanced-stage Non-small cell lung cancer patients. Previously, clinical trials with anti-PD-1/PD-L1 agents had shown prompt and durable response in about 14–20% of pre-treated patients with advanced NSCLC (15-17). The results from Checkmate-017, checkmate-057 and POPLAR study all made fundamental bases for immunotherapy (18-20).
Although statistics supported the conclusion that checkpoint inhibitors did provide clinical efficacy and safety for NSCLC patients, it should be noted that only less than one fourth of all cancer patients respond to immunotherapy. Yet, biomarkers such as TMB, T cell-inflamed GEP score and PD-1/PD-L1 expression were used for screening patients for immunotherapy (21,22). However, the results of these biomarkers still cannot fully summarize and select patients for best clinical response. Some studies had already challenged the role of PD-1/PD-L1 in patients’ selection and prognosis evaluation (23,24). And for now, few studies reported the prognosis value of these biomarkers in resectable NSCLC patients. Thus, some immune biomarkers with regard to stimulation of immune cells should also be focused, such as co-stimulatory factors.
The representing co-stimulatory factors CD134 (OX40), member of TNF receptor super family, showed important role in potentiating antitumoral efficacy (25,26). OX40 highly expressed by activated T cells, B cells, DCs, neutrophils and NKs. The activation of OX40/OX40L axis is an important signal which can provide the co-stimulatory second signal to CD8+ T cell and also diminish the inhibitory effect of Treg, which can create proper anti-tumoral effect (27,28). Recently, OX40 agonistic monoclonal antibodies were investigated in several Phase I/II trials for solid tumor either as monotherapy or in combination with other immunomodulators (NCT03410901, NCT03092856, NCT02559024, NCT02315066, NCT03831295, NCT03390296, NCT02554812). However, those data are still not available. For now, the role of OX40 as a pretreating biomarker in lung cancer is still unclear. Whether it can be used as a biomarker for determine survival and prognosis in NSCLC is unknown. Also, the relationships between OX40 and PD-1/PD-L1 have not been fully analyzed in early-stage NSCLC.
Therefore, in this study, we investigated the relationship between OX40/OX40L, PD-1/PD-L1 and tumor infiltrating lymphocytes (TILs) using surgical samples from 139 patients with NSCLC by immunohistochemistry (IHC). We analyzed the correlation between OX40/OX40L expression and pathological traits. Factors that affected the patients’ survival and recurrence-free survival (RFS) were analyzed. We also conducted the survival analysis of patients by using OX40/OX40L, PD-L1 expression condition, TILs condition and pathological types as grouping factors to determine the significant influential factors. From all the analysis above, we should be able to determine the role of OX40/OX40L in resectable NSCLC patients and its relationship with PD-1, PD-L1, TILs and other clinical-pathological traits.
Methods
Patients
One hundred and thirty-nine NSCLC patients with resectable tumor participated in this study, from Department of Oncology and Radiotherapy in Medical University of Gdansk (Gdansk, Poland) started from April 2008 to August 2010. All the patients were naïve of anti-cancer treatment before surgery. The seventh edition of the IASLC system was used to determine the TMN staging, according to the pathological traits, lymph node status and lung cancer stages. Approval of this study was obtained from the Shanghai Pulmonary Hospital, Tongji University (ethical number 15-235), and the regulation was in accordance with the guidelines of the Helsinki Declaration of 1975, as revised in 1983. All the written consents were provided by the participants in the Medical University of Gdansk.
IHC for PD-1, PD-L1, OX40 and OX40L
Specimen IHC for OX40, OX40L and PD-1 were conducted by Ventana Benchmark XT platform, and PD-L1 was conducted by using Dako platform. All the Paraffin-embedded tissue sections were pretreated baking in drying oven at 60 °C for 1 hour. For OX40/OX40L and PD-1 slides, heat-mediated antigen retrieval was performed, which were labeled and put in a Benchmark XT system (Ventana Medical Systems, Tucson, AZ). Primary antibody [OX40 and OX40L, 1:1,000, EPR4392 (Abcam, Cambridge, MA) or PD-1, predilute, NAT 105 (Cell Marque, Rocklin, CA)] was applied, and followed by incubation at 37 °C for 1 hour. An UltraView DAB detection and amplification kit (Ventana Medical Systems) was applied for staining. Human tonsil was used as the positive control for OX40 and PD-1. For PD-L1 slides, Dako Autostainer (Dako, Carpenteria, CA) was used according to manufacturer’s instructions. Primary antibody [PD-L1, 22C3 (Dako)] was implemented by the Dako Autostainer, and followed by incubation at room temperature for 30 minutes. Slides were stained and treated according to protocol. Negative and positive controls were used provided by the Dako kits. Handling procedures were in accordance with previous published article (29).
Validation of OX40/OX40L, PD-1 and PD-L1 cutoff value
All the pathology and IHC results were checked and reviewed independently by two qualified pathologists. As for OX40/OX40L, the value of more than 20% was chosen, as it is the best value to predict both OS and RFS. The cutoff value for PD-1was at least 8% positive indicated by former research. The cutoff value for PD-L1on tumor cells was at least 50% staining, approved by U.S. Food and Drug Administration.
The evaluation of the abundance of TILs
We calculated the mass of lymphocytes under the microscope’s scope. The infiltrating status was determined by grades from 1+ to 3+, with a score of 1+ (<30%) indicating a low percentage of TILs, 2+ indicating a moderate percentage (30–60%), and 3+ indicating a largely (>60%) increase in TILs, following the semi-quantitative manner. TILs were composed of mononuclear cells, including lymphocytes, macrophages and plasma cells. Intra-alveolar macrophages were not considered as part of the immune infiltration. The discordance between pathologists in TIL category was reviewed jointly and consensus category were also reached.
Statistical analysis
All the data analysis was performed by using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). The expression status of OX40 and OX40L on TILs or tumors were described. Chi-square analysis was used to determine the correlation between OX40 Expression on TILs and PD-1, PD-L1, pathology type OX40 Expression on tumor. Multivariate Linear Regression was used to determine the relationship among PD-1, PD-L1, OX40 and OX40L on TILs or tumors. Bivariate logistic regression was conducted to determine the factor that affect the expression of OX40 on TILs. Cox regression was conducted to find crucial factors that affect the OS and RFS. Subgroup analysis was also conducted to determine OX40/OX40L and other immune biomarker that may also affect patients’ prognosis. All statistics were two sided, and statistical significance was defined as P less than 0.05.
Results
Patients characteristics
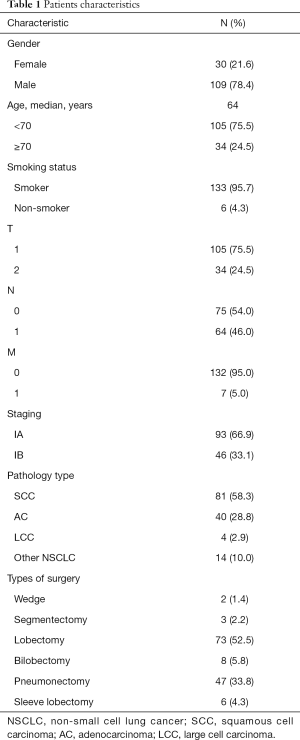
In the cohort of 139 participated patients, 21.6% were female and 78.4% were male. There were 34 patients who were older than 70 years old, with 105 patients less than 70 years. The median age was 64 years. There were only 6 (4.3%) patients who were never-smoker. The staging of all patients fell within I stage, with 93 stage IA and 46 stage IB. The major pathology type was squamous cancer cell, with 81 patients, taking up to 58.3%. The leading types of surgery performed were lobectomy (73, 52.5%) and pneumonectomy (47, 33.8%). Other information should refer to Table 1.
Full table
The expression status of OX40 and OX40L on TILs or tumors and the characterization among OX40, OX40L, PD-1, PD-L1, TILs and pathology type
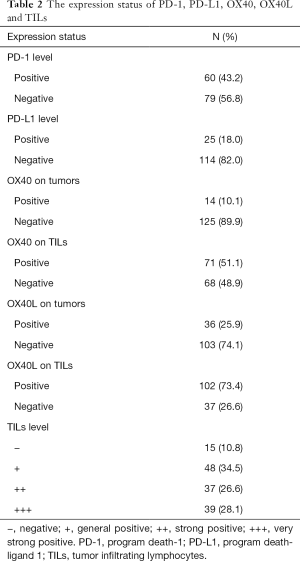
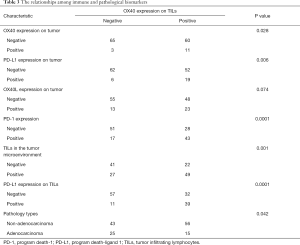
The OX40 expression status on TILs were 71 positives (51.1%) cases and 68 negatives (48.9%) cases. The OX40L expression status on TILs were 102 positives (73.4%) and 37 negatives (26.6%). With 125 negatives (89.9%) and 103 negatives (74.1%), The OX40 and OX40L level status on tumors were relatively low. Only 15 cases (10.8%) were negative status of TILs, with 124 cases (89.2%) of positive cases, grades ranging from “−” and “+” to “+++”. The PD-1 positive expression were 60 cases (43.2%), and the PD-L1 positive expression were 25 cases (18%) (Table 2). Chi-square analysis validated the intergroup differences of TILs OX40 expression between tumor cell PD-L1 status (P=0.006), TILs PD-L1 expression status (P=0.0001), tumor OX40 expression (P=0.028), PD-1 status (P=0.0001), TILs status (positive/negative) (P=0.001) and pathology type (P=0.042). The intergroup comparison results indicated that the status of PD-1, PD-L1, TILs, tumor OX40 and pathology types were important factors that might affect the TILs OX40 expression status (Table 3).
Full table
Full table
The linear correlation between OX40/OX40L and immune factors PD-1/PD-L1/TILs
Univariate linear regression results indicated that PD-1expression was negatively correlated with the TILs OX40 expression [R=0.250, R2=0.063, Y=0.075X+12.227, 95% CI: 0.026–0.125, (P=0.003)]. PD-1expression was also negatively correlated with the TILs OX40L expression [R=0.386, R2=0.149, Y=0.246X+2.936, 95% CI: 0.147–0.346, (P=0.0001)]. The expression of OX40/OX40L was not correlated with TILs grades. Multivariate linear regression results indicated that PD-1expression was positively correlated with TILs grades and negatively correlated with the TILs OX40L expression [R=0.531, R2=0.282, Y=5.864X1+0.450X2+1.373, (X1, 95% CI: 3.552–8.176, P=0.0001; X2, 95% CI: 0.216–0.683), (P=0.0001)].
Bivariate Logistic regression of TILs OX40 expression
In the regression model, 5 independent variables were included and considered risk factors for TILs OX40 expression. OX40L expression on tumors had the OR of 5.675 (1.185-27.171, P=0.030), which were considered as the important factor that had impact on the expression of TILs OX40 expression. Likewise, TILs OX40L expression, PD1 expression, PD-L1 expression and TILs were other risk factors that influenced TILs OX40 expression (Table 4).

Full table
Cox regression analysis for OS and RFS
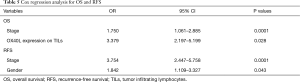
The Cox analysis results indicated that staging and TILs OX40L expression were risk factors for OS, with the OR 1.750 for stage (95% CI: 1.061–2.885, P=0.0001) and OR 3.379 for TILs OX40L expression (95% CI: 2.197–5.199, P=0.028). As for RFS, stage had the OR of 3.754 (95% CI: 2.447–5.758, P=0.0001), and gender had the OR of 1.842 (95% CI: 1.109–3.327, P=0.043) (Table 5).
Full table
Subgroup analysis for OS and RFS
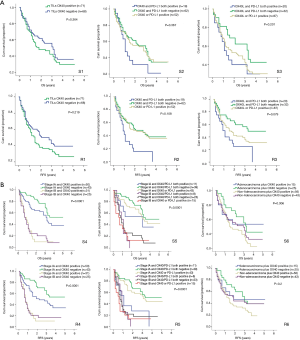
All the patients were allocated into different subgroups by immune biomarker status. The TILs OX40 expression was the main grouping factor (Table 6). In the subgroup 1, the intergroup comparison was not significant, possibly due to limited sample size and follow-up time. However, OX40 expression negative group had RFS surpass the positive group, suggesting the OX40 did have the tendency in reducing the RFS. In subgroup 2, the intergroup comparison between OX40+/PD-L1+ and OX40-/PD-L1- for RFS was significant (P=0.036). Likewise, in subgroup 3, the intergroup comparison between OX40L+/PD-L1+ and OX40L-/PD-L1− for RFS was significant (P=0.036) (Figure 1A). In the subgroup 4, the group with Stage IA and TILs OX40- had the survival of 3.5 years and RFS 4.903 years, which was the leading group. Also, the inter-group comparison for RFS between OX40+ and OX40- in stage IA was significant (P=0.037). The same results were shown by the subgroup 5, in which the intergroup comparison between OX40-/PD-L1− and OX40+/PD-L1+ in stage IA was significant (P=0.017) (Table 6). In subgroup 5, the inter-group comparison for RFS between adenocarcinoma OX40- and non-adenocarcinoma OX40+ was significant (P=0.029) (Figure 1B). All these results imply that TILs OX40 and OX40L expression both served as an important biomarker for RFS prediction in early stage NSCLC.
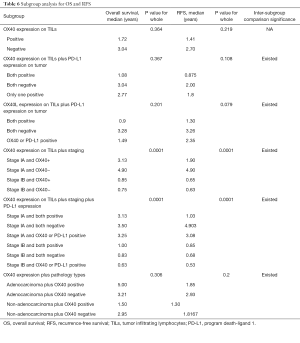
Full table
Discussion
OX40 with its ligand OX40L, member of the tumor necrosis factor receptors/tumor necrosis factor superfamily, plays a pivotal role in regulating immune cells. It is expressed on activated immune cells such as T cells (CD4, CD8, Th1, Th2, Th17, regulatory T cells), B cells, DCs, macrophage, neutrophils, mast cell, even on some antigen-presenting cells and tumor cells (30). The activation of OX40/OX40L can create comprehensive downstream signaling factors, including PI3K/PKB, NF-κB and NFAT, which can contribute to the cell division, maturation and activation of T cells (31-33). Its role as an immune modulator in CD4+ T cell was well studied, as previous studies illustrated OX40 was shown to have effects on cytokine production and lead CD4+ T cell into Th1 or Th2 subsets (34). When it comes to naïve CD4T cell, the OX40/OX40L signal usually lead CD4+T cell converting into Th2 cells due to the CD4+T autocrine of IL-4 (35,36). Several studies illustrated the presence of IL-2 and type I interferon could transform this process into Th1 (35,37,38). It was also proved by a mouse model study that the OX40 KO results in the absence of Th1 immune response, suggesting the role of OX40 in Th1 immune response (39). Data implied OX40 was involved in the production of IL-17 and IL-23, leading to Th17 activation and differentiation (40). So far, less was known about the role of OX40 in Tfh. As for Treg development, studies suggested that OX40 had few contributions to the development of nTreg, and the OX40 signaling was thought to play a much more important role in iTreg formation. Hitherto, studies were divergent on the ideas of whether OX40 directly alter the ability of iTreg or modified the phenotype of the population and cytokine milieu. Some studies put forward that OX40 affect the iTreg in a way of modulating the expression of Foxp3 by the combing the Foxp3 promoter of pSmad3 and Stat3 (41,42). Some other research demonstrated OX40 drive IL-4 and IFN-γ production which could synergize with APC-produced IL-6 and blocked Foxp3 expression (32,43). Recent research also put forward several independent mechanisms concerning the iTreg induction, where OX40 activate AKT-mTOR pathway in order to inhibit Foxp3 expression (44). The role of OX40 in inhibiting iTreg was also observed in an airway tolerance autoimmune model (43). It was believed that the survival of T cells, including Tregs, rely on the activation of OX40/OX40L signaling (28). However, it remained contradictory in whether OX40/OX40L signaling suppressing or promoting the amount of Treg. For now, the majority support the latter idea. Studies showed that the OX40 agonist could induce proliferation of both naive effectors and Tregs in a dose-dependent manner. Also, upregulated OX40L was shown to have intact suppressive function for Treg (45-48). Some research also proposed novel OX40L DCs that selectively expand Tregs (49). In another word, the activation of OX40/OX40L signaling would not only activate the CD4+ and CD8+ T cell, but also Treg. The underlying reason could be that the immune system was programmed to balance the homeostasis in the life entity. Thus, the role of OX40 in expanding Treg could partially explain why the OX40/OX40L was associated with decreased survival and poor prognosis in our study. Due to the fact that the activation of OX40/OX40L would surely proliferate and prime the Treg, and the TILs (mainly T cells and APCs) in the TME could be possibly suppressed by the activated Treg. Also, early expression of OX40 on the TILs in the TME could possibly be the sign of depletion of CD8+T cells and overproduced Tregs. Therefore, it is vital to distinguish T cell types (CD4+, CD8+, Treg, etc.) when OX40 were chosen as a biomarker to determine the survival in patients. Recently, OX40 agonist has already existed in PhaseI/II study clinical trials, alone or in combination with immune checkpoints inhibitors (NCT02315066, NCT02410512, NCT02221960, NCT02705482, NCT02923349, NCT02528357. Data are currently not available). Although the toxicity record was good to perform, the application of OX40 agonist on patients should go through a series of certain biomarker test, including OX40/OX40L expression, staging and cancer types. Further to say, OX40/OX40L signaling is intricate and should be reviewed in different aspects. What’s more, the indication of OX40 agonist should also be fully analyzed. Research found the agonistic OX40 antibody could deplete intra-tumoral Tregs which expressed higher levels of OX40 through FcγR mediated ADCC caused by myeloid and NK cells within the TME (45). On the other hand, in some trials, anti-OX40 agonistic antibody showed increased tumor infiltration of Tregs despite increased Teff cell proliferation, thus compromised the anti-tumoral efficacy (50,51). The explanation was that Tregs were induced by constitutively expressed OX40 upon OX40L binding. Therefore, it is still important to verify role of anti-OX40 agonists in immunological modulation, although it showed promising efficacy for cancer therapy.
To our knowledge, it is the first research to analyze the role of OX40/OX40L in early-stage resectable NSCLC and demonstrate its relationships with PD-1/PD-L1, TILs and patients’ clinical outcomes. OX40/OX40L has already been studied previously as an immune characteristic biomarker in some other cancer types, including lymphoma, hepatocellular carcinoma, Leukemia, ovarian, neuroblastoma, head and neck squamous cell carcinoma, gastric cancer and breast cancer (52-63). The TILs OX40 was reported to correlate with patient survival. The role of OX40 was evaluated in the cohort of 316 patients with hepatocellular carcinoma, in which the high expression of OX40 is associated with poor survival, vascular invasion and high serum AFP level (53). They found the expression of OX40 is an independent predictor of survival, with low-OX40 expression having longer survival. The high-expression of OX40 was characterized by upregulated cytokines and exhaustion-specific markers, which indicated that high expression of OX40 was associated with immunosuppressive factors. Also, the high or low expression status was related to mutations in AKT/mTOR and Wnt/β-catenin signaling, respectively. The OX40 expression was also associated with poor prognosis and shorter survival in the study of acute myeloid leukemia (54). In nearly half of the cases, OX40 expression led to proliferation and release of proleukemic cytokines, which provided a survival benefit for leukemic cells. Some authors proposed that the prognostic value of OX40 was inconsistent, with especially superior prognostic value in melanoma, lung cancer and colorectal cancer but inferior prognosis in cutaneous squamous cell carcinoma (25,64). However, our results showed that the expression of OX40 is negatively correlated with PD-1, PD-L1, and serve as a good biomarker for RFS prediction, with high level of OX40 associated with poor RFS and shorter survival, and vice versa. Most importantly, we found the prognostic value of OX40 was dependent on the tumor staging, as early stage (IA) tumor with different OX40 expression status could have different survival condition. And it was not associated with pathological types and clinicopathological factors as stated before. This was also confirmed by Martins et al., who found T cells in gastric cancer had decreased levels of OX40 that become more pronounced with stages III and IV (57). Although studies indicated that the OX40 served an independent prognosis factors, it should be noted that the expression of OX40 as a prognostic marker must be observed with other important clinicopathological features, such as PD-1/PD-L1 or staging. In another study, a panel of immune checkpoints (including OX40) was analyzed with NSCLC patients, in which they found the OX40 was related with tumor-associated inflammatory cells (TAIC) (65). Although the data did not show the importance of OX40 in the prognosis of NSCLC patients, they suggested that the upregulation of checkpoints markers on the TAIC indicated the exhaustion of immune cell in the TME and tumor cell invasion. Therefore, in early stage resectable NSCLC, the high expression of OX40 should be an indicative of immune cell exhaustion. Thus, Future studies are still needed whether the high expression of OX40 warrant anti-OX40 agonist treatment alone or in combination with present anti-cancer immunomodulators.
The OX40 agonist application has becoming an emerging strategy to potentiate anti-tumoral effect by initiating immune system. These antibodies were designed to provide co-stimulatory signal for exhausted immune cells in the TME, like TILs. Some preclinical studies had witnessed the effect of OX40 agonist in causing tumor regression and tumor rejection in animal models (26,48,50,66-73). 9B12, a murine IgG anti-OX40 antibody, was studied in a phase I clinical trial (NCT01644968) for patients with solid tumor refractory to conventional therapy (50). The study consisted of 3 arms with different dosage of 9B12. The results showed acceptable toxicity profile of the 9B12 and demonstrated OX40 was a potent immune-stimulating target for treatment in patients with solid tumors. Although no patients received partial response by RECIST, regression and stable disease was observed. Mechanistically, the 9B12 increased the immune response of T cell and B cell and also led preferential upregulation of OX40 on CD4+FoxP3+ Treg in the TILs. In line, humoral immunity was also activated according to study results, as flow cytometry indicate Ki-67 upregulation in the CD4+T cells after anti-OX40 treatments. In summary, this study had already shined lights on the anti-tumoral anti-OX40 treatment of human beings and studies with refined design would be anticipated for better results. For now, some antibodies such as BMS 986178, PF-04518600, MEDI6469 are anticipated for promising clinical results in solid malignancies. Although the anti-OX40 therapy was thought to be a promising strategy in anti-tumoral immunotherapy, two issues should be noted. Firstly, the sequence and combination arrangement of anti-OX40-based immunotherapy should be scrutinized. According to Rajeev K. Shrimali et al., the concurrent use of anti-PD-1 antibody combing with anti-OX40 antibody reduced the anti-tumoral effect created by OX40 agonist and induced the T cell apoptosis (74). However, once the sequence was reversed, the anti-tumoral effects remained intact. The possible mechanism was explained by the theory of the PD-1-induced TCR-mediated signaling altered the contact interaction between T cell and antigen-bearing cells. It is possible that the checkpoint inhibitor could render OX40 agonist ineffectual by interfering pivotal factors or receptors that correlated with OX40/OX40L signaling. Although the underlying molecular relationship remains unknown, it is necessary to conduct strict appraisal when using combination therapy of 2 or more immunological modulators. Another research also proposed the importance of the timing and sequence of OX40-based immunotherapy (75). As they found the sequential combination therapy of anti-OX40 followed by anti-PD-1 resulted in remarkable increase in therapeutic efficacy, however the concurrent using of those two antibodies negated the anti-tumoral effect. Secondly, the efficacy of anti-OX40 may vary on the method of administration. Anti-OX40 antibodies is associated with depletion of TILs through an antibody-dependent cell cytotoxicity (71). When the antibody is given systematically, it will mobilize peripheral lymphocyte instead of TILs in the TME. Data are still limited concerning the consequences of different administration method of anti-OX40 in human beings. Thus, it is necessary to analyze whether systematical or in situ administration could hamper the therapeutic efficacy of anti-OX40 therapy in clinical trials.
Moreover, as a pivotal biomarker expressed in immune system, OX40 also serves as an important marker in associate with MEK, Dectin-1, RORγt+ CD8+ T Cells and other immune modulator in the anti-cancer immunology setting (76-82). To our knowledge, the activation of RAS/MAPK pathway plays an important role in tumor growth and escape, and it is a common phenomenon in clinical cancer settings. One study investigated the OX40 acted as an important factor in upregulating inhibited MEK in triple-negative breast cancer, which augmented the anti-tumoral effect (76). The downstream signaling was related to p38/JNK signaling. Putting together, OX40 was the crucial factor in reversing exhausted immune cell and increased the immunogenicity, thus resulting in superior anti-cancer efficacy. OX40 also was upregulated in dectin-1 stimulated DC, which induced potent antitumor immunity response depended on Th9 and IL-9 (80). The underlying mechanism was that dectin-1 activate syk, Raf1 and NF-κB signaling that increased p50 and RelB translocation and OX40 expression. In CD8+ T cell signaling, a subset of RORγt+ CD8+ T cells that expressed high-level OX40 was found to be associated with reduced patients (78). Unlike OX40-expressed Treg, the OX40-expressed RORγt+ CD8+ T cells were associated with promoting pro-carcinogenic inflammation and lead to tolerogenic microenvironment in tumors. Therefore, OX40, as a distinct biomarker, should have important clinical implication for anti-tumoral immunity.
There are some limitations in our study. Firstly, with 139 patients participated in our study, the limited sample size may result in biased outcome and lead to neglect of some latent pattern. Secondly, due to the fact that this study is a retrospective study, some aspects are not perfect, including lack of the driver gene mutation status and follow-up of subsequent immune system alteration. Thirdly, the cell line derived from resected sample were failed to establish due to limited recourses. To make this study better, a verification study should be also conducted on new collected clinical sample. Some large-sample, multi-center with long term follow up clinical study is still needed to verify the role of OX40/OX40L signaling in NSCLC.
Conclusions
To conclude with, the co-stimulatory marker OX40 and OX40L are crucial factors that should be focused. In our study, we analyzed the relationship between OX40/OX40L and other immune modulator and identified its role as the biomarker for RFS and prognosis in early-stage NSCLC. The TILs OX40/OX40L expression is correlated with the PD-1/PD-L1 expression. The expression of TILs OX40 varied significantly among tumor OX40/OX40L, PD-1, PD-L1, TILs and pathological types. The bivariate logistic regression of TILs OX40 expression indicated that tumor OX40L expression, TILs OX40L expression, PD1 expression, PD-L1 expression and TILs were considered as risk factors. In the survival analysis, the staging and TILs OX40L were considered as risk factors for OS while stage and gender were risk factors for RFS. In the subgroup analysis, the TILs OX40 expression status was identified as a major factor for determine the RFS. Although the P value in some subgroup were not significant (>0.05), the tendency of intergroup comparison was distinct and it could be better if the sample size was larger than present. The low-expression of OX40 was related to longer RFS, OS and better prognosis. In short, future studies should focus on the underlying mechanism of OX40/OX40L signaling in anti-tumoral therapy and investigate the synergism effect of anti-OX40 with other checkpoint inhibitors in some large sample size settings.
Acknowledgments
Funding: This study was supported in part by a grant from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131). National key research & development project (2016YFC0902300). Major disease clinical skills enhancement program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), grant of Shanghai Science and Technology Commission (16JC1405900). The fundamental research funds for the central universities.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval of this study was obtained from the Shanghai Pulmonary Hospital, Tongji University (ethical number 15-235), and the regulation was in accordance with the guidelines of the Helsinki Declaration of 1975, as revised in 1983. All the written consents were provided by the participants in the Medical University of Gdansk. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Mok TSK, Kim SW, Wu YL, et al. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. J Clin Oncol 2017;35:4027-34. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer Consensus Statement on Optimizing Management of EGFR Mutation-Positive Non-Small Cell Lung Cancer: Status in 2016. J Thorac Oncol 2016;11:946-63. [Crossref] [PubMed]
- Piotrowska Z, Niederst MJ, Karlovich CA, et al. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov 2015;5:713-22. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Forde PM, Kelly RJ, Brahmer JR. New strategies in lung cancer: translating immunotherapy into clinical practice. Clin Cancer Res 2014;20:1067-73. [Crossref] [PubMed]
- Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:822-35. [Crossref] [PubMed]
- Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol 2016;17:212-23. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44-56. [Crossref] [PubMed]
- Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018. [Crossref] [PubMed]
- Kelly PN. The Cancer Immunotherapy Revolution. Science 2018;359:1344-5. [Crossref] [PubMed]
- Soria JC, Marabelle A, Brahmer JR, et al. Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res 2015;21:2256-62. [Crossref] [PubMed]
- Sapski S, Beha N, Kontermann R, et al. Tumor-targeted costimulation with antibody-fusion proteins improves bispecific antibody-mediated immune response in presence of immunosuppressive factors. Oncoimmunology 2017;6:e1361594. [Crossref] [PubMed]
- Hebb JPO, Mosley AR, Vences-Catalan F, et al. Administration of low-dose combination anti-CTLA4, anti-CD137, and anti-OX40 into murine tumor or proximal to the tumor draining lymph node induces systemic tumor regression. Cancer Immunol Immunother 2018;67:47-60. [Crossref] [PubMed]
- Aspeslagh S, Postel-Vinay S, Rusakiewicz S, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52:50-66. [Crossref] [PubMed]
- Croft M. Control of Immunity by the TNFR-Related Molecule OX40 (CD134). Annu Rev Immunol 2010;28:57-78. [Crossref] [PubMed]
- He Y, Yu H, Rozeboom L, et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J Thorac Oncol 2017;12:814-23. [Crossref] [PubMed]
- Willoughby J, Griffiths J, Tews I, et al. OX40: Structure and function - What questions remain? Mol Immunol 2017;83:13-22. [Crossref] [PubMed]
- Croft M, So T, Duan W, et al. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009;229:173-91. [Crossref] [PubMed]
- Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009;9:271-85. [Crossref] [PubMed]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005;23:23-68. [Crossref] [PubMed]
- Qui HZ, Hagymasi AT, Bandyopadhyay S, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol 2011;187:3555-64. [Crossref] [PubMed]
- So T, Song J, Sugie K, et al. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A 2006;103:3740-5. [Crossref] [PubMed]
- Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005;202:1213-23. [Crossref] [PubMed]
- Ito T, Amakawa R, Inaba M, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol 2004;172:4253-9. [Crossref] [PubMed]
- Rogers PR, Croft M. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J Immunol 2000;164:2955-63. [Crossref] [PubMed]
- Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol 2003;33:861-9. [Crossref] [PubMed]
- Zhang Z, Zhong W, Hinrichs D, et al. Activation of OX40 augments Th17 cytokine expression and antigen-specific uveitis. Am J Pathol 2010;177:2912-20. [Crossref] [PubMed]
- Lu Y, Zhang M, Wang S, et al. p38 MAPK-inhibited dendritic cells induce superior antitumour immune responses and overcome regulatory T-cell-mediated immunosuppression. Nat Commun 2014;5:4229. [Crossref] [PubMed]
- Xu L, Kitani A, Stuelten C, et al. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 2010;33:313-25. [Crossref] [PubMed]
- Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol 2008;181:8650-9. [Crossref] [PubMed]
- Zhang X, Xiao X, Lan P, et al. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep 2018;24:607-18. [Crossref] [PubMed]
- Bulliard Y, Jolicoeur R, Zhang J, et al. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol 2014;92:475-80. [Crossref] [PubMed]
- Marabelle A, Kohrt H, Sagiv-Barfi I, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013;123:2447-63. [Crossref] [PubMed]
- Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med 2009;206:1103-16. [Crossref] [PubMed]
- Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med 2008;205:825-39. [Crossref] [PubMed]
- Marinelarena A, Bhattacharya P, Kumar P, et al. Identification of a Novel OX40L (+) Dendritic Cell Subset That Selectively Expands Regulatory T cells. Sci Rep 2018;8:14940. [Crossref] [PubMed]
- Curti BD, Kovacsovics-Bankowski M, Morris N, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013;73:7189-98. [Crossref] [PubMed]
- Ruby CE, Yates MA, Hirschhorn-Cymerman D, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol 2009;183:4853-7. [Crossref] [PubMed]
- Xu PP, Sun C, Cao X, et al. Immune Characteristics of Chinese Diffuse Large B-Cell Lymphoma Patients: Implications for Cancer Immunotherapies. EBioMedicine 2018;33:94-104. [Crossref] [PubMed]
- Xie K, Xu L, Wu H, et al. OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis. Oncoimmunology 2018;7:e1404214. [Crossref] [PubMed]
- Rothfelder K, Hagelstein I, Roerden M, et al. Expression of the Immune Checkpoint Modulator OX40 in Acute Lymphoblastic Leukemia Is Associated with BCR-ABL Positivity. Neoplasia 2018;20:1150-60. [Crossref] [PubMed]
- Ramser M, Eichelberger S, Daster S, et al. High OX40 expression in recurrent ovarian carcinoma is indicative for response to repeated chemotherapy. BMC Cancer 2018;18:425. [Crossref] [PubMed]
- Nanni P, De Giovanni C, Burocchi A, et al. OX40 triggering concomitant to IL12-engineered cell vaccine hampers the immunoprevention of HER2/neu-driven mammary carcinogenesis. Oncoimmunology 2018;7:e1465164. [Crossref] [PubMed]
- Martins MR, Santos RLD, Jatahy KDN, et al. Could OX40 agonist antibody promote activation of the anti-tumor immune response in gastric cancer? J Surg Oncol 2018;117:840-4. [Crossref] [PubMed]
- Ladányi A, Kapuvari B, Papp E, et al. Local immune parameters as potential predictive markers in head and neck squamous cell carcinoma patients receiving induction chemotherapy and cetuximab. Head Neck 2019;41:1237-45. [Crossref] [PubMed]
- Knaus HA, Berglund S, Hackl H, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018. [Crossref] [PubMed]
- Kinkead HL, Hopkins A, Lutz E, et al. Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer. JCI Insight 2018. [Crossref] [PubMed]
- Jahan N, Talat H, Curry WT. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells. Neuro Oncol 2018;20:44-54. [Crossref] [PubMed]
- Deumelandt K, Platten M, Ochs K. Synergy of vaccination and agonist OX40 treatment-toward a mechanism-driven combination of glioma immunotherapy. Neuro Oncol 2018;20:4-5. [Crossref] [PubMed]
- Bagley SJ, Hwang WT, Brem S, et al. RNA-seq for identification of therapeutically targetable determinants of immune activation in human glioblastoma. J Neurooncol 2019;141:95-102. [Crossref] [PubMed]
- Lai C, August S, Albibas A, et al. OX40+ Regulatory T Cells in Cutaneous Squamous Cell Carcinoma Suppress Effector T-Cell Responses and Associate with Metastatic Potential. Clin Cancer Res 2016;22:4236-48. [Crossref] [PubMed]
- Parra ER, Villalobos P, Zhang J, et al. Immunohistochemical and Image Analysis-Based Study Shows That Several Immune Checkpoints are Co-expressed in Non-Small Cell Lung Carcinoma Tumors. J Thorac Oncol 2018;13:779-91. [Crossref] [PubMed]
- Virani NA, Thavathiru E, McKernan P, et al. Anti-CD73 and anti-OX40 immunotherapy coupled with a novel biocompatible enzyme prodrug system for the treatment of recurrent, metastatic ovarian cancer. Cancer Lett 2018;425:174-82. [Crossref] [PubMed]
- Mazor R, King E, Pastan I. Anti-drug antibodies to LMB-100 are enhanced by mAbs targeting OX40 and CTLA4 but not by mAbs targeting PD1 or PDL-1. Cell Immunol 2018;334:38-41. [Crossref] [PubMed]
- Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci 2008;99:361-7. [Crossref] [PubMed]
- Fear VS, Tilsed C, Chee J, et al. Combination immune checkpoint blockade as an effective therapy for mesothelioma. Oncoimmunology 2018;7:e1494111. [Crossref] [PubMed]
- Yu G, Li Y, Cui Z, et al. Combinational Immunotherapy with Allo-DRibble Vaccines and Anti-OX40 Co-Stimulation Leads to Generation of Cross-Reactive Effector T Cells and Tumor Regression. Sci Rep 2016;6:37558. [Crossref] [PubMed]
- Sagiv-Barfi I, Czerwinski DK, Levy S, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med 2018. [Crossref] [PubMed]
- Niknam S, Barsoumian HB, Schoenhals JE, et al. Radiation Followed by OX40 Stimulation Drives Local and Abscopal Antitumor Effects in an Anti-PD1-Resistant Lung Tumor Model. Clin Cancer Res 2018;24:5735-43. [Crossref] [PubMed]
- Serebrovskaya EO, Yuzhakova DV, Ryumina AP, et al. Soluble OX40L favors tumor rejection in CT26 colon carcinoma model. Cytokine 2016;84:10-6. [Crossref] [PubMed]
- Shrimali RK, Ahmad S, Verma V, et al. Concurrent PD-1 Blockade Negates the Effects of OX40 Agonist Antibody in Combination Immunotherapy through Inducing T-cell Apoptosis. Cancer Immunol Res 2017;5:755-66. [Crossref] [PubMed]
- Messenheimer DJ, Jensen SM, Afentoulis ME, et al. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin Cancer Res 2017;23:6165-77. [Crossref] [PubMed]
- Dushyanthen S, Teo ZL, Caramia F, et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat Commun 2017;8:606. [Crossref] [PubMed]
- Babic M, Romagnani C. Boosting Type 2 Immunity: When OX40L Comes from ILC2s. Immunity 2018;48:1067-9. [Crossref] [PubMed]
- Chellappa S, Hugenschmidt H, Hagness M, et al. CD8+ T Cells That Coexpress RORgammat and T-bet Are Functionally Impaired and Expand in Patients with Distal Bile Duct Cancer. J Immunol 2017;198:1729-39. [Crossref] [PubMed]
- Adler AJ, Mittal P, Ryan JM, et al. Cytokines and metabolic factors regulate tumoricidal T-cell function during cancer immunotherapy. Immunotherapy 2017;9:71-82. [Crossref] [PubMed]
- Zhao Y, Chu X, Chen J, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun 2016;7:12368. [Crossref] [PubMed]
- Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018;33:463-479.e10. [Crossref] [PubMed]
- Triplett TA, Tucker CG, Triplett KC, et al. STAT3 Signaling Is Required for Optimal Regression of Large Established Tumors in Mice Treated with Anti-OX40 and TGFbeta Receptor Blockade. Cancer Immunol Res 2015;3:526-35. [Crossref] [PubMed]


