
Decade in review: a new era for RET-rearranged lung cancers
Introduction
RET alterations are estimated to occur in approximately 2% of all human cancers (1). The oncogenic potential of RET was first identified in 1985 after the discovery that transfection with human lymphoma DNA could transform NIH 3T3 fibroblasts (2). RET fusions were later identified in papillary thyroid cancer in the 1980s, followed by the discovery of germline RET mutations as the causative genetic link in multiple endocrine neoplasia type 2 (MEN2) syndromes. In all, RET alterations are found in 5–10% of papillary thyroid cases (3) and the majority of medullary thyroid cancer (MTC) cases (4).
The RET proto-oncogene on chromosome 10q11.2 encodes for a transmembrane glycoprotein receptor tyrosine kinase whose ligands belong to the glial-derived neurotrophic factor (GDNF) family. In normal cellular functioning, RET signaling is essential for the development and maintenance of the kidneys (5) and enteric nervous system (6). Loss-of-function RET mutations can result in hereditary Hirschsprung disease (7) and some forms of congenital malformations of the kidneys and urinary tract (8). RET activation occurs when its GDNF ligands bind to cell membrane-bound GDNF family receptor (GFR) co-receptors, which induces RET homodimerization and auto-phosphorylation within the RET intracellular tyrosine kinase domains (9). This activates downstream signaling pathways involved in normal cellular differentiation and proliferation, such as the RAS, MAPK, PI3K and JAK-STAT pathways (10-12).
In non-small cell lung cancer (NSCLC), oncogenic activation of RET occurs by chromosomal rearrangement, which fuses the 3’ coding region for the RET kinase domain on chromosome 10 with a 5’ upstream partner gene containing one of several possible domains, such as a coiled-coil or LIS1 homology (13-15). This fusion induces ligand-independent dimerization, constitutive RET activation, and oncogenesis. Intrachromosomal rearrangements are most frequently seen, with partner genes such as KIF5B, CCDC6, and NCOA4 also originating from chromosome 10, although interchromosomal partners have also been identified (16). The specific fusion partner and breakpoints locations in RET or upstream partners impact the properties of the formed oncoprotein, such as its subcellular location. Fusion partners also confer distinctive properties such as higher levels of activated RET oncoprotein (13) and formation of multikinase signaling hubs (17), both of which are seen with KIF5B-RET and are postulated to impact the fusion protein’s drug sensitivity.
In contrast, in other cancer types, namely thyroid cancer but also breast and colorectal cancer (1), the primary mechanism of aberrant RET activation is point mutation. Mutations in the cysteine-rich extracellular domain, for example, define the hereditary syndrome MEN2A. MEN2A is characterized by the development of MTC and pheochromocytomas in the majority of patients, as well as hyperparathyroidism in a select subset (18). The hereditary syndrome MEN2B, which results in pheochromocytomas and MTC as well as a characteristic marfanoid habitus, is defined by point mutations in the intracellular kinase domain, with the most common alteration being RETM918T (19).
RET fusions were not identified in NSCLC until 2012 (Figure 1), when four independent groups from the United States, Japan, and Korea reported RET fusions in 1–2% of lung cancer cases examined (13-15,20). Patients with RET fusions tend to be young, never smokers, and more frequently had adenocarcinomas over other histological types (21), characteristics that have since been further validated (22). Of note, these fusions are also seen, although less frequently, in older patients, those with a substantial smoking history, and non-adenocarcinoma histologies. Molecular profiling should thus be unbiased in relation to clinical or pathologic features. Soon after its identification in NCSLC, RET became a target for molecularly-targeted therapies, the first of which were existing multikinase inhibitors (MKIs).

Multikinase inhibitors
Until recently, there were no approved therapies specifically for RET fusion-positive NSCLCs. Several MKIs have been shown to have modest clinical activity against RET fusions in phase II clinical trials, leading to their use being supported by National Comprehensive Center Network guidelines as category 2A recommendations (23). As their name implies, MKIs target RET as well as kinases including VEGFR2, MET, KIT, BRAF or EGFR (24), depending on the particular agent in question; this contributes both to their off-target effects and decreased effectiveness against RET due to pharmacokinetic limitations.
Cabozantinib was the first MKI studied in RET-rearranged lung cancer in a prospective clinical trial. The clinical response of the first three patients in this single-arm phase II trial were reported in 2013 (25), with the full results of 26 patients published in 2016 (26). Enrolled patients had metastatic or unresectable NSCLC with a RET fusion, 20 of whom had received at least one prior line of chemotherapy. Of the 25 patients analyzed, seven (28%) demonstrated a partial response (PR) with an additional nine (36%) achieving stable disease (SD). There were no complete responses (CR). The median progression-free survival (PFS) was 5.5 months with an overall survival (OS) of 9.9 months.
Vandetanib, lenvatinib, sorafenib and RXDX-105 have also been studied in prospective clinical trials (Table 1). Of these, a Japanese trial of 18 patients treated with vandetanib reported the highest objective response rate (ORR) of 53% (27), with a median PFS of 6.5 months and an OS of 13.5 months (28). Other phase II trials of sorafenib (3 patients), lenvatinib (25 patients), and vandetanib (19 patients) reported ORRs ranging from 0 to 18% and median PFS ranging from 4.7 to 7.3 months (29-32). Of note, of the MKIs, RXDX-105 is the only one that relatively spares VEGFR2, a characteristic which was hypothesized to allow tolerable dose up-titration to more clinically active plasma concentration levels. In a phase I/IB trial, however, the ORR was similar to those reported for other MKIs (ORR =19% or 6/31 patients) (33). RXDX-105 was discontinued later in 2019.

Full table
Retrospective series of MKIs have reported similar response rates. The GLORY database included 169 patients with RET-rearranged NSCLC, with 53 patients receiving at least one MKI (22). Cabozantinib, which 21 patients received, had the highest ORR of 37%, followed by vandetanib (18%) and sunitinib (22%). Responses were also seen with lenvatinib and nintedanib. No responses were seen with sorafenib, alectinib, ponatinib and regorafenib, although the number of patients who received each agent were in the single digits. The median OS for patients in the registry was 6.8 months, which highlighted the need for a more active RET inhibitor beyond standard MKIs. The results of the interventions in GLORY, as well as three other retrospective series of alectinib (34,35) and vandetanib (36), are summarized in Table 2.

Full table
MKI limitations
The response to MKIs in RET-rearranged NSCLC was notably disappointing compared to other targeted therapies in lung cancer. For context, the ORRs for osimertinib, alectinib, and entrectinib for untreated EGFR, ALK, and ROS1-altered NSCLC are 80% (37), 83% (38), and 77% (39), respectively. The relatively modest response and limited overall durability for MKIs in RET-fusion positive NSCLCs is likely due to several factors, the most important of which are non-selectivity for RET, potent inhibition of non-RET targets such as VEGFR2 (which contributes to dose-limiting toxicity and drug discontinuation), and intrinsic and acquired resistance.
Off-target kinase inhibition by MKIs contributes to the comparative lack of efficacy in RET-rearranged disease by several mechanisms. First, the concurrent inhibition of non-RET targets mediates some of the most significant toxicities seen with MKIs. VEGFR2 inhibition can cause hypertension, hand-foot syndrome, and proteinuria (40), while EGFR inhibition can contribute to acneiform rash and diarrhea (41). Cabozantinib, vandetanib and lenvatinib all more potently inhibit VEGFR2 than RET (42,43). These treatment-related toxicities lead to dose reductions and consequently, potentially decreased on-target inhibition of RET (26). Drug reduction rates for MKIs range from 23% to 73%, and are summarized in Table 3. Second, the lack of selectivity for RET makes it difficult for these agents to more meaningfully inhibit RET in patients due to relatively reduced potency in vivo and limited plasma exposures. While the latter issue is not a universal problem among MKIs in driver-positive lung cancers (e.g., crizotinib is active against ALK/ROS1 fusions), this has been a substantial limitation for drugging RET fusions.
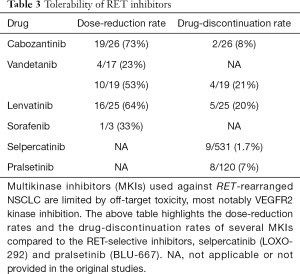
Full table
MKI resistance
There are known mechanisms of both intrinsic and acquired resistance with MKI therapy that may contribute to limited therapeutic response. Several acquired resistance mutations have been identified, although some have only been discovered in cell lines during in vitro treatment with MKIs and are hypothesized, but not proven, to propagate resistance in humans during treatment. These include RETI788N (44), solvent-front mutation RETG810A (45), and gatekeeper mutation RETV804L (45,46). In contrast, both gatekeeper mutation RETV804M (47) and RETS904F (48), a mutation in the activation loop of the RET kinase domain, were discovered in patient samples (plasma and tissue, respectively) after progression on vandetanib. An alternative mechanism of resistance to RET inhibition derived from preclinical models includes activation of the mitogen-activated protein kinase (MAPK) pathway (44), possibly by acquisition of NRAS mutations (49). Finally, certain fusion partners are thought to potentially mediate intrinsic resistance to certain MKIs (50). For example, both RXDX-105 and vandetanib have diminished activity against tumors with KIF5B-RET fusions (27,33), suggesting that this particular fusion may be less susceptible to non-selective RET inhibition.
Combination strategies have been utilized in attempts to overcome innate or acquired resistance postulated with MKI monotherapy. The strategy of combining vandetanib with the mammalian target of rapamycin (mTOR) inhibitor everolimus was tested in the clinic after preclinical data suggested that mTOR inhibition may both improve blood-brain barrier penetration and overcome resistance mediated by AKT amplification (51). Of 13 RET-rearranged patients, 7 had a PR (ORR =54%), including all three patients with brain metastases (52). However, 17/19 patients (89%) required dose reduction after the first cycle due to toxicity, indicating that tolerability may limit combination strategies.
Chemotherapy
In the GLORY registry, 84 patients with advanced disease received platinum-based chemotherapy as first-line treatment. Complete or partial responses were seen in 33 of 65 evaluable patients (51%), with a median PFS of 7.8 months and median OS of 24.8 months in 70 patients with survival data (22). A retrospective series of 18 patients with lung adenocarcinoma also demonstrated an ORR of 45% with median PFS of 19 months in patients treated with pemetrexed-based regimens, which was similar to contemporaneous responses to pemetrexed-based therapy in ROS1- and ALK-rearranged lung cancers (53). Anecdotal success of long-term (>2 years) treatment with single-agent pemetrexed has also been reported (54). In summary, chemotherapy represents a viable treatment option for patients with RET-rearranged NSCLC during their treatment course, and pemetrexed-based chemotherapy should be considered when possible.
Immunotherapy
Responses to immunotherapy in RET-rearranged lung cancer have not been characterized prospectively but are thought to be poor, based on available data from retrospective studies. The IMMUNOTARGET registry included 16 patients with RET fusions who were treated with immune checkpoint blockade as a second or higher line of therapy. A 6% response rate to immune checkpoint blockade was observed, with a median PFS of 2.1 months (55). Similar immunotherapy response rates were observed for patients with ALK and ROS1 driver alterations. A second retrospective series from Memorial Sloan Kettering (MSK) described a 0% response rate among 16 patients with RET-rearranged NSCLC treated with immunotherapy, even among patients with PD-L1 expression ≥1%, including one with PD-L1 expression >50% (56). Notably, the majority of evaluated cases in the MSK series (21/26 or 80.7%) had <50% PD-L1 expression, indicating RET-rearranged tumors may be less immunologically active. In contrast, a separate retrospective series included 9 RET-rearranged NSCLC patients treated with single-agent immunotherapy in the second or third-line setting. Of 8 evaluable patients, 3 (37%) achieved a PR, 2 (25%) achieved SD, and 3 (37%) had PD (57). Data in large, prospective cohorts is thus necessary to draw more definitive conclusions on the activity of single-agent immunotherapy. Notably, outcomes with combination chemotherapy and immunotherapy have not been described to date and represent a highly relevant data set to examine.
Selective RET inhibitors
The modest activity of MKIs against RET both demonstrated a clear need for more selective therapies and highlighted the ideal characteristics needed for such agents to be effective. Selpercatinib, formerly known as LOXO-292, and pralsetinib, formerly known as BLU-667, are two such agents whose early clinical results were reported in 2017–2018 and subsequently updated in 2019. Both are notable for potent in vitro and in vivo selective activity against both wild-type and mutated RET with significantly diminished affinity for VEGFR2 and other kinases, a crucial feature for resolving dose-limiting toxicity. From early clinical results, both agents were granted Breakthrough Therapy designation by the US Federal Drug Administration (FDA) for advanced NSCLC with RET fusions after progression on platinum chemotherapy, in September 2018 for selpercatinib and May 2019 for pralsetinib. In May 2020, selpercatinib was approved by the FDA for adults with advanced lung and thyroid cancers with RET fusions or mutations, making it the first targeted therapy approved for RET-driven cancers.
Selpercatinib is a highly selective, ATP-competitive small molecular RET inhibitor with preliminary in-human results reported in 2017. In preclinical models, selpercatinib demonstrated both >100-fold higher potency against RET compared to non-RET kinase targets and uniform activity in RET-altered xenograft models, independent of kinase fusion partner (58). In particular, the compound demonstrated promising in vivo activity against RETV804M, a known acquired resistance mutation against which MKIs are postulated to be ineffective (59).
The results of LIBRETTO-001, the phase 1/2 dose escalation/expansion trial of selpercatinib in advanced solid tumors with RET-fusion positive alteration, were first reported at the Annual Meeting of the American Society for Clinical Oncology (ASCO) in 2018. In 2019 at the World Conference of Lung Cancer (WCLC), the results of the first 105 patients with NSCLC who had received prior platinum chemotherapy were updated. In contrast to MKIs, the overall ORR was 68%, with 66% achieving PR and only 2% of patients demonstrating PD as their best response (60). The responses did not vary by prior treatment received (chemotherapy, immune checkpoint blockade or MKIs) or by fusion partner. Recognizing that follow up was yet to mature, the median PFS was 18.4 months, with a median 7.5 months of follow-up. The majority of treatment-related toxicities were grades 1 or 2, and included fatigue, diarrhea, constipation, dry mouth, nausea, and dyspnea, highlighting the tolerability of the compound when off-target inhibition is minimized. There were two treatment-related AEs that were grade 3 or higher, which were tumor lysis syndrome and increased ALT.
Preclinical and early clinical results of pralsetinib were published in 2018, and similarly highlighted the drug’s selectivity against RET. In enzymatic assays, pralsetinib inhibited wild-type and mutated RET with sub-nanomolar potency and was 90-fold more selective for RET than VEGFR2 (61). The first results of the global ARROW study, a registrational trial that included both a dose escalation and dose expansion phase in multiple solid tumors, were released at ASCO in 2019. Of 57 evaluable patients, the ORR was 56%, all of which were PRs. Six patients remained on treatment for more than six months (62). The ORR for patients previously treated with chemotherapy was also high at 60% (18/30) and comparable to the results of selpercatinib. The responses seen were independent of prior therapy received and RET fusion partner. Pralsetinib was similarly well-tolerated, with the majority of treatment-related toxicities being grade 1 and reversible, which included constipation, neutropenia, AST elevation, hypertension, diarrhea, and dry mouth. 28% of patients had grade 2 or higher treatment-related toxicity events.
Intracranial disease
In addition to the comparatively higher response rates and tolerability of both RET-selective compounds, the improvement in intracranial response is particularly noteworthy. About 45% of patients with RET alterations in NSCLC develop brain metastases during the lifetime of treatment, demonstrating a crucial need for therapies with adequate CNS penetration (63). The CNS response rate with MKIs has been poor. In a retrospective series of 11 patients with CNS metastases treated with MKIs, only two had an intracranial response (63). In contrast, of the 11 patients with CNS metastases in LIBRETTO-001, two (18%) achieved intracranial CR, eight (73%) PR, and one SD (9%). In addition, a case report from MSK in 2019 described complete resolution of leptomeningeal disease with initiation of selpercatinib, in addition to partial response of CNS parenchymal lesions (64). Similarly, in the ARROW study, seven of nine patients with measurable brain metastases achieved shrinkage of CNS disease and no patients developed new brain metastases while on treatment.
Selective inhibitor resistance
As expected, acquired resistance eventually emerges even with highly selective RET inhibitors. A case series published in January 2020 described RETG810R/S/C mutations in the RET solvent front detected in circulating tumor DNA in two patients just prior to progression on selpercatinib (65). The authors used structural modeling to elucidate that the mutations sterically hinder binding of selpercatinib, thus resulting in loss of activity. A possible solution to resistance mediated by RET solvent-front mutations is already in the clinic. TPX-0046 is a novel RET/SRC inhibitor that in enzymatic assays demonstrated high potency against RETG810R, with a mean IC50 of 17 nM, compared to IC50 >500 nM with pralsetinib or selpercatinib (16). Therefore, TPX-0046 may be able to overcome solvent-front mutation-mediated resistance after treatment with selpercatinib or pralsetinib. A phase I clinical trial testing TPX-0046 in multiple solid tumors, including NSCLC, has recently opened (NCT04161391).
Future directions and conclusions
From the initial identification of RET rearrangements in NSCLC in 2012 to the present, investigation into therapeutic options has rapidly grown. While treatment with repurposed MKIs held modest promise initially, the substantial increase in activity and favorable safety profile of the RET-selective agents selpercatinib and pralsetinib have made MKIs significantly less desirable first-line RET TKI options. The future of first-line RET TKI therapy for NSCLC with RET fusions clearly lies in selective RET inhibition, which seems to have overcome the major deficiencies seen with MKIs.
In early 2020, a randomized phase III trial of selpercatinib compared to platinum-pemetrexed with or without pembrolizumab in treatment-naïve RET fusion positive NSCLC is expected to open (NCT04194944). In addition, a phase III open-label trial of pralsetinib in first-line treatment of RET fusion positive NSCLC compared to platinum chemotherapy-based regimen is also planned for early 2020 (NCT04222972). With these trials, in addition to expanded recruitment of the existing phase II trials for both drugs, it is likely that not only one, but two drugs will likely soon be approved in a variety of regulatory environments for use in NSCLC with RET alterations. The beginning of the new decade, therefore, is likely the beginning of an unprecedented era for patients whose cancers harbor RET alterations.
Acknowledgments
Funding: The authors would like to thank the MSK Craig Thompson Cancer Center Support Grant (P30 CA008748).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Silvia Novello, Francesco Passiglia) for the series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-346). The series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” was commissioned by the editorial office without any funding or sponsorship. AD reports PERSONAL FEES from Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Axis, Peerview Institute, OncLive, Paradigm Medical Communications, LLC, Remedica Ltd., ArcherDX, Foundation Medicine, PeerVoice, Research to Practice, Medscape, and WebMD, outside the submitted work; ASSOCIATED RESEARCH PAID TO THE FOLLOWING INSTITUTIONS: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; RESEARCH collaboration with Foundation Medicine; ROYALTIES to Wolters Kluwer; OTHER relationships with Merck - Food/Beverage, Puma - Food/Beverage, Merus and Boehringer Ingelheim; CME HONORARIA from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology and Research to Practice. The author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kato S, Subbiah V, Marchlik E, et al. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res 2017;23:1988-97. [Crossref] [PubMed]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985;42:581-8. [Crossref] [PubMed]
- Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676-90. [Crossref] [PubMed]
- Drosten M, Putzer BM. Mechanisms of Disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat Clin Pract Oncol 2006;3:564-74. [Crossref] [PubMed]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994;367:380-3. [Crossref] [PubMed]
- Tsuzuki T, Takahashi M, Asai N, et al. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene 1995;10:191-8. [PubMed]
- Edery P, Lyonnet S, Mulligan LM, et al. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature 1994;367:378-80. [Crossref] [PubMed]
- Chatterjee R, Ramos E, Hoffman M, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet 2012;131:1725-38. [Crossref] [PubMed]
- Wang X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim Biophys Acta 2013;1834:2205-12. [Crossref] [PubMed]
- Worby CA, Vega QC, Zhao Y, et al. Glial cell line-derived neurotrophic factor signals through the RET receptor and activates mitogen-activated protein kinase. J Biol Chem 1996;271:23619-22. [Crossref] [PubMed]
- Qian Y, Chai S, Liang Z, et al. KIF5B-RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer 2014;13:176. [Crossref] [PubMed]
- Trupp M, Scott R, Whittemore SR, et al. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 1999;274:20885-94. [Crossref] [PubMed]
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7. [Crossref] [PubMed]
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4. [Crossref] [PubMed]
- Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res 2012;22:436-45. [Crossref] [PubMed]
- Drilon A, Rogers E, Zhai D, et al. 506P-TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann Oncol 2019;30. [Crossref]
- Das TK, Cagan RL. KIF5B-RET Oncoprotein Signals through a Multi-kinase Signaling Hub. Cell Rep 2017;20:2368-83. [Crossref] [PubMed]
- Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993;363:458-60. [Crossref] [PubMed]
- Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996;276:1575-9. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [Crossref] [PubMed]
- Gautschi O, Milia J, Filleron T, et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol 2017;35:1403-10. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer: NCCN2017 March 16, 2017.
- Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018;15:150. [Crossref] [PubMed]
- Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630-5. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653-60. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017;5:42-50. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. 1487PLURET: Final survival results of the phase II trial of vandetanib in patients with advanced RET-rearranged non-small cell lung cancer. Ann Oncol 2018;29:vii493-547. [Crossref]
- Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer 2019;138:124-30. [Crossref] [PubMed]
- Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017;28:292-7. [Crossref] [PubMed]
- Horiike A, Takeuchi K, Uenami T, et al. Sorafenib treatment for patients with RET fusion-positive non-small cell lung cancer. Lung Cancer 2016;93:43-6. [Crossref] [PubMed]
- Nokihara H, Nishio M, Yamamoto N, et al. Phase 1 Study of Cabozantinib in Japanese Patients With Expansion Cohorts in Non-Small-Cell Lung Cancer. Clin Lung Cancer 2019;20:e317-28. [Crossref] [PubMed]
- Drilon A, Fu S, Patel MR, et al. A Phase I/Ib Trial of the VEGFR-Sparing Multikinase RET Inhibitor RXDX-105. Cancer Discov 2019;9:384-95. [Crossref] [PubMed]
- Ribeiro M, Alessi JVM, Oliveira LJC, et al. Alectinib activity in chemotherapy-refractory metastatic RET-rearranged non-small cell lung carcinomas: A case series. Lung Cancer 2020;139:9-12. [Crossref] [PubMed]
- Lin JJ, Kennedy E, Sequist LV, et al. Clinical Activity of Alectinib in Advanced RET-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:2027-32. [Crossref] [PubMed]
- Platt A, Morten J, Ji Q, et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 2015;15:171. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Hayman SR, Leung N, Grande JP, et al. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 2012;14:285-94. [Crossref] [PubMed]
- Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011;19:1079-95. [Crossref] [PubMed]
- Ferrara R, Auger N, Auclin E, et al. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27-45. [Crossref] [PubMed]
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298-308. [Crossref] [PubMed]
- Plenker D, Riedel M, Bragelmann J, et al. Drugging the catalytically inactive state of RET kinase in RET-rearranged tumors. Sci Transl Med 2017;9. [Crossref] [PubMed]
- Huang Q, Schneeberger VE, Luetteke N, et al. Preclinical Modeling of KIF5B-RET Fusion Lung Adenocarcinoma. Mol Cancer Ther 2016;15:2521-9. [Crossref] [PubMed]
- Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004;23:6056-63. [Crossref] [PubMed]
- Dagogo-Jack I, Stevens SE, Lin JJ, et al. Emergence of a RET V804M Gatekeeper Mutation During Treatment With Vandetanib in RET-Rearranged NSCLC. J Thorac Oncol 2018;13:e226-7. [Crossref] [PubMed]
- Nakaoku T, Kohno T, Araki M, et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun 2018;9:625. [Crossref] [PubMed]
- Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-Inhibition in RET-Rearranged NSCLC Is Mediated By Reactivation of RAS/MAPK Signaling. Mol Cancer Ther 2017;16:1623-33. [Crossref] [PubMed]
- Rich TA, Reckamp KL, Chae YK, et al. Analysis of Cell-Free DNA from 32,989 Advanced Cancers Reveals Novel Co-occurring Activating RET Alterations and Oncogenic Signaling Pathway Aberrations. Clin Cancer Res 2019;25:5832-42. [Crossref] [PubMed]
- Subbiah V, Berry J, Roxas M, et al. Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer 2015;89:76-9. [Crossref] [PubMed]
- Subbiah V, Cascone T, Hess KR, et al. Multi-kinase RET inhibitor vandetanib combined with mTOR inhibitor everolimus in patients with RET rearranged non-small cell lung cancer. J Clin Oncol 2018;36:9035. [Crossref]
- Drilon A, Bergagnini I, Delasos L, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol 2016;27:1286-91. [Crossref] [PubMed]
- Takeda M, Sakai K, Nishio K, et al. Successful long-term treatment of non-small cell lung cancer positive for RET rearrangement with pemetrexed. Onco Targets Ther 2019;12:5355-8. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Offin M, Guo R, Wu SL, et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis Oncol 2019.1-8. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Vinas F, et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018;29:1869-76. [Crossref] [PubMed]
- Velcheti V, Bauer T, Subbiah V, et al. OA 12.07 LOXO-292, a Potent, Highly Selective RET Inhibitor, in MKI-Resistant RET Fusion-Positive Lung Cancer Patients with and without Brain Metastases. J Thorac Oncol 2017;12:S1778. [Crossref]
- Drilon A, Oxnard G, Wirth L, et al. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. J Thorac Oncol 2019;14:S6-7. [Crossref]
- Subbiah V, Gainor JF, Rahal R, et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov 2018;8:836-49. [Crossref] [PubMed]
- Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:9008. [Crossref]
- Drilon A, Lin JJ, Filleron T, et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J Thorac Oncol 2018;13:1595-601. [Crossref] [PubMed]
- Guo R, Schreyer M, Chang JC, et al. Response to Selective RET Inhibition With LOXO-292 in a Patient With RET Fusion-Positive Lung Cancer With Leptomeningeal Metastases. JCO Precis Oncol 2019. [Crossref] [PubMed]
- Solomon BJ, Tan L, Lin JJ, et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-driven malignancies. J Thorac Oncol 2020;15:541-9. [Crossref] [PubMed]


