
GEMSTONE-301: a phase III clinical trial of CS1001 as consolidation therapy in patients with locally advanced/unresectable (stage III) non-small cell lung cancer (NSCLC) who did not have disease progression after prior concurrent/sequential chemoradiotherapy
Introduction
Lung cancer is the most common type of cancer in China. Data published by the National Tumor Registry (1) revealed that in 2015, there were 733,300 new cases of lung cancer in China (509,300 males and 224,000 females), accounting for 17.01% of all new cases, with 610,200 attributed deaths, comprising 21.68% of cancer-related mortality. About 15% of all lung cancer patients have already reached the locally advanced stage by the time they are diagnosed (2) and the 5-year survival rate is a meagre 10–15% (3).
Concurrent chemoradiotherapy (cCRT) has predominantly been used around the world as the combined modality therapy for unresectable non-small cell lung cancer (NSCLC) patients with stage III disease. Meanwhile, in China, platinum-based doublet chemotherapy administered on a concurrent or sequential basis with radiotherapy are the main combined treatment modalities. Despite improved overall survival (OS) stemming from treatment modalities being optimized, the disease inevitably progresses in the majority of patients, who can expect progression-free survival (PFS) of approximately 8–10 months (4-13).
Immune checkpoints are a group of immunosuppressive molecules. Their physical function is to modulate immune reaction, regulating its intensity and extent to prevent normal cells from damage or injury. This feature is often exploited by tumor cells to avoid the attack by immune cells. Clinically validated immune checkpoints include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and PD-1/PD-L1; of the two, PD-1/PD-L1 has greater clinical potential owing to its favorable safety and broader indication range.
The expression of PD-L1, which is the main ligand of T-cell inhibitory receptor PD-1, mainly takes place on the surface of tumor cells and antigen-presenting cells. PD-L1 expressed on tumor cells binds with PD-1 on T cells, triggering a signaling cascade that inhibits T cell proliferation and cytokine secretion by activated T cells, lowers the activity of T cells, and decreases the killing of tumor cells by T cells. Drugs that block PD-1 and PD-L1 from interacting together may restore T cell activity and its ability to kill tumor cells.
CS1001 is the first full-length, fully humanized PD-L1-targeted immunoglobin G4 (IgG4) mAb developed through the OMT transgenic rat platform. It specifically binds to PD-L1, blocking its ligation with PD-1. A phase Ia/Ib study (NCT03312842) carried out an evaluation of the safety, tolerability, pharmacokinetics (PK), and preliminary antitumor activity of CS1001 in patients who had multiple types of solid tumors or lymphomas In the phase Ia portion, CS1001 administered at 5 doses [3 mg/kg, 10 mg/kg, 20 mg/kg, 1,200 mg and 40 mg/kg, every 3 week (Q3W)] was demonstrated to be safe and well tolerated, entailing no dose-limiting toxicity or related serious adverse events (SAEs). The pharmacokinetic profile of CS1001 at 1,200 mg resembles that of atezolizumab, a marketed drug with a similar mechanism. CS1001 1,200 mg fixed-dose administered intravenously Q3W was recommended as the phase II dose (RP2D) based on safety, PK, antitumor activity, and other data.
The data from phase Ib monotherapy reported that 59 (80.0%) patients experienced CS1001-related adverse events (AEs). Of these patients, 11 (15.1%) had Grade 3–5 CS1001-related AEs, and 7 (9.6%) patients had SAEs related to CS1001 as of 1 July 2019, indicating CS1001 to be well tolerated. CS1001 monotherapy in microsatellite instability-high or deficient mismatch repair (MSI-H/dMMR) solid tumors reported objective response rate (ORR) of 38.1% in 21 patients, while CS1001 in combination with chemotherapy reported ORR of 77.8% and 62.1% in esophageal squamous cell carcinoma (ESCC) and gastric and gastro-esophageal junction carcinoma (GC/GEJ), respectively. Together with other phase Ib studies, these data indicate promising antitumor activity in a range of solid tumors.
GEMSTONE-301 was initiated on the basis of the safety and antitumor potential CS1001 had demonstrated in the phase I study. It is a phase III, randomized, double-blind study (NCT03728556) to assess the efficacy and safety of CS1001 as a consolidation therapy for Chinese patients with unresectable NSCLC who have stage III disease after undergoing concurrent or sequential chemoradiotherapy. We present the following article in accordance with the SPIRIT reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-608).
Methods
The study protocol and informed consent documents were approved by the ethical committees of the participating institutions, and informed consent was obtained from all patients. All procedures performed in this study will be in accordance with the Declaration of Helsinki.
Patients
All patients in the GEMSTONE-301 study must have histologically confirmed diagnosis of locally advanced/unresectable stage III NSCLC with no disease progression after chemoradiotherapy delivered concurrently/sequentially. Patients with epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) translocation, or c-ros pro-oncogene 1-receptor tyrosine kinase (ROS1) translocation are excluded. The criteria for inclusion and exclusion are summarized in Table 1. Patients for the GEMSTONE-301 study are presently being recruited at 37 trial sites in China with a target sample size of 402. Written informed consent must be obtained from all patients before the commencement of any activities related to the trial.

Full table
Study design and treatment
Figure 1 summarizes the GEMSTONE-301 study design. This is a phase III, randomized, double-blind, placebo-controlled, multicenter study. Eligible patients are randomized at 2:1 by stratified block randomization method into either the CS1001 group or placebo group. The stratification factors for randomization are ECOG status (0 versus 1), chemoradiotherapy (concurrent versus sequential) and total radiotherapy dose (<60 versus ≥60 Gy).
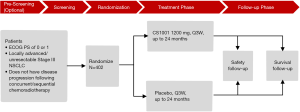
In the treatment phase, patients will receive CS1001 1200 mg or placebo intravenous infusion Q3W (21±3 days), with the first dose administered in the 42 days after concurrent/sequential chemoradiotherapy has ended. The investigational product is in liquid form for injection with specifications of 90 mg/3 mL/vial and 600 mg/20 mL/vial (provided by CStone Pharmaceuticals). The total dose will be diluted with 250 mL of sterile normal saline (0.9% sodium chloride solution) and infused over a period of 60 minutes. Treatment will continue until disease progression is confirmed, intolerable AEs occur, informed consent is withdrawn, the patient is lost to follow-up, death, or the study ends (depending on which happens first), and will last for 24 months at most.
The follow-up phase will include safety follow-up and survival follow-up. The safety follow-up phase will continue for 90 days after the last dose of the investigational product. Survival will be assessed by telephone follow-up visit or field visit every 12 weeks from the last dose of the investigational product until death or end of trial.
Safety, anti-CS1001 antibody, and efficacy will be monitored throughout the course of the entire trial. Safety assessment will be performed at each site visit. The severity, time of AE and causality with the investigational product will be evaluated. Tumor evaluation will be conducted and evaluated based on RECIST v1.1. The baseline tumor assessment will be performed after chemoradiotherapy and within the 28 days preceding the initial dose of study treatment. The tumor will be assessed every 9 weeks in the first year after the initial dose and every 12 weeks thereafter until the disease progresses, the patient dies, or the study ends, whichever comes first.
Study objectives
Table 2 summarizes the study’s objectives. The central objective is to investigate the efficacy of CS1001 in terms of investigator-assessed PFS compared to placebo. Secondary objectives include comparing OS, PFS assessed by BICR, safety, and other efficacy measurements between the CS1001 and placebo groups.

Full table
Statistics
The efficacy population will include all randomized patients (intent-to-treat, ITT), grouped according to their allocated treatment. Safety assessments will be carried out in all randomized patients who received at least 1 dose of any study drug, grouped by allocated treatment. A comparison of PFS and OS between treatment groups will be performed through a two-sided log-rank test with a significance level of 5%, with the same stratification factors used for randomization. A stratified Cox regression model will be used for estimating the efficacy in risk ratio, and event rates over time will be estimated within each treatment group using Kaplan-Meier method.
Interim analysis will be performed. Safety and efficacy data will be periodically reviewed by an independent data monitoring committee (iDMC), which will make recommendations according to pre-set criteria.
Discussion
Radiotherapy could enhance inflammatory reaction, facilitate antigen presentation and help to convert “cold” tumors into “hot” tumors; this might be the mechanism that lies behind its synergic effect with immunotherapy (14). Both preclinical evidence and clinical hints indicate that anti-PD-(L)1 mAb may offer clinical benefit after chemoradiotherapy.
A phase III trial, “PACIFIC”, conducted by AstraZeneca, investigated durvalumab, an anti-PD-L1 mAb, as consolidation treatment for locally advanced/unresectable NSCLC patients with stage III disease who did not have disease progression after cCRT, and reported statistically significant and clinically meaningful improvement of durvalumab compared with placebo not only in PFS [stratified hazard ratio for disease progression or death, 0.51; 95% confidence interval (CI), 0.41 to 0.63], but also in OS (stratified hazard ratio for death, 0.68; 99.73% CI, 0.47 to 0.997; P=0.0025) (15). A retrospective analysis of KEYNOTE-001 in patients with NSCLC also confirmed the synergic effect of pembrolizumab, an anti-PD-1 antibody with radiotherapy (16). In 97 patients who received pembrolizumab, 42 had previously received radiotherapy (radiotherapy group), while 55 had never received radiotherapy (non-radiotherapy group). Those who had undergone radiotherapy to treat NSCLC achieved significantly longer PFS with pembrolizumab than those who had not received radiotherapy, which suggests that the combination of radiotherapy and anti-PD-1 antibody for the treatment of advanced NSCLC is acceptably safe and holds promise.
CS1001 has been demonstrated to be well tolerated in the phase Ia/Ib study, with promising antitumor activity in multiple solid tumor types, either when received alone or when combined with chemotherapy. This evidence supports the initiation of GEMSTONE-301 for the evaluation of the CS1001’s efficacy and safety in a larger Chinese patient population who have unresectable stage III NSCLC. After chemoradiotherapy, these patients have a 5-year survival rate of only 10–15%, which reflects a desperate unmet need for more treatment alternatives for this group, to improve OS and PFS (17).
The target population of the GEMSTONE-301 study includes patients who did not have disease progression after cCRT or sCRT. Compared to the PACIFIC trial, which only enrolled patients treated with cCRT, the design is optimized to take into account that the overall utilization of concurrent CRT is generally lower in China than that in the US (78%) and Western Europe (53%) (18). In China, the proportion of sequential CRT utilization varies widely, from as low as 20–30% in most hospitals to as high as 80–90% in a small minority. As such, the utilization of sequential CRT represents an important combination modality in China. This phenomenon might be attributed to multiple factors, such as the inherent idea that sCRT may be more suitable for frail patients who are less tolerant of cCRT. Also, the lack of multidisciplinary treatment in certain areas and hospitals leads to great diversity in patient treatment flows and schedules, which further results in the limited utilization of cCRT. Whether NSCLC patients who receive sCRT could benefit from immunotherapy will be evaluated for the first time in GEMSTONE-301. Considering the different long-term survival benefit between the concurrent or sequential delivery of chemotherapy with radiotherapy (17), whether a patient has received sCRT or cCRT has been set as a stratification factor.
In the PACIFIC study, durvalumab treatment as consolidation therapy following CRT continued for 1 year (12). The CheckMate 153 study is a randomized study to evaluate the efficacy of continuous treatment vs. 1-year fixed duration of Nivolumab treatment for NSCLC patients. Those who were treated continuously with Nivolumab experienced vastly improved PFS and OS compared with those who stopped after 1 year (19). Given these previous findings, the recommended treatment duration of GEMSTONE-301 is set at 2 years and it could be prolonged if patients continue to show clinical benefits, as determined by the investigators. This design enables the optimization of CS1001 treatment length for curative purposes, and could potentially bring more clinical benefits for patients.
The correlation between EGFR mutation and the efficacy of anti-PD-L1 antibody (hazard ratio for disease progression or death) in NSCLC patients has been retrospectively analyzed in the PACIFIC trial (12). Patients with positive EGFR mutations exhibited a higher hazard ratio (0.76; 95% CI, of 0.35–1.64) than EGFR negative patients (0.47; 95% CI, 0.36–0.60), which indicated that the patients with EGFR mutations benefited less from anti-PD-L1 antibody treatment compared with those of wild type. The PACIFIC trial enrolled mostly non-Asian (~75%), with EGFR-mutant patients accounting for 6% of the total patients enrolled in the trial (the EGFR mutation status of 26.6% patients was unknown). Since Chinese NSCLC adenocarcinoma patients typically carry a higher EGFR mutation rate (38.3%) compared with Western populations (14–21%) (20), it is thus medically reasonable to exclude the EGFR-mutant population from the study to support the benefit for patients.
In conclusion, GEMSTONE-301 is the first phase III trial in China to evaluate an anti-PD-L1 mAb as consolidation therapy for unresectable stage III NSCLC that does not have disease progression following chemoradiotherapy. It will provide robust evidence of ability of chemoradiotherapy to enhance antitumor immune response and offer valuable insight into the potential role of CS1001 in patients with Stage III NSCLC. Most importantly, for the first time, it will generate clinical data on how anti-PD-L1 mAb works in patients who receive definitive sequential chemoradiotherapy.
Acknowledgments
Funding: The trial is being supported by CStone Pharmaceuticals (Su Zhou) Co., Ltd. and National Key R&D Program of China (Grant No. 2016YFC1303800).
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-608
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-608). QZ reports honorary from AstraZeneca and Roche, outside the submitted work; Dr. YLW reports personal fees from AstraZeneca, grants and personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Roche, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and informed consent documents were approved by the ethical committees of the participating institutions, and informed consent was obtained from all patients.
Sponsor Statement: CStone Pharmaceuticals will carefully fulfill the duties of sponsors according to ethics principles in Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements during the study, and be responsible for the initiating, submitting, organizing, sponsoring and monitoring this clinical study. Treatments that the investigator considers necessary for a subject’s welfare may be administered except for the prohibited therapies. Subjects with serious adverse events during the clinical study will be provided with active intervention and the corresponding treatment expense will be covered by sponsor according to applicable national regulatory requirements. Adequate financial compensation will be offered to subjects who experience damage confirmed to be consequence of investigational product-induced serious adverse effect.
Trial Statement: Under the general Principle of Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements, the developments and amendments of the protocol, all protocol related process and dissemination of the study results will follow CStone Standard Operating Procedure and applicable regulatory authority requirements. The data will be collected and validated as defined in the Data Management Plan. Adverse events and medical/ surgical history will be coded using the most current version of Medical Dictionary for Regulatory Activities. Medications will be coded using the World Health Organization Drug Dictionary. The data analysis will be performed following statistical analysis plan. Authorized representatives may perform audits to examine all study related activities. The authorship guidance of International Committee of Medical Journal Editors (ICMJE) is followed to guide authorship and publication principle of trial readout.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer Statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cancer in China. 2016. Available online: www.gbihealth.com
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Lau DH, Crowley JJ, Gandara DR, et al. Southwest Oncology Group phase II trial of concurrent carboplatin, etoposide, and radiation for poor-risk stage III non-small-cell lung cancer. J Clin Oncol 1998;16:3078-81. [Crossref] [PubMed]
- Davies AM, Chansky K, Lau DHM, et al. Phase II Study of Consolidation Paclitaxel After Concurrent Chemoradiation in Poor-Risk Stage III Non–Small-Cell Lung Cancer: SWOG S9712. J Clin Oncol 2006;24:5242-6. [Crossref] [PubMed]
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7. [Crossref] [PubMed]
- Fournel P. RTOG 94-10: Keenly Awaited Results Validating the Best Therapeutic Strategy for Locally Advanced Non-Small Cell Lung Cancer. J Natl Cancer Inst 2011;103:1425-7. [Crossref] [PubMed]
- Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13:671-8. [Crossref] [PubMed]
- Liang J, Bi N, Wu S, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol 2017;28:777-83. [Crossref] [PubMed]
- Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:953-62. [Crossref] [PubMed]
- Bao Y, Peng F, Zhou QC, et al. Phase II trial of recombinant human endostatin in combination with concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer. Radiother Oncol 2015;114:161-6. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Ahn JS, Ahn YC, Kim JH, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol 2015;33:2660-6. [Crossref] [PubMed]
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice: Radiation-Immunotherapy Combinations. CA Cancer J Clin 2017;67:65-85. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Treatment Architecture: United States Lung Cancer, Non-Small Cell. Kantar Health 2016;14.
- Spigel DR, Mcleod M, Hussein MA, et al. Randomized results of fixed-duration (1-yr) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol 2017;28:mdx380.002.
- Zhang XC, Wang J, Shao GG, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun 2019;10:1772. [Crossref] [PubMed]


