
Overcoming TKI resistance in fusion-driven NSCLC: new generation inhibitors and rationale for combination strategies
Introduction
The impressive technological progress made in molecular biology during the last two decades and the widespread adoption of next generation sequencing led to a paradigmatic shift in most solid tumors, including non-small cell lung cancer (NSCLC), moving from a large indistinct histological entity to a constellation of low-frequent molecularly-defined subgroups of patients. Oncogenic gene fusions were initially described in hematological tumors and result from chromosomal inversion, interstitial deletions, duplications, and translocations (1,2). During the last several years, multiple gene rearrangements with oncogenic potential have been described in NSCLC, identifying specific clinic-pathological subgroups of patients that benefit from a targeted therapeutic approach, including anaplastic lymphoma kinase (ALK), c-ros protooncogene 1 (ROS1) and, more recently, REarranged during Transfection (RET) and neurotrophic tyrosine receptor kinases (NTRK) genes. Beside these, several other gene fusions are emerging as potential therapeutic target in NSCLC, such as neuregulin-1 (NRG1), fibroblast growth factor receptor 3 (FGFR-3), v-Raf murine sarcoma viral oncogene homolog B (BRAF), although the actionability of these genetic rearrangements is far less defined.
Despite initial impressive antitumor activity, the use of targeted therapies in oncogene-addicted NSCLC subgroups is invariably associated with the development of acquired resistance through multiple mechanisms that can include both on-target and off-target mechanisms (3). Emergence of resistance represents one of the major hurdles for long-term efficacy of these drugs and several different strategies have been implemented or are under active development to overcome mechanisms of resistance, including highly selective TKIs, targeting by-pass track mechanisms, co-targeting of upstream and downstream pathways, and combinatorial approaches with chemotherapy and/or immunotherapy. Herein, we provide a comprehensive overview on the therapeutic strategies for overcoming acquired resistance to tyrosine kinase inhibitors (TKIs) targeting the most well-established oncogenic gene fusions in advanced NSCLC, including ALK, ROS1, RET, and NTRK rearrangements.
Overcoming resistance to ALK inhibitors
ALK rearrangements are found in ~3–5% of all NSCLCs and represent a distinct clinic-pathologic subgroup of patients that is associated with high sensitivity to ALK TKIs (4). Over the last five years the therapeutic landscape of advanced ALK-rearranged NSCLC profoundly changed, moving from first generation ALK TKI crizotinib, the first-in-class ALK inhibitor with proved superiority compared with 1st line platinum-based chemotherapy (5,6), followed by 2nd generation TKIs that demonstrated higher efficacy compared with platinum-based chemotherapy (ceritinib) (7) or crizotinib (alectinib and brigatinib) (8-10) in the upfront setting in comparative phase III trials. Second generation ALK TKIs are associated with longer PFS compared with crizotinib in ALK TKI-naïve patients and higher central nervous system (CNS) penetration. Therefore, the use of 2nd generation ALK TKIs is preferable and alectinib is the current standard of care in most of the countries due to its more favorable safety profile and wider availability (brigatinib is not FDA/EMA approved in treatment-naïve patients). Crizotinib is still a valuable first line option, mainly in countries where second generation ALK TKIs have not approved yet (11).
Distinct patterns of resistance have been identified for crizotinib and next generation ALK TKIs (Figure 1), but can be recapitulated to two major classes: ALK-dependent (on-target) and ALK-independent (off-target) mechanisms. Furthermore, in some cases, acquired resistance can be associated to pharmacological mechanisms rather than biological factors, as in the case of isolated CNS progression during crizotinib therapy (12) that is a consequence of the poor brain penetration of the drug.
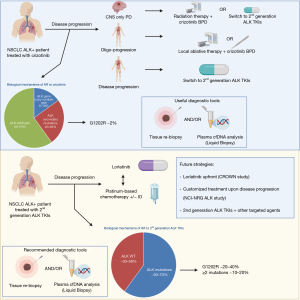
Given the different spectrum and frequency of mechanisms of resistance to the various classes of ALK TKIs, as a result, therapeutic strategies to overcome acquired resistance differ considerably between 1st generation and 2nd generation ALK inhibitors.
Secondary mutations in ALK gene were the first mechanism of acquired resistance described in crizotinib-resistant NSCLC (13) and are approximately found in 20‒36% of all patients after progression (14-16). In contrast with EGFR-mutated NSCLCs progressing on 1st/2nd generation EGFR TKIs, where EGFR T790M mutation is the most frequent mechanism of AR and other acquired mutations are relatively uncommon (17), a multitude of ALK secondary mutations have been described and include L1196M, C1156Y, G1269A, S1206Y/C, G1202R, L1152R, F1171T, F1174V/L/C, I1171T/N/S, E1210K, and 1151T ins (13-16,18). The presence of de novo ALK kinase domain mutations is instead relatively uncommon in ALK TKI-naïve patients (<3% of the cases) and might be responsible of intrinsic resistance to crizotinib (19). In addition to ALK secondary mutations, acquired resistance to crizotinib is also associated with copy number gain (CNG) of the gene in a significant proportion of patients (6–18%) either alone or in association with ALK mutations (15,16). Crizotinib-resistant tumors are still highly sensitive to ALK inhibition, as demonstrated by the relatively high ORRs (37.5–54%) in with 2nd generation ALK TKIs (alectinib, ceritinib, ensartinib and brigatinib) in crizotinib pretreated patients in multiple phase II/III clinical trials, regardless of the mechanisms of resistance (20-25). For these reasons, after crizotinib failure, treatment with 2nd generation ALK TKIs could be evaluated independently of a novel tumor genotype assessment though tissue re-biopsy and/or liquid biopsy.
However, the complexity of ALK-dependent mechanisms of resistance grows with increasingly potent ALK TKIs, since the use of 2nd generation ALK TKIs is associated with a higher frequency of ALK secondary mutations (~50–70%) and a different spectrum of resistant mutations in tissue and/or liquid biopsies. Indeed, the G1202R mutation that is relatively uncommon after crizotinib progression seems to be the most frequent ALK mutation after 2nd generation TKIs (21–43% vs. 2% with crizotinib) and confers resistance to most of the available ALK TKIs, while other mutations are associated with resistance to some 2nd generation ALK TKIs, but are sensitive to others, such as the F1174 that confers resistance to ceritinib but its sensitive to alectinib, or the I1171 that is associated with the inverse sensitivity. In addition, the sequential use of different ALK TKIs is associated with the development of compound mutations (≥2 mutations) in 12.5–23% of the cases, conferring high levels of resistance to ALK inhibitors (14,26). The use of liquid biopsy might better recapitulate the complexity and dynamics of the mutational status of the tumor at progression following 2nd generation ALK TKIs than tissue re-biopsy, with a significant discordance in the incidence of compound mutations, likely due to the spatial heterogeneity of the mechanisms of resistance (26).
The 3rd generation inhibitor lorlatinib is an ATP-competitive, macrocyclic TKI targeting both ALK and ROS1 rearrangements and designed to overcome ALK resistance mutations, including G1202R, and higher CNS penetration (cerebrospinal fluid-plasma ratio of 0.75 vs. 0.03 with crizotinib). Lorlatinib showed promising activity in 41 heavily pretreated ALK-rearranged NSCLC in a phase I study, with a 46% ORR (57% after one prior ALK TKI and 42% after ≥2 prior ALK TKIs) and a median PFS of 9.6 months. Activity was seen also in patients with brain metastases (intracranial ORR 46%), with higher efficacy in patients with secondary ALK mutations than those without evidence of mutations (27). These results were confirmed in a global phase II study enrolling 278 ALK-positive patients in five expansion cohorts, including 30 treatment-naïve patients (EXP1), 27 crizotinib-pretreated (EXP2), 32 crizotinib- and chemotherapy-pretreated (EXP3A), 28 progressing after a 2nd generation ALK TKI and/or chemotherapy (EXP3B), 65 patients treated with 2 previous ALK TKIs +/- chemotherapy (EXP4), and finally 46 patients treated with 3 previous ALK TKIs +/– chemotherapy (EXP5) (28). Higher ORR were observed in treatment-naïve (ORR 90%) and crizotinib-pretreated only patients (ORR 69.5%), while ORR ranged from 32.1% to 47% in patients who had received a previous 2nd generation ALK TKI or up ≥2 previous ALK TKIs and/or chemotherapy (28). The analysis with NGS of plasma and tissue samples from 198 ALK-positive NSCLC patients enrolled into the phase II study revealed that tumor genotyping for ALK mutations after failure of a 2nd generation ALK TKI may identify patients who are more likely to derive clinical benefit from lorlatinib, since patients harboring an ALK mutation had higher ORR than those without detectable mutations in tissue or plasma at baseline (62% vs. 32% in cfDNA and 69% vs. 27% in tissue). Furthermore, in patients harboring compound mutations (~one third of patients) the ORR was inferior than those observed in patients with only one ALK mutation (56% vs. 75%), with a shorter duration of response (DoR) (6.1 vs. 24.4 months) (29).
The NCI-NRG ALK study (NCT03737994) is a master protocol that includes multiple phase II studies that are testing different biomarker/ALK inhibitor combinations thought the use of tissue and plasma NGS results after progression on a next generation ALK TKI after or not prior crizotinib. Patients with L1198F mutation (alone or in combination with another ALK mutation) will receive crizotinib, patients with C1156Y or F1174 mutations will receive either lorlatinib, alectinib, or brigatinib, patients with a compound mutation will receive lorlatinib, patients with G1202 (including G1202del and G1202R) will receive either lorlatinib or brigatinib, patients with I1171 or V1180 mutations will receive either lorlatinib, ceritinib, or brigatinib, patients with L1196 (including L1196M) mutation will receive either lorlatinib, ceritinib, alectinib, brigatinib, or ensartinib, patients with MET amplification will receive crizotinib, and finally patients with no ALK-resistant mutations will receive either lorlatinib, ceritinib, alectinib, brigatinib, ensartinib, or pemetrexed with or without carboplatin/cisplatin.
After progression on lorlatinib, multiple compound mutations have been described either in preclinical models (30) and in clinical settings (26). Some of these mutations are particularly recalcitrant and are associated with resistance to all currently available ALK TKIs, as for example the G1202R/L1196M, whereas others can restore the sensitivity to other ALK TKIs, as for instance the L1198F mutation that paradoxically enhances binding to crizotinib, negating the effect of the associated C1156Y mutation (31). Since the sequential treatment with increasingly potent ALK TKIs fosters the emergence of compound ALK resistance mutations refractory to available ALK TKIs (26,30), the change of position of the 3rd generation ALK TKI lorlatinib, which has a broader spectrum of activity against most of the ALK mutations, to the upfront setting might be associated with represent a more effective strategy and is under clinical evaluation in the randomized phase III trial CROWN (NCT03052608).
In addition to ALK-dependent mechanisms of resistance, different off-target mechanisms of acquired resistance have been described in crizotinib-resistant patients and in preclinical models, including the activation of by-pass track signaling pathways, such as KIT amplification (16), KRAS mutations (15), EGFR mutation and/or amplification (16,32), IGF-1R activation (33), RAS/MEK activation (34), and histological (small cell lung cancer transformation) and/or phenotypical (epithelial-to-mesenchymal transition) changes (16). Due to their higher ALK inhibition potency and selectivity, 2nd generation ALK TKIs and lorlatinib are more associated also with a different spectrum of ALK-independent mechanisms of resistance. Recently, MET amplification has emerged as a mechanism of acquired resistance to 2nd generation ALK TKIs (12%) and lorlatinib (22%), but is not evident after crizotinib (0%). Furthermore, MET amplification seems more common after front-line use of 2nd generation ALK TKIs than after sequential use of crizotinib-next generation ALK TKIs (P=0.019) (35). These results are similar to those observed with EGFR TKIs (36), suggesting that the likelihood of developing target-independent mechanisms increases with TKI potency. Activation of MET pathway can also occur through alternative mechanisms, including gene fusions (ST7-MET rearrangements) that might co-exist with MET amplification as well. ALK resistance with both MET amplification and ST7-MET rearrangement is reversed with dual ALK/MET inhibition in in vitro studies (35), providing the rational for combinatorial approaches or the use of crizotinib. Other bypass track mechanisms described in preclinical models include also RAS/MEK activation (37,38), protein kinase C (PKC) activation (39), SRC activation and EMT transformation (40), activation of EGFR and HER4 pathways (38), SHP2 activation (41), and NF2 loss (40). The use of combinatorial strategies has been shown to overcome acquired resistance due to bypass track mechanism in multiple preclinical models and different combinatorial strategies are under clinical evaluation to overcome or prevent the emergence of these off-target mechanisms of resistance. The combination of ALK TKI + MEK inhibitors is under evaluation in three phase I/II studies with ceritinib-trametinib (NCT03087448), alectinib-cobimetinib (NCT03202940), and brigatinib-binimetinib (NCT04005144). Other studies are evaluating the addition of the antiangiogenic agent bevacizumab plus alectinib (NCT02521051, NCT03779191) or brigatinib (NCT04227028), while others are combining the mTOR inhibitor everolimus with ceritinib (NCT02321501). Moreover, a cohort of the NCI-NRG ALK master protocol (NCT03737994) is evaluating crizotinib monotherapy in patients with MET amplification after resistance to 2nd generation ALK TKIs.
It has been reported that in some models that harbor EML4-ALK rearrangements PD-L1 expression can be induced due to constitutive oncogenic signaling contributing to immune escape (42), providing the rationale for investigating immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 in ALK-rearranged NSCLCs either in combination with ALK TKIs or with chemotherapy.
A phase IB multicenter, dose escalation and expansion study, assessed the safety and activity of ceritinib plus nivolumab in 36 patients with advanced ALK-positive NSCLCs, including both previously treated and treatment-naive patients. Nivolumab was given intravenously at 3 mg/kg dose every two weeks and Ceritinib was orally given at doses 300 or 450 mg per day with low fat meal. 2 patients in the Ceritinib 300 mg cohort experienced dose-limiting toxicities (DLT) and 4 in the 450 mg cohort. ORR was 83% in the 450 mg and 60% in the 300 mg of ceritinib cohort respectively, 50% for the ALK TKI pretreated patients in the Ceritinib 450 mg arm and 25% in the Ceritinib 300 mg arm. Despite overlapping curves, response trended to be greater among PD-L1 positive patients compared with PD-L1 negative (ORR 64% and 31% respectively). Most common grade 3–4 toxicities were transaminase increase, amylase and lipase increase and maculopapular rash (43). Another phase I/II trial (CheckMate-370, cohort E) assessed the safety and tolerability of nivolumab-crizotinib combination as first line therapy. Unfortunately, the study was prematurely discontinued due to the evidence of severe hepatotoxicity in 38% of patients, including two treatment-related deaths (44). Safety concerns with the use of ICIs immediately after crizotinib also emerged in a retrospective study that showed an unusual incidence of grade 3–4 ALT elevation (36.3%) in ALK-positive NSCLC treated with sequential ICIs after crizotinib vs. only 3.4% in those who received crizotinib alone (45). Recently, growing interest emerged on the use of chemo-immunotherapy combinations in oncogene-addicted NSCLCs, including ALK-rearranged NSCLCs. The randomized phase III trial IMpower150 recently showed promising activity for the combination carboplatin-paclitaxel-bevacizumab plus atezolizumab in a small subgroup of patients with EGFR mutations or ALK translocations who failed or were intolerant for at least one line of TKI. The combination was associated with a statistically significant longer PFS compared with carboplatin-paclitaxel-bevacizumab alone (11.3 vs. 6.8 months; HR 0.51; P<0.001) (46). These results are hypothesis-generating, but requires further confirmation in larger patient cohorts.
Finally, another potential strategy after acquired resistance to 2nd generation ALK TKIs is the addition of platinum-based chemotherapy to ALK inhibition. This strategy has been recently reported in a small retrospective study of three institutions, demonstrating that patients who received platinum/pemetrexed in combination with an ALK TKI beyond progression had a longer PFS compared to those who received platinum/pemetrexed alone (6.8 vs. 3.2 months, respectively; HR 0.33; P=0.025) (47). These results are hypothesis generating and deserve further investigation.
Overcoming resistance to ROS1 inhibitors
ROS1 (ROS proto-oncogene 1) rearrangements were first reported in NSCLC in 2007 (48) and identify a small subset of lung adenocarcinoma (~1%) with peculiar clinicopathological characteristics, including predominance of solid, papillary, acinar, cribriform and mucinous histology patterns, younger age, never smoking status (49), and high sensitivity to pemetrexed-based chemotherapy (50). ROS1 rearrangements were identified as a potential target for TKIs on the basis of preclinical evidences in cell lines (48,51), with high sensitivity in both preclinical and clinical models to the MET/ALK inhibitor crizotinib (49). Based on these data, an expanded cohort of ROS1-rearranged NSCLCs was enrolled into the phase I PROFILE 1001 study with crizotinib. The preliminary results of this study showed an impressive 72% ORR and a 19.2 months PFS among 51 ROS1-translocated patients harboring 7 different fusion partners for ROS1 (52). The remarkable activity of crizotinib in this molecularly defined subgroup of patients is further confirmed by the recently published updated analysis of the PROFILE 1001 that continue to show the clinically meaningful benefit and safety of crizotinib after a follow-up period of 62.6 months with a median OS of 51.4 months (95% CI, 29.3 to not reached) and survival probabilities at 12, 24, 36, and 48 months of 79%, 67%, 53%, and 51%, respectively (53). The role of crizotinib in this subgroup of patients is further supported by a large retrospective study (54) and four single arm phase II trials in both Caucasian and Asian patients, demonstrating ORR ranging from 54% to 80% and median PFS between 5.5 to 20.0 months (55-58) (Table 1).

Full table
As in other NSCLC scenarios with targetable genomic alterations, ROS1-translocated patients develop acquired resistance after the use of crizotinib. Pooling retrospective and prospective published experiences of crizotinib treatment in ROS1-rearranged NSCLC patients, around 10 cases have been reported as primary refractory to the inhibitor (59), due to different biological or pharmacological mechanisms that include KRAS mutations acquisition (60), limited CNS penetration and metabolic alterations due to liver impairment (54), and BIM deletion polymorphisms (61).
The first mechanism of acquired resistance to crizotinib in ROS1-rearranged NSCLCs was a glycine-to-arginine substitution at codon 2032 in the ROS1 kinase domain (G2032R) (62) that confers resistance to ROS1 kinase inhibition through steric interference with drug binding (>100-fold increase in crizotinib half-maximal inhibitory concentration-IC50). According to a small series recently published, G2032R is the most commonly observed mutation accounting for crizotinib resistance in ROS1-positive NSCLC patients, occurring in 41% of cases, followed by D2033N (6%), and S1986F (6%) (63). The presence of an aspartic acid-to-asparagine substitution occurring at ROS1 codon 2033 (D2033N) is associated with acquired resistance to crizotinib, but has in vitro and in vivo sensitivity to cabozantinib (64). The serine at 1986 ROS1 position can be substituted by either tyrosine (S1986Y) or phenylalanine (S1986F) residues, leading to crizotinib exhaustion in an EZR-ROS1-rearranged NSCLC (65). Differently from the previous reported codons involved, the 1986 does not correspond to ROS1 active site and S1986Y/F substitutions appear to induce crizotinib resistance by both preventing its access to the enzyme active site and by increasing kinase activity, the latter event reported for the corresponding ALK C1156Y mutation. Functional in vitro studies demonstrated that ROS1 harboring either the S1986Y or the S1986F mutation, while conferring resistance to crizotinib and ceritinib, was inhibited by lorlatinib (65).
Another documented crizotinib resistance mutation in ROS1-positive NSCLCs is the gatekeeper ROS1 L2026M mutation that is an analogue to the ALK L1196M that has been largely approached in in vitro studies (66). Additional preclinical assays have indicated that crizotinib resistant cells harboring this mutation are sensitive to lorlatinib, repotrectinib and foretinib (67,68).
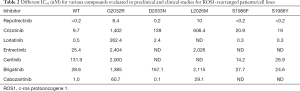
As described for other mutations studied in vitro before their clinical appearance, preclinical evidence concerning mechanisms of acquired resistance to crizotinib in ROS1-rearranged cells flanked clinical reports (59). The cell lines HCC78CR1 and -CR2 harbored the L2155S mutation (67), while a molecular screen with alkylating agent N-ethyl-N-nitrosourea upon ROS1 signaling-dependent Ba/F3 cells reveal a few further substitutions (69). The ROS1 secondary mutations E1990G, M2128V, L1951R, L2026M, K2003I and G2032R showed IC50 values for crizotinib more than 3-fold higher with regard to the native ROS1 form (69). Table 2 shows the different IC50 (nM) for various compounds evaluated in pre-clinical and clinical studies under development in ROS1-positive NSCLC TKI-naïve and previously treated with crizotinib.
Full table
In addition to mutations in ROS1 kinase domain, other crizotinib resistance mechanisms have recently been described. Davies et al. observed a switch in the control of growth and survival signaling pathways from ROS1 to EGFR in the HCC78 ROS1 resistant cell line (70). As a result of this switch, ROS1 inhibition-resistant cells became sensitive to EGFR inhibition (gefitinib ~1 µM), an effect that was enhanced by co-treatment with a ROS1 inhibitor. The mechanism behind this change remains unclear, although it occurred in the absence of a significant increase in EGFR autophosphorylation, suggesting that an autocrine signaling mechanism was not responsible. It is noteworthy that EGFR kinase activity is not always dependent on autophosphorylation and thus low levels of EGFR phosphorylation do not preclude its signaling activity (71). Prolonged exposure of HCC78 cell to the specific preclinical inhibitor JNJ-ROS1i-A or crizotinib has been associated with the emergence of G12C KRAS and Q61K NRAS mutations, respectively, both associated with a markedly decreased expression level of both mRNA and protein of SLC34A2-ROS1 in crizotinib-resistant models (72). Furthermore, KRAS amplification has been described in vivo in a ROS1-positive NSCLC with acquired resistance to crizotinib, support these preclinical evidence (60). Another bypass track mechanism of resistance to crizotinib in ROS1-positive NSCLC is the acquisition of an activating KIT mutation (D816G) that leads to constitutive activation of the tyrosine kinase receptor. The acquisition of KIT D816G renders the HCC78 and CUTO2 cell lines resistant to crizotinib, and only dual inhibition of ROS1 and KIT with crizotinib plus ponatinib could resensitize the cells to ROS1 blockage (73). As observed in other oncogene addicted tumors after resistance to targeted agents, another potential mechanism of resistance to crizotinib in ROS1-translocated NSCLCs is the activation of an epithelial-to-mesenchymal transition (EMT) (67).
Until recently, crizotinib was the only approved targeted drug directed against ROS1. Several other molecules, already in clinical use or in late phases of development in ALK-driven disease, are currently evaluated in ROS1-positive populations. Ceritinib, a 2nd generation ALK/ROS1 TKI, is able to overcome in vitro and in vivo crizotinib resistance in ALK- and ROS1-rearranged NSCLC, although, the activity in the second group has been less promising. Although not fully exhaustive, preclinical evidence revealed IC50 values of ceritinib for the native isoforms of ROS1 are significantly higher (74). Taking into account the structural and functional data of crizotinib resistance conferred by specific ALK mutations (75) and the predictive model for ROS1, only the expected M2001T and G2101A and the reported L2026M can be overcame by ceritinib, as they interfere with crizotinib binding only (65). Analogously to the corresponding ALK substitutions, ROS1 1981Tins, L1982F, S1986Y/F and F2004C/V cannot be inhibited by ceritinib, as they increase enzyme activity or induce conformational changes in ROS1 catalytic domain (65). Furthermore, ROS1 G2032R and D2033N mutations are not susceptible of ceritinib control as they induce deep structural changes in drug-binding site (61,63). As ceritinib does not boost the silencing of ROS1 signaling compared with crizotinib, its contribution when a bypass mechanism occurs would be negligible. The updated data of the ceritinib phase II study showed an ORR of 63% and a median PFS of 19.3 months in TKI-naïve patients (76), overlapping with data obtained with crizotinib (52).
The 3rd generation ALK/ROS1 inhibitor lorlatinib, which has shown sustained activity against almost all ALK resistant forms in in vitro and in vivo models (77), has demonstrated significant activity in cell experiments against ROS1 L2026M, D2033 and S1986Y/F (65,78).
The reduced inhibitory effect on cell viability upon ROS1 G2032R mutants, with IC50 of 17,747, 27,042 and 508 nM (65) suggests that lorlatinib may not overcome crizotinib resistance generated by the most important substitution. These preclinical data were recently confirmed in the preliminary report of the phase II PFROST study, evaluating lorlatinib in ROS1 fusion-positive patients crizotinib-resistant. No responses were observed among patients harboring a secondary ROS1 mutation (n=1 ROS1 S1861I, n=1 ROS1 V2054A, n=3 ROS1 G2032R) and all the patients harboring the ROS1G2032R mutation rapidly progressed, maintaining this aberration in liquid biopsy at the time of lorlatinib failure (79). Nevertheless, the reported efficacy and the preclinical evidence of the ≈100-fold potency against the native ROS1, compared to the 1st generation inhibitor, sustain that lorlatinib could overcome bypass signaling-driven crizotinib resistance (65).
Entrectinib is a pan-TRK, ROS1 and ALK inhibitor that has shown potent anti-neoplastic activity and tolerability in various neoplastic conditions, particularly NSCLC (80). The integrated analysis of three ongoing phase I/II trials of entrectinib (ALKA-372-001, STARTRK-1, and STARTRK-2) in 53 locally advanced or metastatic ROS1 fusion-positive NSCLCs recently reported a 77% ORR and a median DoR of 24.6 months at a median follow-up of 15.5 months (81). Based on these results, on August 2019 the US FDA approved entrectinib for ROS1 fusion-positive NSCLC. However, all patients enrolled in these studies were ROS1 TKI-naïve and preclinical data suggest that entrectinib lacks of activity against ROS1 G2032R and L2026M mutants (74,80), suggesting that this agent is not a suitable candidate to reverse acquired crizotinib resistance.
The multikinase inhibitor cabozantinib demonstrated activity against the crizotinib-resistant ROS1 D2033N mutation (64) and preclinical evidence suggest that cabozantinib, while inhibiting the native enzyme at doses inferior to crizotinib (74), has a direct activity upon several ROS1 mutants, abrogating the hypothesis of a potential off-target effect of the drug. In absence of clinical validation, cabozantinib showed IC50 values against ROS1 G2032R mutant between 13.5336, 15.351 and 26 nM (74). Cabozantinib clinical efficacy could therefore be attributed so far to either the inhibition of a putative bypass signaling or a more pronounced inhibition of the wild-type ROS1 kinase. An ongoing phase II trial is evaluating cabozantinib efficacy in advanced NSCLC harboring RET, ROS1 or NTRK fusions, as well as increased MET or AXL activity (NCT01639508).
Another multitarget inhibitor, foretinib, has shown in vitro activity against native ROS1 and several of its mutant forms. However, the suboptimal toxicity profile of the compound, together with the upcoming availability of specific, effective and safe inhibitors, does not ostensibly allow the allocation of foretinib among the most relevant ROS1 inhibitors (59). The 2nd generation ALK inhibitor brigatinib also inhibits ROS1 at concentrations clinically achievable in patients (74). Nevertheless, lack of effectiveness against G2032R and L2026M, disprove its regular use in the clinic. Repotrectinib is a low-molecular-weight, macrocyclic TKI that is selective and highly potent against ROS1, TRKA-C, and ALK. Importantly, repotrectinib exhibits activity against a variety of solvent-front substitutions in vitro and in vivo (68), with a substantial increased activity against ROS1 G2032R mutants compared with lorlatinib (IC50 values of 3.3 nmol/L vs. 160.7 nmol/L). Furthermore, repotrectinib repotrectinib was slightly less potent than cabozantinib (1.3 vs. 0.2 nmol/L) against the ROS1 D2033N mutation, but more potent than lorlatinib (3.3 nmol/L) (68). During the 2019 ASCO annual meeting, Cho et al. showed preliminary results of the TRIDENT-1 trial; among 10 evaluable TKI-naïve ROS1 NSCLC patients, repotrectinib was associated with a confirmed ORR of 90% and a median DoR not reached (range 5.5+ – 14.9+ months). Among 18 pretreated patients, confirmed ORR was 28% with a DoR of 10.2 months. In 7 patients with measurable target brain lesions at baseline, the intracranial ORR was 100% with a DoR (5.5+; 7.2+; 14.85+ months) in TKI-naïve patients and 50% with DoR (5.5+;14.8+, months) in TKI-pretreated patients, respectively (82).
DS-6051b is a new-generation selective ROS1/NTRK inhibitor that inhibits the intracellular phosphorylation of these kinases in a concentration-dependent manner and induces dramatic growth inhibition of both wild-type and G2032R mutant ROS1–rearranged cancers in vitro and in vivo, with an IC50 of 13.5 nM (83). Besides the G2032R mutants, DS-6051b had single-digit nanomolar IC50 against L1951R, S1986F, and L2026M, but had a relatively high IC50 against D2033N (IC50 ~30 nM) (84). In a clinical trial of DS-6051b, a crizotinib-naive ROS1-rearranged NSCLC patient with brain metastasis showed a partial response in the primary lung and brain metastasized tumors, suggesting that DS-6051b would be effective in brain metastasized tumors, although the blood–brain barrier penetration of this compound is still unclear in humans (84).
The role of combinatorial approaches to overcome by-pass mechanisms of resistance to ROS1 inhibitors has been investigated only in preclinical models, combining the ROS1 inhibitor TAE684 with gefitinib (70) or crizotinib plus dacomitinib or afatinib (67) in resistance models with EGFR activation and ponatinib plus crizotinib in cell lines with KIT D816G acquisition (73). A phase I study is investing the safety of the combination of brigatinib with the MEK inhibitor binimetinib in ALK or ROS1-rearranged NSCLC (NCT04005144)
Overcoming resistance to RET inhibitors
RET (REarranged during Transfection) fusion-positive NSCLCs represent a small subgroup of patients (~1–2%) that correlates with adenocarcinoma histology, never-smoking status, younger age, more advanced disease stage, potentially higher chemosensitivity (in particular, to pemetrexed-based regimens), and coexistence of other genomic alterations (85). Different fusion partners have been reported, but the most common RET fusions in lung cancer are kinesin family member 5B (KIF5B)-RET (70–90%) and CCDC6-RET (10–25%), followed by other less common variants (NCOA4-RET, TRIM33-RET, ZNF477P-RET, ERCC1-RET, HTR4-RET, and CLIP1-RET) (86). The mechanism of activation of RET fusion proteins is analogous to the oncogenic activation of rearranged ALK in NSCLC, but clearly differs from ROS1. In the EML4-ALK fusion gene, a coiled-coil domain in EML4 is fused to the ALK kinase domain, conferring oligomerization and constitutive kinase activation, while coil-coiled domains are not consistently present in ROS1 fusion genes in NSCLC and are not necessary to drive oncogenesis (87). The tumorigenic potential of RET fusion proteins has been demonstrated in vitro in Ba/F3 (pro-B lymphocyte) (88) or NIH3T3 (fibroblast) cell lines (87,89), and in CCDC6-RET-positive LC-2 lung adenocarcinoma cells (90).
Several preclinical studies reported on the activity of different multikinase inhibitors in RET-fusion-positive cell lines. Ba/F3 cells harboring the KIF5B-RET fusions common in RET-fusion-positive NSCLC were found to be sensitive to sorafenib, vandetanib, regorafenib, ponatinib, and lenvatinib (91-93). In 2016, a global multicenter network study (GLORY) included 165 patients with RET-rearranged NSCLC from 29 centers across Europe, Asia, and the United States. Seventy-two percent of the patients had KIF5B-RET fusion and 53 received one or more RET inhibitors in sequence, including cabozantinib (n=21), vandetanib (n=11), sunitinib (n=11), sorafenib (n=2), alectinib (n=2), lenvatinib (n=2), nintedanib (n=2), ponatinib (n=2), and regorafenib (n=1). The ORR with cabozantinib, vandetanib, and sunitinib was 37%, 18%, and 22%, respectively. Considering the main outcomes, median PFS was 2.3 months (95% CI, 1.6 to 5.0 months), and median OS was 6.8 months (95% CI, 3.9 to 14.3 months) (94). Moreover, this registry also provided information regarding the efficacy of first-line platinum-based chemotherapy in RET-rearranged NSCLC that was associated with an ORR of 50% (94).
Vandetanib predominantly inhibits VEGFR 2-3, EGFR and RET (IC50 for RET 100nM). This compound demonstrated in vitro (87,89,90,92) and in vivo (88,90,95) activity, suppressing the growth of KIF5B-RET-transfected NIH3T3 fibroblasts, KIF5B-RET transfected Ba/F3 lymphocytes, and CCDC6-RET-positive LC-2 lung adenocarcinoma cells, as well as, athymic mice transplanted with CCDC6-RET lung adenocarcinoma tumors and in immunocompetent KIF5B-RET transgenic mice. In unselected population, vandetanib was associated with low therapeutic efficacy either as monotherapy (ZEST and ZEPHYR) or in combination with docetaxel (ZODIAC) or pemetrexed (ZEAL) (85). Nevertheless, in selected RET-translocated NSCLC two small single arm phase II studies in Asian patients reported some signals of activity with ORR ranging from 18% to 47% and median PFS of 4.54–6.5 months (96-98). However, the efficacy seen in these studies seems less impressive than usually observed in oncogene-addicted NSCLCs treated with targeted therapies and a differential sensitivity for vandetanib was reported in the LURET study for KIF5B-RET and CCDC6-RET rearrangements (96,97).
Another multitarget TKI, lenvatinib, was identified through the exploratory research of agents with various tyrosine kinase inhibitory activities related to angiogenesis, including VEGFR1-3, FGFR1-4, PDGFRα, KIT, and RET (99). Interestingly, lenvatinib has the lowest IC50 (1.5 nM) among RET multikinase inhibitors. In vitro, lenvatinib suppresses the growth of KIF5B-RET and CCDC6-RET-transfected NIH3T3 fibroblasts and of CCDC6-RET LC-2 lung adenocarcinoma cells, with in vivo, antitumor activity seen also in mice transplanted with KIF5B-RET and CCDC6-RET transfected NIH3T3 cell lines (100). Velcheti et al. reported a phase II trial of lenvatinib in 25 RET-rearranged NSCLC patients, including a 52% with KIF5B-RET and 48% with less frequent RET fusion genes. The trial included a 28% of patients that received lenvatinib after a previous RET inhibitor. The trial reported a modest activity, with 16% ORR (14% in patients who had been treated with a previous RET inhibitor), 76% DCR and a median PFS of 7.3 months. Although the ORR was equivalent (~15%) between patients harboring the KIF5B-RET gene fusion and those with other known RET rearrangements, median PFS was lower in KIF5B-RET compared to the second group variants (3.6 vs. 9.1 months) (101).
Cabozantinib (XL184) is a small-molecule kinase inhibitor with potent activity toward MET and VEGFR2, as well as a number of other receptor tyrosine kinases that have also been implicated in tumor pathobiology, such as RET (IC50 for RET 5-20 nM), KIT, AXL, and FLT3. Treatment with cabozantinib inhibits MET and VEGFR2 phosphorylation in both in vitro and in vivo models and leads to significant reductions in cell invasion. In mouse models, cabozantinib dramatically alters tumor pathology, resulting in decreased tumor and endothelial cell proliferation coupled with increased apoptosis and dose-dependent inhibition of tumor growth in lung cancer models (102). Clinical activity of cabozantinib in RET-rearranged NSCLC patients was evaluated in a phase II study (n=26) that included both KIF5B-RET rearrangements (62%), other rarer rearrangements (CCDC6-RET, CLIP1-RET, TRIM33-RET, and ERC1-RET) (15%) or unknown fusion partners (23%). Preliminary results showed a 28% ORR, and median PFS and OS of 5.5 and 9.9 months, respectively. ORR in KIF5B-RET-rearranged NSCLC patients was 20% and 50% in patients with different known RET fusion genes (103).
Alectinib is an orally active TKI originally developed to target ALK rearrangements, but also inhibits RET with a half maximal inhibitory concentration of 4.8 nM (104). Alectinib demonstrates significant in vitro and in vivo antitumor activity in RET-rearranged models and is active against two common RET resistance mutations, which usually confer resistance to vandetanib in cell lines V804L (32 nM IC50 for RET V804L) and V804M (53 nM IC50 for RET V804M) (105). Furthermore, alectinib inhibits KIF5B-RET V804L and KIF5B-RET V804M more potently than cabozantinib and vandetanib (104). Some signals of activity have been reported in retrospective studies, with two partial responses among six RET fusion-positive NSCLC patients (94). A prospective phase II study (ALERT-lung, NCT03445000) is evaluating alectinib activity in RET-rearraged NSCLCs.
Ponatinib is a broad-spectrum multikinase inhibitor that targets BCR-ABL, FLT-3, c-KIT, FGFR, sarcoma viral oncogene homolog (SRC), VEGFR, PDGFR, RET (IC50 for RET inhibition 25.8 nM) (106). Preclinical data from ponatinib support the potential role for RET-TKI-resistant cancer cell models harboring diverse mutations (V840L, V840M, and G810A). In vivo, ponatinib efficiently inhibited the wo patients treated with ponatinib in the GLORY cohort experienced disease stabilization as the best response (95). A Phase II study (NCT01813734) investigating ponatinib in RET-rearranged NSCLC patients prematurely closed enrollment after the recruitment of nine patients and the results are awaited.
More recently, two selective RET inhibitors entered clinical development with promising results.
Selpercatinib (LOXO-292) is a highly selective TKI against RET-rearranged tumors. The Phase I/II LIBRETTO-001 basket trial (NCT03157128) investigated the safety, tolerability, pharmacokinetics and preliminary antitumor activity of selpercatinib in solid tumors. First results of RET-driven NSCLC patients were recently reported and updated at the 19th IASLC World Conference of Lung Cancer (WCLC). So far, 38 patients with RET-rearranged NSCLC were evaluated. The study included heavily pretreated patients with a median of three lines of previous therapies, including multikinase inhibitors (55%), platinum-based chemotherapy and anti-PD-(L)1 therapy. The most common RET fusion partner was KIF5B (16 patients), followed by CCDC6 (11 patients). The study showed a 68% ORR, with 26 patients showing a partial response (6 additional cases showed tumor shrinkage between −3% and −29%). All patients with target lesions in the brain showed intracranial responses, with one CR and three PRs. Antitumor activity was observed regardless of previous treatment and, after a median follow-up of 8.5 months, 25 of 26 (96%) responding patients remained on treatment. The longest duration of response was >14 months (107).
Pralsetinib (BLU-667) is a highly potent, selective RET inhibitor that inhibits wild type RET, RET mutants V804L, V804M, M918T and CCDC6-RET fusion with IC50s of 0.4, 0.3, 0.4, 0.4, and 0.4 nM, respectively. Pralsetinib has been investigated in the phase I ARROW basket study (NCT03037385) to define safety, tolerability and preliminary antitumor activity. Recently, preliminary data for RET-rearranged NSCLC were reported, demonstrating a 56% ORR among 57 response-evaluable patients (60% in 30 patients pretreated with platinum chemotherapy) with durable responses (91% of responding patients were on treatment at the time of the analysis) and a DCR of 91%. Responses were seen regardless of prior treatment, RET fusion type and brain metastases presence (108).
PD-L1 expression has been described in RET-rearranged lung adenocarcinomas and correlates with the presence of concomitant mutations (109). However, the activity of ICIs targeting PD(L)-1 seems relatively modest in this subgroup of patients, as recently reported in retrospective studies (110,111), even in patients with high PD-L1 expression (112).
Overcoming resistance to TRK inhibitors
Rearrangements of neurotrophic tyrosine receptor kinases (NTRK) gene in NSCLC were initially described in 2013 (113) and identify a relatively uncommon subgroup of patients that accounts for ~0.5% of lung cancer patients (114). NTRK1, NTRK2 and NTRK3 are three genes coding for transmembrane proteins belonging to the tropomyosin receptor kinase (Trk) family. Fusions involving these genes can lead to the pathological activation of oncogenic pathways and were described in different cancers (115). Targeting NTRK gene fusions is a successful example of tumor-agnostic treatment, with entrectinib (116) and larotrectinib (117) being the first generation of this kind of compounds. Given the relatively rarity of these alterations, much of the existing evidence concerning NTRK targeting is not specific to NSCLC but, rather, encompasses different histologies. Similar to previously-described mechanisms of resistance in other molecularly-defined subgroup of patients, also for NTRK both target mutations and bypass signaling activation were described (118).
Among the resistance mutations involving NTRK genes, NTRK3 G623R and NTRK1 G595R mutations were the most frequent resistance mutations in 7 out of 9 patients in a pooled cohort of patients treated with larotrectinib (117) and are also called “solvent front” mutations as they alter a hydrophilic portion of the NTRK kinase domain (119). Less frequently, xDFG mutations—which affect the kinase-activation loop—and gatekeeper domain mutations can also be found (117,120). The 2 patients with NSCLC from the phase I study experiencing progressive disease during larotrectinib were found to have the solvent front NTRK1 G595R and the xDFG NTRK1 G667S mutations (117). Both are a paralogue of previously described ALK (16) and EGFR (121) mutations. Among the few patients with primary progressive disease to larotrectinib in the phase I study (117), one patient with mammary analogue secretory carcinoma (MASC) was shown to carry the NTRK3 G623R mutation. Intriguingly, this patient was previously treated with entrectinib—showing a very good response—and was found to carry this mutation at the moment of Entrectinib progression (122).
Off-target mechanism of acquired resistance to TRK inhibitors have been described as well. In a patient with pancreatic cancer who developed resistance to larotrectinib matched pre and post treatment biopsies revealed the occurrence of BRAF V600E and KRAS G12D mutations (123); expectedly, with this patient the use of a second-generation NTRK inhibitor was unsuccessful. In the same work (123), a patient with a single liver metastasis from a colorectal primary showed to retain—during NTRK inhibitor treatment—a KRAS G12A mutation at the progression site. Finally, in a patient with cholangiocarcinoma with NTRK rearrangement and MET amplification, single NTRK inhibition did not achieve any response (123).
New generation NTRK inhibitors with higher affinity to mutant NTRK isoforms have already demonstrated clinical activity. Selitrectinib (LOXO-195) is a selective TRK TKI designed to overcome acquired resistance mediated by recurrent kinase domain (solvent front and xDFG) mutations, as demonstrated in both in vitro and in vivo models. Early clinical activity in larotrectinib-resistant patients were recently reported in the first two NTRK-fusion positive patients who developed acquired resistance mutations on larotrectinib who were treated with selitrectinib on a first-in-human phase I study, including a LMNA–NTRK1-rearranged colorectal cancer with a G595R acquired resistance mutation and a pediatric patient with recurrent ETV6–NTRK3-rearranged infantile fibrosarcoma harboring a G623R acquired resistance mutation (119). Selitrectinib is being tested in a phase I trial on patients progressing during larotrectinib treatment; preliminary results of this study showed an objective response rate of 34% (10 out of 29 patients) in the overall population and of 45% (9 out of 20 patients) in the subgroup in which an NTRK mutation was found (120). The mechanism of acquired resistance to selitrectinib are not well known, but recently the acquisition of a gain-of-function KRAS G12V mutation was reported in a metastatic undifferentiated sarcoma harboring a TMP3-NTRK1 fusion and the solvent-front mutation G595R (124). Additional data are eagerly awaited.
Another second generation TRK inhibitor, repotrectinib, showed in vitro the highest affinity for different NTRK mutations when compared to selitrectinib, entrectinib and larotrectinib, and was the only drug active against NTRK1 G595R and F589L mutations. Clinical activity was reported in an entrectinib-resistant patient with MASC harboring a NTRK3 G623E mutation who experienced a long-term response to repotrectinib lasting more than 17 months (68). Repotrectinib is being investigated in the phase I/II trial TRIDENT-1 (NCT03093116) in patients with NTRK1, NTRK2, NTRK3, ROS1 and ALK fusions.
Finally, also some attempts to overcome NTRK bypass mechanisms have been reported, as for example the use of dual inhibition with the MET inhibitor crizotinib plus selitrectinib that was associated with clinical activity in a NTRK-rearranged cholangiocarcinoma carrying a MET amplification (123). Future studies investigating more extensively the mechanisms of acquired resistance to both 1st and 2nd generation TRK inhibitors, using tissue re-biopsies and/or cfDNA, are expected in the next future and could provide more insights on the molecular basis of TRK resistance and how to overcome it.
Conclusions
Despite initial impressive antitumor activity, the use of targeted therapies in gene fusion-positive NSCLCs is invariably associated with the development of acquired resistance through multiple mechanisms. However, the process of acquired resistance is a rapidly evolving clinical scenario that constantly evolves under the selective pressure of tyrosine kinase inhibitors. The development of increasingly higher selective and potent inhibitors, traditionally used to overcome resistance to first generation inhibitors, is associated with the development of novel mechanisms of resistance that encompass complex resistance mutations, highly recalcitrant to available TKIs, and bypass track mechanisms. Tissue re-biopsies at disease progression have been extensively used to identify the emergence of mechanisms of resistance to targeted agents, albeit the growing use of liquid biopsies provides an extraordinary opportunity for a more comprehensive study of the genotyping changes occurring during resistance, going beyond temporal and spatial heterogeneity. The design of innovative master protocols with adaptive design could provide in the next future further evidence on the best therapeutic approach and sequence in gene fusion-positive NSCLCs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Silvia Novello, Francesco Passiglia) for the series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-2019-cnsclc-06). The series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” was commissioned by the editorial office without any funding or sponsorship. OA serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. CR serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2018 to Sep 2020. Dr. Russo reports advisory board role for Astra Zeneca, MSD and Roche, outside the submitted work. Dr. Cardona reports personal fees from Abbvie, Celldex, Roche, MSD, Novartis, Astra Zeneca, Bristol Myers Squibb, Foundation Medicine, Boehringer Ingelheim, Foundation for Clinical and Applied Cancer Research, and other from MSD, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Foundation Medicine, outside the submitted work. Dr. Caglevic reports honoraria from Andes Biotechnologies; consulting or advisory role for Bristol-Myers Squibb, MSD, Roche, and Boehringer-Ingelheim; speakers' bureau for Bristol-Myers Squibb, MSD, Lilly and Roche, research funding from Merck Sharp & Dohme, Medivation, AstraZeneca, Roche, Astellas Pharma, Bristol-Myers Squibb; Travel, Accommodations, Expenses from Bristol-Myers Squibb, outside the submitted work. Dr. Arrieta reports personal fees from MSD, Roche, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, and Pfizer, and grants from AstraZeneca, Roche and MSD, outside the submitted work, outside the submitted work. Dr. Rolfo reports advisor board role for Inivata, ARCHER, MD Serono, Oncompass (non-financial), and Mylan; speakers’ bureau for Astra Zeneca and MSD; honoraria from Elsevier; grants/research support from LCRF (Lung Cancer Research Foundation), ACS (American Cancer Society), GuardantHealth (non-financial), and Biomarkers (non-financial), outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nikanjam M, Okamura R, Barkauskas DA, et al. Targeting fusions for improved outcomes in oncology treatment. Cancer 2020;126:1315-21. [Crossref] [PubMed]
- Schram AM, Chang MT, Jonsson P, et al. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 2017;14:735-48. [Crossref] [PubMed]
- Sini C, Tuzi A, Rossi G, Russo A, Pezzuto A. Acquired resistance in oncogene-addicted non-small-cell lung cancer. Future Oncol 2018;14:29-40. [Crossref] [PubMed]
- Genova C, Rossi G, Tagliamento M, et al. Targeted therapy of oncogenic-driven advanced non-small cell lung cancer: recent advances and new perspectives. Expert Rev Respir Med 2020;14:367-83. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- McCusker MG, Russo A, Scilla KA, et al. How I treat ALK-positive non-small cell lung cancer. ESMO Open 2019;4:e000524. [Crossref] [PubMed]
- Russo A, Franchina T, Ricciardi GRR, et al. Central nervous system involvement in ALK-rearranged NSCLC: promising strategies to overcome crizotinib resistance. Expert Rev Anticancer Ther 2016;16:615-23. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Russo A, Franchina T, Ricciardi GRR, et al. Third generation EGFR TKIs in EGFR-mutated NSCLC: Where are we now and where are we going. Crit Rev Oncol Hematol 2017;117:38-47. [Crossref] [PubMed]
- Lin YT, Yu CJ, Yang JCH, et al. Anaplastic Lymphoma Kinase (ALK) Kinase Domain Mutation Following ALK Inhibitor(s) Failure in Advanced ALK Positive Non-Small-Cell Lung Cancer: Analysis and Literature Review. Clin Lung Cancer 2016;17:e77-94. [Crossref] [PubMed]
- Lucena-Araujo AR, Moran JP, VanderLaan PA, et al. De novo ALK kinase domain mutations are uncommon in kinase inhibitor-naive ALK rearranged lung cancers. Lung Cancer 2016;99:17-22. [Crossref] [PubMed]
- Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Yang JCH, Ou SHI, De Petris L, et al. Pooled Systemic Efficacy and Safety Data from the Pivotal Phase II Studies (NP28673 and NP28761) of Alectinib in ALK-positive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1552-60. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Yang Y, Zhou J, Zhou J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant. Lancet Respir Med 2020;8:45-53. [Crossref] [PubMed]
- Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res 2019;25:6662-70. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Kim S, Kim TM, Kim DW, et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol 2013;8:415-22. [Crossref] [PubMed]
- Lovly CM, McDonald NT, Chen H, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med 2014;20:1027-34. [Crossref] [PubMed]
- Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med 2015;21:1038-47. [Crossref] [PubMed]
- Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations are a Recurring and Actionable Resistance Mechanism in. Clin Cancer Res 2020;26:2535-45. [Crossref] [PubMed]
- Ramalingam SS, Cheng Y, Zhou C, et al. LBA50Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018. [Crossref]
- Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014;346:1480-6. [Crossref] [PubMed]
- Redaelli S, Ceccon M, Zappa M, et al. Lorlatinib Treatment Elicits Multiple On- and Off-Target Mechanisms of Resistance in ALK-Driven Cancer. Cancer Res 2018;78:6866-80. [Crossref] [PubMed]
- Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015;27:397-408. [Crossref] [PubMed]
- Recondo G, Mezquita L, Facchinetti F, et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in. Clin Cancer Res 2020;26:242-55. [Crossref] [PubMed]
- Dardaei L, Wang HQ, Singh M, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med 2018;24:512-7. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Felip E, de Braud FG, Maur M, et al. Ceritinib plus Nivolumab in Patients with Advanced ALK-Rearranged Non-Small Cell Lung Cancer: Results of an Open-Label, Multicenter, Phase 1B Study. J Thorac Oncol 2020;15:392-403. [Crossref] [PubMed]
- Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370). J Thorac Oncol 2018;13:682-8. [Crossref] [PubMed]
- Lin JJ, Chin E, Yeap BY, et al. Increased Hepatotoxicity Associated with Sequential Immune Checkpoint Inhibitor and Crizotinib Therapy in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:135-40. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Lin JJ, Schoenfeld AJ, Zhu VW, et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol 2020;15:258-65. [Crossref] [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Bergethon K, Shaw AT, Ou SHI, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Franchina T, Russo A, Ricciardi GR, et al. Long time response with chemotherapy in ROS1 NSCLC patient with unusual metastatic site. Cancer Biol Ther 2016;17:1089-93. [Crossref] [PubMed]
- Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, et al. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol 2012;7:1086-90. [Crossref] [PubMed]
- Shaw AT, Ou SHI, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019;30:1121-6. [Crossref] [PubMed]
- Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015;33:992-9. [Crossref] [PubMed]
- Michels S, Massuti B, Schildhaus HU, et al. Safety and Efficacy of Crizotinib in Patients With Advanced or Metastatic. J Thorac Oncol 2019;14:1266-76. [Crossref] [PubMed]
- Moro-Sibilot D, Cozic N, Perol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSe phase II trial. Ann Oncol 2019;30:1985-91. [Crossref] [PubMed]
- Wu YL, Yang JCH, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405-11. [Crossref] [PubMed]
- Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin Cancer Res 2019;25:7312-9. [Crossref] [PubMed]
- Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non-small cell lung cancer: Focus on ROS1 inhibition. Cancer Treat Rev 2017;55:83-95. [Crossref] [PubMed]
- Mengoli MC, Barbieri F, Bertolini F, et al. K-RAS mutations indicating primary resistance to crizotinib in ALK-rearranged adenocarcinomas of the lung: Report of two cases and review of the literature. Lung Cancer 2016;93:55-8. [Crossref] [PubMed]
- Zhang L, Jiang T, Li X, et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer 2017;123:2927-35. [Crossref] [PubMed]
- Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med 2013;368:2395-401. [Crossref] [PubMed]
- Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017. [Crossref] [PubMed]
- Drilon A, Somwar R, Wagner JP, et al. A Novel Crizotinib-Resistant Solvent-Front Mutation Responsive to Cabozantinib Therapy in a Patient with ROS1-Rearranged Lung Cancer. Clin Cancer Res 2016;22:2351-8. [Crossref] [PubMed]
- Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin Cancer Res 2016;22:5983-91. [Crossref] [PubMed]
- McCoach CE, Le AT, Gowan K, et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin Cancer Res 2018;24:3334-47. [Crossref] [PubMed]
- Song A, Kim TM, Kim D-W, et al. Molecular Changes Associated with Acquired Resistance to Crizotinib in. Clin Cancer Res 2015;21:2379-87. [Crossref] [PubMed]
- Drilon A, Ou S-HI, Cho BC, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov 2018;8:1227-36. [Crossref] [PubMed]
- Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res 2015;21:166-74. [Crossref] [PubMed]
- Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One 2013;8:e82236. [Crossref] [PubMed]
- Zhang X, Gureasko J, Shen K, et al. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006;125:1137-49. [Crossref] [PubMed]
- Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget 2015;6:5182-94. [Crossref] [PubMed]
- Dziadziuszko R, Le AT, Wrona A, et al. An Activating KIT Mutation Induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J Thorac Oncol 2016;11:1273-81. [Crossref] [PubMed]
- Chong CR, Bahcall M, Capelletti M, et al. Identification of Existing Drugs That Effectively Target NTRK1 and ROS1 Rearrangements in Lung Cancer. Clin Cancer Res 2017;23:204-13. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 2017;35:2613-8. [Crossref] [PubMed]
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015;28:70-81. [Crossref] [PubMed]
- Zou HY, Li Q, Engstrom LD, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A 2015;112:3493-8. [Crossref] [PubMed]
- Landi L, Tiseo M, Heukamp LC, et al. Secondary ROS1 mutations and lorlatinib sensitivity in crizotinib-refractory ROS1 positive NSCLC: Results of the prospective PFROST trial. Ann Oncol 2019;30:v602-v660. [Crossref]
- Rolfo C, Ruiz R, Giovannetti E, et al. Entrectinib: a potent new TRK, ROS1, and ALK inhibitor. Expert Opin Investig Drugs 2015;24:1493-500. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Cho BC, Drilon AE, Doebele RC, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J Clin Oncol 2019;37:9011. [Crossref]
- Katayama R, Gong B, Togashi N, et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun 2019;10:3604. [Crossref] [PubMed]
- Fujiwara Y, Takeda M, Yamamoto N, et al. Safety and pharmacokinetics of DS-6051b in Japanese patients with non-small cell lung cancer harboring ROS1 fusions: a phase I study. Oncotarget 2018;9:23729-37. [Crossref] [PubMed]
- Ferrara R, Auger N, Auclin E, et al. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27-45. [Crossref] [PubMed]
- Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev 2019;81:101911. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4. [Crossref] [PubMed]
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7. [Crossref] [PubMed]
- Suzuki M, Makinoshima H, Matsumoto S, et al. Identification of a lung adenocarcinoma cell line with CCDC6-RET fusion gene and the effect of RET inhibitors in vitro and in vivo. Cancer Sci 2013;104:896-903. [Crossref] [PubMed]
- Horiike A, Takeuchi K, Uenami T, et al. Sorafenib treatment for patients with RET fusion-positive non-small cell lung cancer. Lung Cancer. 2016;93:43-6. [Crossref] [PubMed]
- Matsubara D, Kanai Y, Ishikawa S, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line J Thorac Oncol 2012;7:1872-6. [Crossref] [PubMed]
- Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-Inhibition in RET-Rearranged NSCLC Is Mediated By Reactivation of RAS/MAPK Signaling. Mol Cancer Ther 2017;16:1623-33. [Crossref] [PubMed]
- Gautschi O, Milia J, Filleron T, et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol 2017;35:1403-10. [Crossref] [PubMed]
- Saito M, Ishigame T, Tsuta K, et al. A mouse model of KIF5B-RET fusion-dependent lung tumorigenesis. Carcinogenesis 2014;35:2452-6. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017;5:42-50. [Crossref] [PubMed]
- Yoh K, Seto T, Satouchi M, et al. 1487PLURET: Final survival results of the phase II trial of vandetanib in patients with advanced RET-rearranged non-small cell lung cancer. Ann Oncol 2018. [Crossref]
- Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017;28:292-7. [Crossref] [PubMed]
- Suyama K, Iwase H. Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control 2018;25:1073274818789361. [Crossref] [PubMed]
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett 2013;340:97-103. [Crossref] [PubMed]
- Velcheti V, Hida T, Reckamp KL, et al. Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. Ann Oncol 2016;27:vi416-vi454. [Crossref]
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298-308. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653-60. [Crossref] [PubMed]
- Kodama T, Tsukaguchi T, Satoh Y, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther 2014;13:2910-8. [Crossref] [PubMed]
- Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018;15:151-67. [Crossref] [PubMed]
- Gozgit JM, Chen TH, Song Y, et al. RET fusions observed in lung and colorectal cancers are sensitive to ponatinib. Oncotarget 2018;9:29654-64. [Crossref] [PubMed]
- Drilon A, Oxnard G, Wirth L, et al. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. Jf Thorac Oncol 2019;14:S6-7. [Crossref]
- Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:abstr 9008.
- Song Z, Yu X, Cheng G, et al. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med 2016;14:188. [Crossref] [PubMed]
- Hegde A, Huang L, Liu S, et al. Abstract 4997: Responsiveness to immune checkpoint inhibitors in RET dependent cancers. Cancer Res 2019;79:4997.
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Offin M, Guo R, Wu SL, et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis Oncol 2019. [Crossref] [PubMed]
- Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013;19:1469-72. [Crossref] [PubMed]
- Russo A, Lopes AR, McCusker MG, et al. New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1). Curr Oncol Rep 2020;22:48. [Crossref] [PubMed]
- Gatalica Z, Xiu J, Swensen J, et al. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol 2019;32:147-53. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Doebele RC. Acquired Resistance Is Oncogene and Drug Agnostic. Cancer Cell 2019;36:347-9. [Crossref] [PubMed]
- Drilon A, Nagasubramanian R, Blake JF, et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov 2017;7:963-72. [Crossref] [PubMed]
- Hyman D, Kummar S, Farago A, et al. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res 2019;79:CT127.
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol 2016;27:920-6. [Crossref] [PubMed]
- Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 2019;25:1422-7. [Crossref] [PubMed]
- Hemming ML, Nathenson MJ, Lin JR, et al. Response and Mechanisms of Resistance to Larotrectinib and Selitrectinib in Metastatic Undifferentiated Sarcoma Harboring Oncogenic Fusion of NTRK1. JCO Precis Oncol 2020;4:79-90. [Crossref] [PubMed]


