Is flexible bronchoscopy necessary in the preoperative workup of patients with peripheral cT1N0 subsolid lung cancer? —a prospective multi-center cohort study
Introduction
For patients with lung cancer, value of preoperative flexible bronchoscopy (FB) examination is to identify the underlying pathology, unsuspected endobronchial involvement and anatomical variation. However, as an invasive examination tool, disadvantages of FB include rate of procedural risk, discomfort of patients and addition of time and costs. Utility of FB as a routine preoperative work-up for patients with pulmonary nodules is under debate (1,2). According to the American College of Chest Physicians (ACCP) guideline, preoperative FB examination is not recommended except for pulmonary nodules with the presence of an air bronchogram (3). According to the European Society for Medical Oncology (ESMO) guideline, bronchoscopy is the recommended test for centrally located tumors in Stage I-III lung cancers (4). However, according to National Comprehensive Cancer Network (NCCN) guideline for non-small cell lung cancer (Version 7, 2019), FB examination is recommended for peripheral stage IA non-small cell lung cancer (5).
In 2015, we retrospectively reviewed 1,026 patients with solitary pulmonary nodules (SPN) who receiving FB examination before surgery. We found the diagnostic sensitivity of FB examination for lung cancer was only 5.9%, and the diagnostic accuracy was 24.3%. Only 0.2% (2 cases) surgeries were canceled and 3.5% (36 cases) surgical plans were changed because of bronchoscopic findings. Additionally, for 268 patients with pure ground glass opacity nodules (GGNs), FB examination was unrevealing (6). Empirically, preoperative FB examination is thought to be unnecessary for patients with GGNs because most lesions are small, peripheral and endobronchial involvements are hardly seen. However, there are not enough evidences to support this accustomed idea. Therefore, we performed this prospective, multi-center cohort study to evaluate the necessity of FB examination as a routine assessment for patients with peripheral clinical T1N0 subsolid lung cancer before surgery. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-1122).
Methods
Eligibility criteria
The eligibility criteria included patients between 18-80 years old, peripheral subsolid nodules considering malignancy on thoracic thin-section computed tomography (TS-CT) scan, clinical stage T1N0M0, no contraindication for FB examination and no contraindication for surgery. The exclusion criteria included history of tracheobronchial diseases and severe smoking history (smoking index ≥400/year). Primary endpoint of this study was the incidence rate that planned surgery was changed by positive FB findings which was defined as amount of patients (planned surgery was changed by positive FB findings)/total amount of patients. Secondary endpoints were rate of positive FB findings and rate of procedural complications.
Statistical analysis
Sample size estimation
One of our retrospective studies indicated the proportion that preoperative FB examination changed the strategy of a planned surgical procedure for patient with a SPN was 3.7% (6). We hypothesized the rate that the bronchoscopic findings would have changed the rate of established surgical plans as less than 2.0% (P0), then preoperative FB examination would have limited impact on the surgical plan for patients with radiological subsolid lung cancer; If the bronchoscopic findings would have changed the rate of established surgical plans as more than 4.0% (P1), then preoperative FB examination would be regarded as one routine preoperative assessment for patients with radiological subsolid lung cancer. Null hypothesis: H0: P ≤ P0; Alternative hypothesis: HA: P ≥ P1. α =0.05, 1-β =0.9. Using Simon’s two-stage approach, the first phase enrolled 612 patients. If ≥18 patients supported the alternative hypothesis, it entered the second phase, otherwise the trial was terminated; the second phase enrolled 451 patients. A total of 1,063 patients completed the verification. If the patient number who eventually changed the surgical plan was ≤27, and the null hypothesis was accepted, that preoperative FB examination would be unnecessary for patients with subsolid lung cancer.
Baseline characteristics of patients were reported as number (%) for categorical variables. All statistical analyses were performed by using Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and SPSS version 19.0 (IBM, Chicago, IL, USA).
Clinical practice
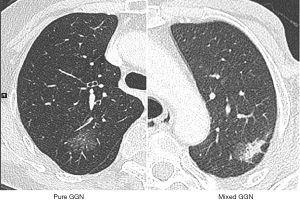
Two chest radiologists preoperatively evaluated the TS-CT images on lung window settings (window width, 1,600 HU; window level, −600 HU; width and interval, 1.0 and 1.0 mm) for measuring radiologic characteristics of the nodules. Maximum diameter of the nodules was measured on the single largest axial dimension measured on a lung window and an edge-enhancing (sharp) filter was recorded for the size of solid component and whole nodule. Subsolid nodules were diagnosed depending on the presence of ground glass opacity (GGO). In the subsolid nodules, pure GGN was defined as a nodule without a solid part, and part solid nodules (PSN) was defined as a lung lesion with both a GGO and solid part (7) (Figure 1). Anatomic variations of bronchus and vessels were also evaluated using 3-D reconstruction of CT images, especially when segmentectomy was conducted. PET/CT scan was optional for patients with subsolid nodules in this study.
Patients received FB examinations before the day of surgery. The procedure of FB examination was described before (6). Biopsies were taken using a brush, needle, forceps or cytologic washing. Biopsies were performed for patients with visualized intrabronchial lesions and suspicious bronchiarctia. Fluoroscopy was not used in this study. All bronchoscopic findings and procedural complications were evaluated and recorded.
Sublobar resection (wedge/segmental) or lobectomy was mainly selected according to the radiologic characteristics including nodule size, nodule location and percentage of GGO component on TS-CT. Sublobar resection was selected for AIS/MIA and lobectomy was selected for invasive adenocarcinoma (IAD) according to intraoperative frozen pathology (8). And for radiologically GGO predominant IAD ≤2 cm, segmentectomy was also selected. The planned surgery could be changed if any aberrant histologic and anatomic findings were detected by FB examinations. Postoperative pathologic assessment was made according to the 2015 World Health Organization (WHO) Classification of Tumors of the Lung, Pleura, Thymus and Heart (9). Lung adenocarcinoma was classified according to the 2011 IASLC/ATS/ERS classification of lung adenocarcinoma as adenocarcinoma in situ (AIS), minimal invasive adenocarcinoma (MIA), IAD (10). Pathologic staging was according to the eighth edition of the TNM classification of lung cancer (11).
This prospective, multi-center clinical trial was conducted in ten Chinese hospitals including Fudan University Shanghai Cancer Center, Shanghai Zhongshan Hospital, Anhui Chest Hospital, Guanxian Central Hospital of Shandong Province, Affiliated Hospital of Jiangnan University, Jilin Provincial Tumor Hospital, Henan Cancer Hospital, Jiangdu People’s Hospital of Yangzhou Jiangsu Province, The Third People’s Hospital of Jieyang and Queen Mary Hospital of Hongkong in China. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Review Boards of Fudan University Shanghai Cancer Center (the primary investigation institution) (IRB number: 1809191-19-1810). This clinical trial was registered in https://clinicaltrials.gov (NCT03591445). All enrolled patients provided the written informed consent.
Results
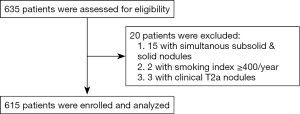
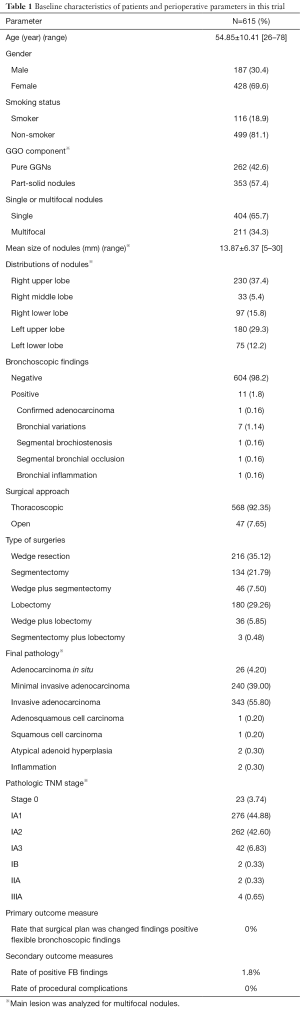
From November 2018 to November 2019, 615 patients were included in this clinical trial (Figure 2). There were 187 (30.4%) male and 428 (69.6%) female patients, the mean age was 54.85±10.41 years old (range, 26–78). 499 patients (81.1%) were non-smokers, and 116 patients (18.9%) were smokers. Of these patients, 404 (65.7%) patients had solitary nodules and 211 (34.3%) patients had multifocal nodules (Table 1).
Full table
There were 262 (42.6%) pure GGNs and 353 (57.4%) part-solid nodules confirmed by the radiologists in this study. For multifocal nodules, only the predominant nodule was analyzed, and its characteristics were recorded. Mean size of these nodules was 13.87±6.37 mm (range, 5–30). 230 (37.4%) nodules were located in right upper lobe, 33 (5.4%) were located in right middle lobe, 97 (15.8%) were located in right lower lobe, 180 (29.3%) were located in left upper lobe and 75 (12.2%) were located in left lower lobe. There were no N1/2 diseases assessed by HRCT in this study (Table 1).
All patients received FB examination before the surgery. Eleven positive findings of FB examinations were indicated, including one (0.16%) pathologically confirmed adenocarcinoma, seven (1.14%) bronchial variations (also detected by TS-CT scan), one (0.16%) segmental bronchostenosis, one (0.16%) segmental bronchial occlusion and one (0.16%) bronchial inflammation. No related complications including hypoxemia, haemorrhage and pneumothorax were found. Five hundred and sixty-eight (92.35%) thoracoscopic and 47 (7.65%) open surgeries were performed subsequently, including 216 (35.12%) wedge resections, 134 (21.79%) segmentectomies, 46 (7.50%) wedge resection plus segmentectomies, 180 (29.26%) lobectomies, 36 (5.85%) wedge resection plus lobectomies and 3 (0.48%) segmentectomies plus lobectomies. All surgical plans were made according to the radiologic features of these nodules before FB examinations. No established surgical plans were changed by positive FB findings. Final pathologies confirmed 26 (4.2%) AISs, 240 (39%) MIAs, 340 (55.80%) IADs, one (0.2%) adenosquamous cell carcinoma, one (0.2%) squamous cell carcinoma, two (0.3%) atypical adenoid hyperplasia and two (0.3%) inflammations. Pathologic TNM staging showed that there were 23 (3.74%) stage 0, 276 (44.88%) stage IA1, 259 (42.60%) IA2, 42 (6.83%) IA3, 2 (0.33%) IB, 2 (0.33%) IIA and 4 (0.65%) IIIA diseases (Table 1).
Discussion
The clinical practice of FB examination is quite different in China and in the western countries. In China, FB examination is routinely performed in the endoscopic room before the day of the surgery, so the surgeons do not use the FB in the operation room. However, in the US and Europe, it is fairly standard practice for the anesthesiologist or surgeon to perform FB examination in the process of anesthesia, or after the anesthesia before the surgery. Intraoperative bronchoscopy offers several advantages such as position control of the double lumen tubes, especially for anaesthetists who are not experienced in single lung ventilation, suction of secretions before ventilation of the lung and final evaluation of the bronchial stump after the surgery. However, for patients in western countries, FB examination could be simplified in the operation room, and this would save the time of the operation, according to the findings in this study. And for Chinese patients, it could reduce the latency time for the surgery, and save the average medical expense of about 144 dollar per person.
Most previous studies analyzed the diagnostic value of FB for SPN, but not subsolid nodules. In 2013, Schwarz C and his colleagues prospectively evaluated 225 patients with SPNs, and their results showed that unsuspected endobronchial involvement was found in 5.5% of patients with lung cancer. FB examination changed the planned surgical approach in five cases. Accordingly, they suggested the FB examination as a regular preoperative assessment of patients with SPN (2). However, in 2014, Jo and his colleagues retrospectively analyzed 668 NSCLC patients with no evidence of endobronchial lesions in the airways other than the primary cancer site on both thoracic CT and PET/CT scan. They found unsuspected malignant endobronchial lesions on FB was found in only two cases (0.3%), and they indicated that preoperative FB was not necessary for assessing the airways of early staged lung cancer patients without evidence of endobronchial malignant involvement (12). In 2015, we retrospectively reviewed 1,026 patients with SPNs, and we found the diagnostic sensitivity of FB examination for lung cancer was 5.9%, only 0.2% (2 cases) surgeries were canceled and 3.5% (36 cases) surgical plans were changed because of bronchoscopic findings (6). The different sample sizes and different patients inclusion criteria of these studies might account for the different conclusions. In Schwarz C’s study, the rate of biopsies was relatively higher (77.7%) for patients with SPNs compared with other studies. In Jo KW’s study, they excluded patients with endobronchial diseases. Recently, Lim and his colleagues showed that bronchial brushing and washing could increase the diagnostic yield for patients with invisible endobronchial tumors (13). Possibly, this procedure may be beneficial for solid tumors. But we have no ideas whether this is beneficial for GGO tumors, considering that there were few studies analyzing FB examination for patients with GGNs. In our study in 2015, subgroup analysis showed that FB examination was unrevealing for 268 patients with pure GGNs (6). Because this analysis was retrospective, we performed this prospective multi-center clinical trial. And in this trial, FB examination showed limited values as a tool for pathologic diagnosis or a means for making surgical plan for patients with peripheral subsolid lung cancer. A total of 11 patients (1.8%) had the positive findings of FB examination in this study. Of these patients, only one patient obtained pathologic diagnosis of adenocarcinoma by FB examination. He was planned to have right upper lobectomy because the lesion was part solid and the maximal diameter was 3 cm. Another patient was detected the right dorsal bronchus stenosis, and he was planned to receive right lower lobectomy because the lesion was part-solid and the maximal diameter was 2.5 cm. The other patient was detected right bronchus occlusion of anterior basal segment. He was planned to receive right S6 segmentectomy because of a 6 mm part-solid nodule. Moreover, seven patients were detected anatomical variations of right bronchus. These bronchial variations were previously found by CT scan, and the surgical plans were decided accordingly. Therefore, these positive findings of FB examinations did not change the planned surgeries which had been decided before.
Limitation of this trial was that the object of this study was the patient with subsolid lung cancer. The prevalence of subsolid lung cancers is quite high in China, while it is relatively low in USA and Europe. In addition, it is unclear whether subsolid lung cancers are similar between Eastern and Western patients, since most studies of subsolid lung cancers are from Eastern countries. Moreover, results of this study could be applied for patients with subsolid tumors, but not for patients with pure-solid lung cancers.
Conclusions
This was the first, prospective, multicenter clinical trial evaluating the necessity of preoperative FB examination for patients with peripheral radiological subsolid lung cancer. As the primary endpoint of this trial, the incidence rate that planned surgery was changed by positive FB findings was 0%. As the secondary endpoints, rate of positive FB findings was 1.8%, and rate of procedural complications was 0%. Therefore, FB examination was unnecessary in the preoperative assessment for patients with peripheral clinical T1N0 subsolid lung cancer.
Acknowledgments
Funding: This study was funded by The National Natural Science Foundation of China (81572253), Shanghai Shenkang Hospital Development Center City Hospital Emerging Cutting-edge Technology Joint Research Project (SHDC12017102) and Shanghai Municipal Health Commission Key Discipline Project (2017ZZ02025 and 2017ZZ01019).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-1122
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-1122
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-1122
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1122). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Review Boards of Fudan University Shanghai Cancer Center (the primary investigation institution) (IRB number: 1809191-19-1810). This clinical trial was registered in https://clinicaltrials.gov (NCT03591445). All enrolled patients provided the written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silvestri GA. Bronchoscopy for the solitary pulmonary nodule: friend or foe? Chest 2012;142:276-7. [Crossref] [PubMed]
- Schwarz C, Schönfeld N, Bittner RC, et al. Value of flexible bronchoscopy in the pre-operative work-up of solitary pulmonary nodules. Eur Respir J 2013;41:177-82. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnois, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines: Non-Small Cell Lung Cancer, Version 7. 2019 J Natl Compr Canc Netw. 2019;
- Zhang Y, Zhang Y, Chen S, et al. Is bronchoscopy necessary in the preoperative workup of a solitary pulmonary nodule? J Thorac Cardiovasc Surg 2015;150:36-40. [Crossref] [PubMed]
- Ye T, Deng L, Wang SP, et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol 2019;14:617-27. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise diagnosis of intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, International Agency for Research on Cancer, Lyon (2015).
- Travis WD, Brambilla E, Noguchi M, et al. International association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Jo KW, Kim HR, Kim DK, et al. Value of flexible bronchoscopy for the preoperative assessment of NSCLC diagnosed using percutanous core needle biopsy. Thorac Cardiovasc Surg 2014;62:593-8. [Crossref] [PubMed]
- Lim JH, Kim MJ, Jeon SH, et al. The optimal sequence of bronchial brushing and washing for diagnosing peripheral lung cancer using non-guided flexible bronchoscopy. Sci Rep 2020;10:1036. [Crossref] [PubMed]