
Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo
Introduction
Lung cancer has the highest incidence of any malignant tumor worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, and around 70% of patients are already at the advanced stage of the disease at the time of diagnosis (1).
Although there have been great breakthroughs in treatment technology, including palliative surgery, radiotherapy and chemotherapy, immunotherapy, and molecular targeted therapies [including epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors], some patients with advanced NSCLC have not benefited from these treatments. The 5-year overall survival rate (OS) of patients with lung cancer is a meager 11%. In particular, patients who receive targeted drug therapy can acquire resistance to treatment (2).
EGFR driver genes play an important role in the occurrence and development of lung cancer. Studies have shown that abnormal activation of the EGFR can promote tumor cell proliferation, migration, and differentiation, and inhibits tumor cell apoptosis (3). EGFR gene mutations are a strong predictor of EGFR sensitivity in NSCLC patients. EGFR tyrosine kinase inhibitors (EGFR TKIs) have shown a promising response for NSCLC patients with EGFR mutations, with a response rate of74.8%. In China, approximately 40% of NSCLC patients have EGFR gene mutations (4).
Authoritative guidelines at home and abroad recommend the first-line use of EGFR TKIs for the treatment of EGFR-positive advanced NSCLC. EGFR TKIs currently in clinical use in China include gefitinib, erlotinib, and icotinib (5,6). However, although EGFR TKIs have an extremely high tumor response rate and can achieve longer progression free survival (PFS) in patients with EGFR-positive advanced NSCLC, most of EGFR-positive patients will develop drug resistance in 10–12 months after TKI treatment and disease progression within a month (7). Therefore, there is an urgent need to discover a drug that can overcome EGFR-TKI resistance, in order to improve the outcomes of patients with EGFR-positive advanced NSCLC.
Vascular endothelial growth factor (VEGF) is highly expressed in various tumor tissues, resulting in the generation of a large number of abnormal blood vessels that lack normal structure and permeability, thereby promoting abnormal tumor proliferation (8). In the study of the resistance mechanism of EGFR TKIs, TKI resistance is usually accompanied by the up-regulation of VEGF expression, and by blocking the EGFR and VEGF pathways, resistance can be delayed or reversed (9). In NSCLC with mutations in the EGFR gene, simultaneously inhibiting the EGFR and VEGF/VEGFR signaling pathways has a synergistic effect (10). Clinical studies have shown that the VEGF inhibitor bevacizumab combined with erlotinib, a first-generation EGFR TKI, can significantly prolong the PFS of non-squamous NSCLC patients with EGFR activating mutations (11). Preclinical studies have shown that EGFR-TKI resistance caused by Thr790Met EGFR mutations can be overcome by blocking the VEGF receptor signaling pathway (12).
Anlotinib is a new, small-molecule, multi-target TKI that can effectively inhibit VEGFR, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), c-Kit, and other kinases. It also has an anti-angiogenic effect and can inhibit tumor growth. Anlotinib has been recommended as a third-line treatment for stage IV non-squamous NSCLC without a driver gene (13,14). Although clinical trials of anlotinib have shown it to achieve a good curative effect for tumors such as metastatic soft tissue sarcoma, metastatic small cell lung cancer, and advanced NSCLC, its effect on NSCLC with acquired resistance to EGFR-TKIs needs further exploration (13,15).
The related studies of lung cancer are divided into first-line, second-line, back-line, and neoadjuvant and adjuvant studies. The research object of this paper is patients with advanced non-small cell EGFR mutation positive. After targeted therapy, it falls between first-line therapy and second-line therapy, which is called line 1.5. The target population is the potential acquired drug resistance after EGFR TKI treatment, which is shown as: tumor markers slowly increase, lesions gradually increase, and clinical efficacy evaluation has not reached the progress standard. The combination of small molecule multi-target antiangiogenic drugs at line 1.5 is used to try to delay the time of drug resistance and prolong the patients’ PFS and OS (16,17). This study aimed to evaluate the clinical efficacy and safety of the combination of EGFR-TKIs with anlotinib in patients with EGFR-mutant NSCLC with acquired EGFR-TKI resistance. The anti-tumor effect of this treatment combination and the underlying molecular mechanism were also assessed in a xenograft mouse model of NSCLC with potential secondary resistance to EGFR TKIs, in the hope of providing a theoretical basis and guidance for its clinical application. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tlcr-21-192).
Methods
Enrollment of clinical patients
From April 2018 to June 2020, we screened NSCLC patients with potential drug resistance to EGFR-TKI therapy. All eligible patients had a pathological diagnosis of lung adenocarcinoma. Twenty patients (10 men and 10 women; age range: 47–80 years, median age: 69 years) with advanced NSCLC who had received EGFR-TKI treatment for more than 6 months and had elevated tumor marker levels during follow-up [including carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and VEGF) were enrolled. Imaging revealed enlarged lesions and stable disease. All enrolled patients were fully informed and signed an informed consent form before receiving anlotinib and EGFR-TKI combination therapy. This study was approved by the ethics committee of the unit.
Patient treatment
The patients were clinically evaluated as having potential secondary drug resistance. The criteria for this included: a continuous increase in tumor marker levels; an increase in lesion size; and stable efficacy evaluation. In addition to their original targeted drug therapy (gefitinib or icotinib), patients were orally administered 12 mg of anlotinib capsules on an empty stomach daily, for 2 weeks followed by a 1-week break. It was recommended that gefitinib or icotinib was taken at intervals about 6 hours (±1 hour), until progressive disease (PD) or intolerance occurred when a patient showed intolerance to the treatment, the dose was reduced to 10 mg, and if intolerance still occurred, the dose was further reduced to 8 mg. Routine blood pressure and urine tests were performed before the administration of medication. The doctor instructed the patients to monitor their blood pressure daily, check their urine routine weekly, and to pay close attention to cough, hemoptysis, and skin conditions of the hands and feet. The lesions were reviewed every 8 weeks by imaging, and the time of disease progression was recorded.
Evaluation of the patients’ condition
Response evaluation criteria in solid tumors (RECIST, version 1.1) was used as the standard of clinical efficacy. PFS was defined as the time from the first day of treatment to disease progression or death, whichever occurred first. OS was defined as the duration from the first day of combination therapy to the date of death or date of the last follow-up. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Toxicity Standard Version 4.0. This study was approved by the Ethics Committee of the Cancer Hospital of Nantong University and was carried out in accordance with the principles of the Declaration of Helsinki (as revised in 2013).
Cell culture and viability experiment
NSCLC cells (A549 cells: EGFR wild-type, H1975 cells: L858R and T790M mutations) were supplied by the Cell Resource Center of Shanghai Academy of Biological Sciences, Chinese Academy of Sciences. The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Gibco BRL, Carlsbad, CA, USA)) complete medium supplemented with 10% fetal bovine serum (Gibco BRL, Carlsbad, CA, USA) and 1% double antibodies (penicillin and streptomycin) in an incubator with 5% CO2 at 37 °C. Anlotinib and gefitinib were purchased from Selleck (Shanghai, China), dissolved in dimethyl sulfoxide (DMSO) to 10 mM, and frozen at −20 °C for later use. A549 and H1975 cells in the exponential growth phase were collected, seeded into 96-well plates (3,000 cells/well), and cultured for 12 hours. The cells were cultured with different concentrations of anlotinib and gefitinib. After 24 hours, 10 µL of Cell Counting Kit-8 (CCK-8) stock solution was added, and the cells were incubated at 37 °C for 3 hours. Finally, a full wavelength microplate reader was used to detect the absorbance at 490 nm, and cell viability was calculated.
Clone formation experiment
A549 and H1975 cells in the exponential growth phase were collected, seeded into a six-well plate (600 cells/well), and cultured for 12 hours. The cells were treated with 20 µM anlotinib and 40 µM gefitinib, and cultured at 37 °C in an incubator with 5% CO2. The medium was changed every 3 days for continuous culture for 2 weeks. After that, the cells were fixed with 1% formaldehyde for 10 minutes, washed three times with phosphate-buffered saline (PBS), and then stained with 0.1% crystal violet for 10 minutes. Finally, the number of cell clones (a clone is more than 50 cells) was counted under a microscope.
Nude mouse tumor xenotransplantation model
Four-to-six-week-old BALB/c male athymic nude mice (weight, 18–22 g) were obtained from the animal experiment center of our hospital. All the mice were individually caged and kept in a specific-pathogen-free animal room. The relative humidity in the animal room was maintained at (50%±10%), and the temperature was 22±2 °C. The animal room is kept on a 12 hours light/12 hours dark cycle. The cages of the mice were cleaned regularly and disinfected with ultraviolet light for 30 minutes. The mice were fed quantitatively, given free access to drinking water, and the litter was replaced every 2 days. The food, drinking water, litter, and other materials used by the mice were autoclaved.
H1975 cells in the exponential growth phase were collected, and the cell density was adjusted to 2×107 cells/mL. The cells were mixed with 50 µL cell suspension and an equal volume of Matrigel, and injected under the skin of the nude mice. The injection site was checked every 3 days for the formation of foreign bodies. When a bulge was observed under the skin, a vernier caliper was used to record the long diameter (l) and short diameter (d) of the tumor. The tumor volume was calculated using the formula V=[(a×b2)/2], where V represents transplantability, ‘a’ represents the long diameter of the transplanted tumor, and ‘b’ represents the short diameter of the transplanted tumor. When the tumor volume reached 50 mm3, the mice were randomly divided into the following four groups (n=4 in each group): the blank control group (control, PBS), the anlotinib group (anlotinib, 50 mg/kg), the gefitinib group (gefitinib, 50 mg/kg, administered by oral gavage), and the anlotinib + gefitinib group (anlotinib, 50 mg/kg + gefitinib, 50 mg/kg). The mice were given these treatments once a day for 31 consecutive days. At the end of the experiment, the mice were killed by cervical dislocation. The tumors were harvested, and the wet weight was weighed. All tumor tissues were cut into two parts, and one part fixed in 10% paraformaldehyde buffer and the other part was stored in liquid nitrogen for later use.
Immunohistochemistry
After harvesting, tumor tissues from the mice were fixed with 10% formalin, and embedded in the hospital pathology department, and then cut into slices and blocked with peroxidase. The antibody (Ki-67, CD31, EGFR, p-EGFR, VEGFR2 and p-VEGFR2) were purchased from Cell Signaling Technology Inc (Beverly, MA, USA), and was fixed at the concentration recommended by the manufacturer’s protocol. The antibody dilution solution was used, and 50–100 uL antibody working solution was added, depending on the area of the tissue section. The tissue sections were then incubated overnight in a refrigerator at 4 °C. After that, a microscope was used to observe the protein expression, distribution, andlocation. Images were captured of three randomly selected fields of view on each section.
Statistical analysis
Continuous variables were described as the number of people, mean, median, standard deviation, and minimum and maximum values. Categorical variables were described as frequency and percentage, and the 95% confidence interval (CI) was calculated. For temporal variables, including PFS, onset time, and duration of remission, the Kaplan-Meier (KM) method was used to estimate the median value and the corresponding 95% CI, and KM curves were used to depict the time change trend. All data were analyzed using SPSS19.0 (SAS Institute, Cary, NC, USA) and Graphpad Prism 6.0 software. Quantitative data were expressed as mean ± standard error (). Comparison between two groups was performed using Student’s t-test. The χ2 test was used to analyze the count data. P<0.05 was considered to show statistical significance.
Results
Anlotinib combined with EGFR TKIs (gefitinib or icotinib) can overcome EGFR-TKI resistance
Acquired resistance
From April 2018 to June 2020, 20 patients with potential acquired EGFR-TKI resistance after treatment with gefitinib (n=13, 65%) or icotinib (n=7, 35%) were enrolled in this study. The main clinical feature of NSCLC patients with EGFR-TKI resistance is an increase in the levels of tumor markers, including CEA, NSE, and VEGF, during treatment. In such patients, imaging shows a gradual increase in the size of the lesions, and the efficacy evaluation shows that PD has not yet been reached. Clinical characteristics of the patients are shown in Table 1. EGFR testing of the 20 patients before treatment found 11 cases of exon 21 L858R mutation, 8 cases of exon 19 deletion, and 1 case of exon 20 S768I mutation.

Full table
Based on the above results (EGFR testing of the 20 patients), patients with drug resistance were given EGFR-TKI therapy combined with anlotinib as a drug-resistant treatment. The best response of the 20 tumor patients in this study is shown in Figure 1A,B. None of the patients showed a complete response (CR), 3 cases were evaluated as partial response (PR), and 17 cases were evaluated as stable disease (SD). The disease control rate (DCR) was 100%. The median follow-up time was 6.6 months [interquartile range (IQR), 4.08–8.28 month]. On the deadline of July 06 2020, the median PFS was 15.7 months (Figure 1C; 95% CI, 10.19–18.87 months), while the median OS has not been reached. After treatment, all the patients were reexamined by imaging. The duration of SD was more than 8 weeks. The last follow-up showed that 2 patients progressed within 12 weeks, 1 patient stopped treatment 1 patient was lost to follow-up, and 8 patients are still in a stable condition and are continuing to receive treatment.

The main adverse reactions to the combination treatment were hypertension, hand-foot skin reaction (HFSR), diarrhea, fatigue, oral ulcers, and anorexia. Four cases (36.3%) developed grade III hypertension, one case (9.1%) developed grade III HFSR, and one case (9.1%) experienced grade III diarrhea. No proteinuria, abnormal liver function, pulmonary interstitial disease, pulmonary hemorrhage, gastrointestinal hemorrhage, or thromboembolic events were observed. The levels of CEA decreased significantly in nine patients. Among them, the dynamic expression of VEGF was detected in four patients, and after the administration of the combination medication, the levels of VEGF decreased significantly. NES showed a downward trend in 4 patients, considering that the resistance mechanism may lead to pathological changes.
Case presentation 1
A 78-year-old male smoker had experienced repeated cough and sputum for 2 years. Chest enhanced computed tomography (CT) showed nodules in the upper lobe of the right lung, and the possibility of right lung cancer was considered; Biopsy pathology indicated non-small cell lung cancer, EGFR gene detection revealed exon 21 mutations (Table 1, patient no. 4). The patient was diagnosed with stage IV right lung cancer. Icotinib 125 mg three times a day (tid) was used as the first-line treatment, and stable PR was achieved. An increase in CEA levels was detected in July 2018, and the lesions in the upper and lower lobes of the right lung showed a gradual increase. At the last follow-up, the patient’s PFS=34 months the serum CEA level was 48.48 ng/mL, which suggested that the disease had a progressive trend. Therefore, on August 2, 2018, the patient commenced treatment with anlotinib 12 mg/day, combined with icotinib 125 mg tid. Chest CT performed on September 20, 2018 showed stable SD (Figure 2A), and the tumor marker levels showed a significant decrease (Figure 2B). Before the data cutoff, the patient’s PFS had reached 26 months, with no obvious adverse reactions observed.
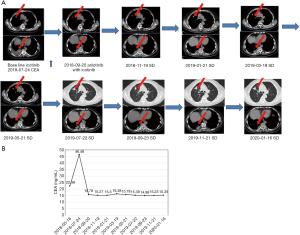
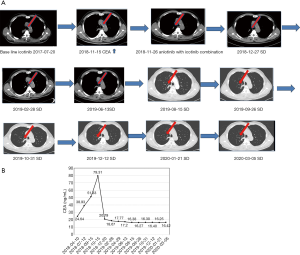
Case presentation 2
A 73-year-old male patient with a PS (Performance status) score of 2 and a 30 pack-year smoking history (Table 1, patient no. 5). A PET-CT (Positron Emission Tomography-Computed Tomography) scan in May 2017 showed central lung cancer in the right lung, lymph nodes in the mediastinum, multiple bone lesions throughout the body, and metastasis to the left adrenal gland. A needle biopsy of the right lung revealed lung adenocarcinoma, and genetic testing revealed a mutation in exon 21. The first-line treatment was icotinib 125 mg tid, which was commenced on July 20, 2017, and SD was reached. Palliative radiotherapy was performed on the upper femur on the right side of the weight-bearing bone. Increased CEA levels were detected in November 2018, and the lesion size slowly increased. At the last follow-up, the patient’s PFS=16 months. From November 11 20181.5-line treatment combined anlotinib (12 mg/day) with icotinib targeted therapy. On May 03 2020, imaging revealed SD (Figure 3A), the CEA levels were decreased (Figure 3B), as were the levels of VEGF and NSE (Table 1), the PFS was 19 months. The main adverse reactions is grade 2–3 oral ulcers.
Effects of gefitinib and anlotinib on NSCLC cell lines in vitro
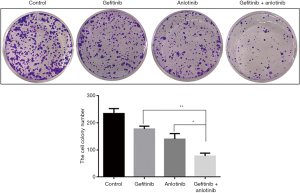
The effects of different concentrations of anlotinib (5, 10, 20, 40, and 80 µM) and gefitinib (2.5, 5, 10, 20, and 40 µM) on NSCLC cells (A549 cells: EGFR wild-type; H1975 cells: with L858R and T790M mutations) were also studied. A CCK-8 assay was performed to investigate cell viability. The results showed that compared with anlotinib or gefitinib treatment alone, the combined treatment of 20 µM anlotinib and 40 µM gefitinib, 40 µM anlotinib and 20 µM gefitinib, 80 µM anlotinib and40 µM gefitinib combination group significantly inhibited the activity of A549 cells and H1975 cells (Figure 4). Further experiments were performed using H1975 cells. The clone formation experiment showed (Figure 5) that compared with anlotinib or gefitinib treatment alone, the combined treatment of 20 µM anlotinib and 40 µM gefitinib significantly inhibited the clone formation ability of H1795 cells. The above results indicated that anlotinib combined with gefitinib could significantly inhibit the drug resistance and proliferation ability of NSCLC cells.

Antitumor activity of gefitinib plus anlotinib in an NSCLC xenograft model
To further evaluate the effect of anlotinib combined with gefitinib on the proliferation of NSCLC cells in vivo, H1795 cells were injected into nude mice to establish a tumor-bearing model. The mice were treated with anlotinib combined with gefitinib or PBS buffer. The tumor volume of the mice was monitored, and the combination treatment of anlotinib with gefitinib was observed to significantly inhibit the tumor growth rate compared to treatment with anlotinib or gefitinib alone (Figure 6A,B).
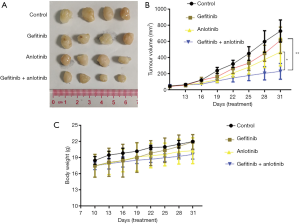
At the same time, we tested the weight change of the mice during the course of treatment. The inspection curve showed that compared with the control group, treatment with anlotinib and gefitinib, either alone or in combination, did not result in a significant change in the body weight of the nude mice (P>0.05) (Figure 6C). Together, these results suggested that the combination treatment could significantly inhibit the proliferation of colon cancer tumors in vivo, thus providing a theoretical basis for subsequent clinical studies.
Effects of gefitinib and anlotinib on the expression of related protein molecules in tumor tissues in nude mice
Immunohistochemistry was carried out to detect the expression of tumor proliferation and angiogenesis-related protein molecules in tumor tissues harvested from the mice. Antigen Ki-67 is currently a widely used biological indicator for various malignant tumors. The detection of Ki-67 can reflect the cell proliferation activity of malignant tumors, and the expression level of Ki-67 in transplanted tumor tissues can be detected by immunohistochemistry, as shown in Figure 7. The results showed that compared with that in the anlotinib or gefitinib alone groups, the Ki-67 expression level in the combination group was significantly reduced, and the difference was statistically significant (P<0.05).

To further investigate the effect of the combination treatment on the formation of blood vessels in tumor tissues, the expression of CD31, EGFR, p-EGFR, VEGFR2 and p-VEGFR2 was performed using immunohistochemistry. The results showed that compared with those in the anlotinib or gefitinib alone groups, the expression levels of CD31, p-EGFR, VEGFR2, and p-VEGFR2 in the combination treatment group were significantly reduced, and the difference was statistically significant (P<0.05) (Figures 8,9). These results showed that the combined application of anlotinib and gefitinib could significantly inhibit the expression of Ki-67, CD31, EGFR, p-EGFR, VEGFR2, and p-VEGFR2 in tumor-bearing tissues in vivo.


Discussion
Several randomized phase III clinical trials of first-line treatments (including Iressa Pan-Asia Study (IPASS), West Japan Thoracic Oncology 3405 (WJTOG 3405), LUX-Lung 3(LUXLUNG 3), and erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC) have explored the use of EGFR TKIs as a first-line treatment for patients with advanced NSCLC. Patients with EGFR gene mutations can obtain 9.5–13.7 months of PFS using EGFR-TKIs. However, Patients with EGFR gene mutations also acquired drug resistance is inevitable. Combinations of different drugs that can delay or reverse potential drug resistance urgently need to be investigated.
Clinically, potential drug resistance manifests as gradual increases in tumor marker levels and lesion size during follow-up, with the response evaluated as stable (18). At present, EGFR-TKI monotherapy has not made significant progress, so combining other anti-tumor drugs, including anti-angiogenic drugs, to delay drug resistance is an inevitable strategy. As a unified marker does not currently exist for the population included in this study, patient inclusion was based on the clinical observations of a gradual increase in the relevant lung cancer markers, as well as a trend toward disease progression without disease progression being reached.
Anlotinib, as a multi-target receptor tyrosine kinase inhibitor, can target vascular endothelial growth factor receptor (VEGFR1/2/3), fibroblast growth factor receptor (FGFR1/2/3), platelet-derived growth factor receptor (PDGFRα/β), c-Kit, and other targets. Meanwhile, anlotinib also can inhibit angiogenesis through inhibiting the expression of VEGFR, PDGFR, FGFR (13,15).
The EGFR and VEGFR signaling pathways are closely connected, with the phosphorylation of EGFR up-regulating the expression of VEGF (19). These signaling pathways have cross and synergistic effects. For instance, activation of EGFR can promote the secretion of VEGF. Simultaneous inhibition of the EGFR and VEGF/VEGFR signaling pathways may have a synergistic effect in NSCLC with sensitive mutations of the EGFR gene, playing important roles in cell proliferation, tumor growth, migration, and metabolism, as well as in the regulation of the tumor microenvironment and tumor growth (20,21).
VEGFR, PDGFR, and FGFR are three tyrosine kinases that regulate angiogenesis. Anlotinib simultaneously inhibits the signaling pathways of VEGFR, PDGFR, and FGFR to overcome drug resistance caused by the complementation of signaling pathways and to efficiently inhibit angiogenesis (13). Anlotinib inhibits tumor growth by acting on c-Kit targets. The amplification of the c-Kit gene is associated with drug resistance in NSCLC. Anlotinib inhibits c-Kit and blocks the downstream Akt (serine/threonine-protein kinase and ERK (extracellular signal-regulated kinase) signaling pathways to inhibit the growth of tumor cells (22).
Although there is a synergistic anti-tumor effect between EGFR inhibitors and VEGF pathways, only one retrospective study has reported that EGFR TKIs re-challenge to bevacizumab achieved an objective response rate (ORR) of 13%, a DCR of 88%, and a median PFS of 4.1 months (95% CI, 2.3–4.9 months) (23).
The clinical study of the combined application of EGFR TKIs and anlotinib has not yet been reported. In this study of 20 cases of advanced lung adenocarcinoma, with mutations including exon 19 mutation, exon 21858R deletion, and exon 20 S768I mutation, the lesions were quickly controlled after first-generation EGFR TKI targeted therapy. However, after several months of EGFR-TKI treatment, tumor cells began to replicate and resist apoptosis, resulting in disease progression, an increase in lesion size, and elevated levels of serum CEA and other tumor markers. Our study included a patient with S768I mutation in exon 20 who was not treated with a second-generation EGFR TKI at baseline due to economic reasons but was treated with gefitinib 250 mg as the first-line treatment. After 2.5 months, the tumor marker NES increased, and the lesions gradually increased in size. After the administration of 12 mg of anlotinib, the efficacy evaluation showed obvious cavitation of the lesions and tumor shrinkage after 8 weeks, as well as partial response. At the last follow-up, the PFS reached 10 months, and exceeded the second generation EGFR-TKI line.
Because the population was included in this study and the imaging efficacy evaluation did not meet the criteria for disease progression, the T790 mutation was not routinely detected. The T790 mutation is a common mechanism of first-generation EGFR-TKI resistance. In a randomized clinical trial comparing second-line osimertinib combined with bevacizumab versus osimertinib alone in Japan, the combination group failed to show prolonged PFS in patients with advanced lung adenocarcinoma with EGFR T790M mutation. This single-arm study suggests that osimertinib plus an anti-angiogenic inhibitor may not work together. The T790 mutation rate is 50 percent in patients with late-stage driver gene positivity, and according to these studies, the benefit may be greater if antiangiologic drugs are used earlier. In our study, the use of first-generation EGFR TKI in combination with amlotinib in patients with potential continued drug resistance could theoretically prolong the use of second-line osimertinib and prolong the overall survival of patients (24).
The 1.5-line drug therapy combined with anlotinib can theoretically extend the ‘second-line osimertinib’ use time, and prolong the OS of patients. For the patients in this study, the available tumor markers for clinical guidance mainly include CEA and VEGF. In patients with PR and SD, CEA and VEGF are significantly reduced after treatment with anlotinib, which often indicates a good prognosis. NSE can also be used as a selective reference tumor indicator. In the EGFR-TKI resistance mechanism, some patients may exhibit a small cell pathological type conversion (25).
Previous study suggests that there is potential acquired resistance, and NSCLC patients with acquired resistance to EGFR TKIs can also benefit from comprehensive treatment with TKIs, achieving disease control for months to years. In the mechanism of EGFR resistance, PD-L1 and TMB may also have an impact on the treatment of EGFR-TKIs. PD-L1 and TMB are the two most important therapeutic markers for immunotherapy at present, and they show the immunological characteristics of tumors and the immune status of TMB. High PD-L1 expression is one of the possible causes of primary resistance to EGFR-TKIs, and TMB identifies patients who benefit from immunotherapy by reflecting the immunogenicity of the tumor. Studies have shown that in patients with advanced lung adenocarcinoma, TMB is negatively correlated with the clinical response to EGFR-TKIs. In view of the difference in efficacy among patients in this paper, PD-L1 and TMB expression can be detected simultaneously in the follow-up to further clarify the mechanism of drug resistance and individual differences (26). After acquiring resistance to EGFR TKIs in NSCLC, we discussed the re-challenge of anlotinib to EGFR TKIs. The ORR and DCR were 25% and 100%, respectively, and the median PFS was 15.7 months (95% CI, 10.19–18.87 months). Therefore, anlotinib has good anti-tumor activity at a dose of 12 or 10 mg per day.
However, based on the general situation of our patients and our concerns regarding their intolerance to toxicity, we introduced a targeted treatment for them, including a daily dose of 12 mg combined with a first-generation EGFR TKI daily. After treatment, the duration of SD was more than 8 weeks after imaging review, and the median time to progression reached 12 weeks. Of the 20 patients, 2 progressed within 12 weeks, and 8 were in stable condition and continued to receive treatment at the last follow-up. The most common adverse reactions were hypertension, hand-foot syndrome, proteinuria, fatigue, anorexia, and elevated transaminases. These results indicate that the combination of EGFR-TKI therapy and anlotinib may be another treatment option for patients with acquired resistance to EGFR TKIs. This study found that for patients with exon 21 mutations, a large tumor burden, and obvious symptoms, small molecule anti-vascular drugs combined with TKIs prolonged disease progression.
However, this study has some limitations. For instance, the sample size was limited d due to a lack of prospective research and clinical trials with larger sample sizes are needed to verify our results.
In this study, our data showed that in patients with advanced NSCLC, anlotinib delayed resistance to a first-generation EGFR TKI. These findings provide a promising combination treatment strategy for advanced driver gene-positive NSCLC and a solid foundation for the design of future clinical trials. In the future, the study group and the control group will be prospectively designed, the genomics of tissue samples will be tested at baseline and after progression, and the targeted markers for patients with potential EGFR-TKI resistance will be screened, in order to provide new strategies for the clinical treatment of advanced NSCLC that will improve patient survival.
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group.
Funding: This work was supported by the Youth Fund of Nantong Municipal Health and Family Planning Commission (QA2019030). The other major funding came from Nantong Science and Technology Bureau (MSZ18135, MS12020046).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-21-192
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-21-192
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-21-192). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Cancer Hospital of Nantong University and was carried out in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All enrolled patients were fully informed and signed an informed consent form before receiving anlotinib and EGFR-TKI combination therapy.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Hui R, Ozguroglu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1670-80. [Crossref] [PubMed]
- Liu Q, Yu S, Zhao W, et al. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer 2018;17:53. [Crossref] [PubMed]
- Wang S, Gao A, Liu J, et al. First-line therapy for advanced non-small cell lung cancer with activating EGFR mutation: is combined EGFR-TKIs and chemotherapy a better choice? Cancer Chemother Pharmacol 2018;81:443-53. [Crossref] [PubMed]
- Yoneda K, Imanishi N, Ichiki Y, et al. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J UOEH 2019;41:153-63. [Crossref] [PubMed]
- Shi Y, Sun Y, Yu J, et al. China Experts Consensus on the Diagnosis and Treatment of Advanced Stage Primary Lung Cancer (2016 Version). Zhongguo Fei Ai Za Zhi 2016;19:1-15. [PubMed]
- Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. [Crossref] [PubMed]
- Frezzetti D, Gallo M, Maiello MR, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets 2017;21:959-66. [Crossref] [PubMed]
- Reckamp KL, Frankel PH, Ruel N, et al. Phase II Trial of Cabozantinib Plus Erlotinib in Patients With Advanced Epidermal Growth Factor Receptor (EGFR)-Mutant Non-small Cell Lung Cancer With Progressive Disease on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy: A California Cancer Consortium Phase II Trial (NCI 9303). Front Oncol 2019;9:132. [Crossref] [PubMed]
- Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer 2017;84:184-92. [Crossref] [PubMed]
- Masuda C, Yanagisawa M, Yorozu K, et al. Bevacizumab counteracts VEGF-dependent resistance to erlotinib in an EGFR-mutated NSCLC xenograft model. Int J Oncol 2017;51:425-34. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Syed YY. Anlotinib: First Global Approval. Drugs 2018;78:1057-62. [Crossref] [PubMed]
- Desai S, Kim C, Veytsman I. Role of Anti-EGFR Targeted Therapies in Stage III Locally Advanced Non-small Cell Lung Cancer: Give or Not to Give? Curr Oncol Rep 2019;21:84. [Crossref] [PubMed]
- Urata Y, Katakami N, Morita S, et al. Randomized Phase III Study Comparing Gefitinib With Erlotinib in Patients With Previously Treated Advanced Lung Adenocarcinoma: WJOG 5108L. J Clin Oncol 2016;34:3248-57. [Crossref] [PubMed]
- Tomasello C, Baldessari C, Napolitano M, et al. Resistance to EGFR inhibitors in non-small cell lung cancer: Clinical management and future perspectives. Crit Rev Oncol Hematol 2018;123:149-61. [Crossref] [PubMed]
- Karaman S, Leppanen VM, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development 2018;145:dev151019 [Crossref] [PubMed]
- Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res 2018;136:97-107. [Crossref] [PubMed]
- Siveen KS, Prabhu K, Krishnankutty R, et al. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr Vasc Pharmacol 2017;15:339-51. [Crossref] [PubMed]
- Si X, Zhang L, Wang H, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer 2018;122:32-7. [Crossref] [PubMed]
- Feng PH, Chen KY, Huang YC, et al. Bevacizumab Reduces S100A9-Positive MDSCs Linked to Intracranial Control in Patients with EGFR-Mutant Lung Adenocarcinoma. J Thorac Oncol 2018;13:958-67. [Crossref] [PubMed]
- Akamatsu H, Toi Y, Hayashi H, et al. Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:386-94. [Crossref] [PubMed]
- Suh KJ, Keam B, Kim M, et al. Serum Neuron-Specific Enolase Levels Predict the Efficacy of First-Line Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors in Patients With Non-Small Cell Lung Cancer Harboring EGFR Mutations. Clin Lung Cancer 2016;17:245-52.e1. [Crossref] [PubMed]
- Hsu KH, Huang YH, Tseng JS, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naive advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer 2019;127:37-43. [Crossref] [PubMed]


