
Safety and efficacy of anti-PD-1 inhibitors in Chinese patients with advanced lung cancer and hepatitis B virus infection: a retrospective single-center study
Introduction
Lung cancer is the most frequently diagnosed cancer and major cause of cancer-related deaths worldwide (1-3). Conventional chemotherapy and radiation therapy for patients in the advanced stage may help to provide palliation in the setting of widespread disease but can be difficult for patients to tolerate. Over the past few decades, molecularly targeted therapy has greatly improved the survival time and life quality of lung cancer patients with targetable driver mutations (4,5). However, tumor response is often not durable with drug resistance, leading to therapeutic failure (6). Immune checkpoint inhibitors, known as a switch for restoring antitumor immunity, have ushered in a new era of anti-cancer therapy (7-9). The programmed cell death protein 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1) axis has been widely investigated and anti-PD-1/PD-L1 therapy approved as a standard treatment for advanced lung cancer (10). Clinical trials have indicated immunotherapy, in combination of chemotherapy, showed a significant overall survival (OS) benefit to patients with advanced stage lung cancer patients. Chronic hepatitis B virus (HBV) is a serious global public health problem and endemic in East Asia (11). Those either carrying or infected with HBV are defined as hepatitis B surface antigen positive (HBsAg-positive). According to the World Health Organization (WHO), approximately 250 million people are living with chronic hepatitis B infection worldwide (12), and China accounts for 10% of the global HBV carrier population (13).
Host immune responses play a critical role in HBV control (14,15). Impaired immunity leads to HBV reactivation, showing active virus replication or immune-mediated hepatic injury (16). HBV reactivation can be triggered by cancer chemotherapy (17,18) with an incidence as high as 20–50% in patients who are chronic HBV carriers, and infection has been reported in this setting (19). With the advent of cancer immunotherapy, anti-PD-1/PD-L1 immune checkpoint inhibitors have become an effective method for lung cancer treatment. In the context of the cancer immunoediting theory, however, immunotherapy-related HBV reactivation is a potential threat to this therapy. There are few studies investigating the risk of viral reactivation and hepatotoxicity of PD-1/PD-L1 inhibitors for HBsAg-positive patients because such patients are initially excluded from clinical trials. Previous retrospective studies evaluated the safety of immunotherapy mainly in Caucasian cancer populations (multiple tumor types, such as melanoma and hepatocellular carcinoma) with HBV surface antigen expression. As the prevalence of HBV infection in China is relatively higher than Western countries and the increasing incidence of lung cancer, the safety and efficacy of immunotherapy in these patients needs to be urgently explored. In addition, the question of immunotherapy in the setting of HBV presents an important safety dilemma regarding subsequent therapies for lung cancer patients with immune-related liver dysfunction in the setting of potentially prolonged survival outcomes. Unfortunately, there are limited published data. Therefore, we conducted a single-center retrospective analysis of Chinese lung cancer patients with HBV surface antigen expression to evaluate the safety and efficacy of PD-1 checkpoint inhibitor immunotherapy. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-21-79).
Methods
Patients
Patients with histologically confirmed lung cancer who had been administered PD-1 immune therapy checkpoint inhibitors (ICIs) at the First Affiliated Hospital, College of Medicine, Zhejiang University between September 2018 and May 2020 were reviewed, with the final follow up occurring on August 14, 2020. Metastases in the porta hepatis or other causes of biliary obstruction or baseline liver cirrhosis were ruled out. Clinical and treatment information was collected from electronic medical records. Individual consent for our study was waived as the privacy of the patients has not been disclosed. This study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Response evaluation and statistical analysis
Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Management of immune-related AEs (irAEs) included discontinuation of treatment and administration of corticosteroids based on AE severity following standard protocol guidelines (20). The response evaluation of PD-1 antibody was based on the immune-related Response Evaluation Criteria in Solid Tumors (21). The objective response rate (ORR) was defined as the proportion of patients who had achieved either complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as CR, PR, or stable disease (SD). Progression-free survival (PFS) was calculated from the first day of treatment to the first radiological evidence of disease progression or death. The follow up interval was 3 months, at which time CT scan, MRI and bone scan were performed. OS was defined as the interval from the start of first line anti-cancer treatment to death from any cause. Censored data were defined as data from patients who were alive and had no evidence of disease progression at the last follow-up visit.
Baseline clinical characteristics, including age at diagnosis, gender, smoking/alcohol status, clinical stage, tumor histology, liver metastasis or not, baseline liver function test, history of anti-HBV therapy, the number of previous lines of treatment, adverse events, and abnormal changes of liver function were recorded during ICIs by retrospective chart review. Qualitative variables were reported as the frequency (percentage), and continuous variables were described as the median (range). The denominator for calculating ORR included all patients in our study who could be evaluated for response. Kaplan-Meier methodology was used to determine median PFS and all analyses were conducted using the SPSS software (ver. 13.0). The treating physician made all treatment strategy decisions.
Results
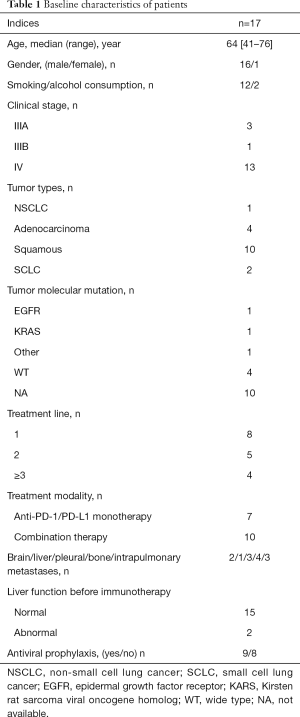
In total, 330 consecutive lung cancer patients who received PD-1 ICIs were reviewed, 17 of whom had a history of HBV. The clinical characteristics of the 17 HBV patients are summarized in Table 1. The median age of patients was 64 (range, 41–76) years old, and 47.1% [8/17] were ≥65 years old. The majority of patients (94.1%, 16/17) were male and reported a history of current or prior smoking (70.1%, 12/17). Metastases were present in 13 patients with advanced lung cancer (Figure S1). Extrapulmonary metastases were most commonly found in the bone (23.5%, 4/17), followed by pleura (17.6%, 3/17), brain (11.8%, 2/17) and liver (6.0%, 1/17). Ten patients were diagnosed squamous carcinoma, whilst four had adenocarcinoma and two had small cell lung cancer (SCLC). The one left was classified as non-small cell lung cancer (NSCLC) due to low tumor differentiation. Molecular testing, including EGFR/KRAS/NRAS/BRAF/HER-2/MET/PI3KCA mutation and ALK/ROS1/RET fusion were performed in seven patients. Among four adenocarcinoma patients, one patient harbored EGFR 19 deletion combined with T790M mutation, another one harbored HER2 mutation, and the other two had no known positive genetic mutation. One NSCLC patient harbored KRAS. Two squamous carcinoma patients were wild type (Table 2). Routine tumor staining for PD-L1 was not performed. All patients were treated with PD-1 inhibitors monotherapy or combination therapy, including 2 Tislelizumab, 2 Sintilimab 2 Nivolumab, 3 Camrelizumab, 3 Triprizumab, and 5 Pembrolizumab. Eight patients received anti-PD-1 immunotherapy as first-line treatment, and the other nine patients received it as second or later line immunotherapy. Ten patients received anti-PD-1 in combination with chemotherapy. Seven patients were treated with single anti-PD-1 agent, and nine patients received anti-HBV treatment when anti-PD-1 immunotherapy started according to infectious disease consultation, due to the lack of specific antiviral strategy for cancer immunotherapy.
Full table
Full table
Baseline liver function test abnormalities were seen in 11.8% [2/17] of patients before immunotherapy. One patient had grade 2 ALT elevation before anti-PD-1 immunotherapy and developed grade 3 elevation of ALT after immunotherapy, while another one with grade 1 baseline ALT elevation also experienced a mild rise in ALT.
Treatment
Single agent anti-PD-1 immunotherapy was given to 41.2% of patients (7/17). Only one patient received single agent anti-PD-1 immunotherapy as first line therapy, because of their poor ECOG status. Six patients received single agent anti-PD-1 immunotherapy as subsequent treatment (Table 1) and seven received chemotherapy combined with anti-PD-1 immunotherapy as first-line treatment. Of these, five patients diagnosed as having lung squamous cell carcinoma received nano-albumin bound paclitaxel plus carboplatin and two lung adenocarcinoma patients received pemetrexed plus carboplatin.
Effectiveness
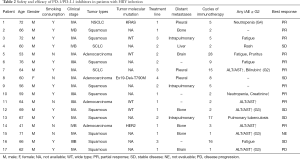
Among the 17 patients, 16 were available for anti-PD-1 immunotherapy response evaluation (Table 2), and one was lost to follow-up. The median number of cycles of immunotherapy used was 5 (range, 1–28). PR was achieved in 10 of 16 patients (62.5%), and five patients (31.2%) had SD. The ORR of the cohort was 62.5%, and DCR was 93.7%.
At the end of the last follow-up, eight patients were still receiving anti-PD-1 immunotherapy, six patients discontinued anti-PD-1 immunotherapy for various reasons including treatment-related adverse events, three had disease progression and two had died (Figure 1). The median PFS was 3 (range, 1 to 16.5) months and the median OS was 7.5 (range, 1.2 to 46.0) months.
Safety
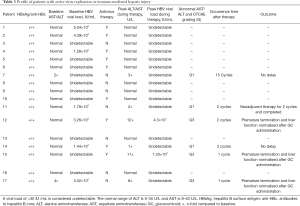
Generally, anti-PD-1 immunotherapy was well tolerated in lung cancer patients with HBV infection. There was no treatment related death in our study. The most prevalent side-effect was hepatic transaminase elevation in six patients (35.3%). Other treatment related AEs were neutropenia, seen in patients who received chemotherapy combined with ICIs, and which was considered a chemotherapy related AE as well as fatigue and rash. One patient developed active pulmonary tuberculosis (TB) after 12 cycles of anti-PD-1 immunotherapy. The patient then ceased anti-PD-1 immunotherapy and is receiving anti-TB treatment (Table 2). Liver function abnormalities were specifically analyzed (Table 3). Among six patients who were observed to have hepatic transaminase elevation, three patients developed grade 3 elevation transaminase and in all patients, anti-PD-1 agent was withheld and systemic corticosteroid therapy initiated. Aminotransferase reverted to normal in all patients and no adverse events were observed in subsequent treatment. The remaining three patients exhibited grade 1-2 aminotransferase elevation, with or without a concomitant increase in bilirubin levels. One patient developed grade 1 aminotransferase elevation after 2 cycles of neoadjuvant chemotherapy combined with immunotherapy; however aminotransferase levels slowly returned to baseline without intervention or treatment breaks. The patient received single agent mono-chemotherapy as postoperative adjuvant therapy and no aminotransferase abnormalities were observed.
Full table
Discussion
The results of the Chinese National Hepatitis seroepidemiological survey of 2019 (22) indicate the overall prevalence of HBV infection in the general population to be 5–6% with around 70 million people chronically infected. HBV infection represents a significant public health burden in China in spite of the success of HBV vaccination (23). Due to the immunocompromised status, patients with hematological malignancies and lymphomas are at high risk for reactivation of HBV (24,25). Previous studies report HBV reactivation and subsequent liver function impairment in lung cancer patients who received chemotherapy and targeted therapy. In the case report of Qin et al., fulminant viral hepatitis occurred in a patient with SCLC during chemotherapy and the patient succumbed to liver failure (26). In the study by Yao et al. 16 of 171 patients developed HBV reactivation during EGFR TKI treatment, with an annual incidence of 7.86% (27). It is generally assumed that HBV mediates chronic liver injury through abnormal immune attack. HBsAg seropositive patients are considered to have impaired baseline liver function and are more prone to trigger immune reconstitution inflammatory syndrome, although the initial ALT/AST are within the normal range.
Recently, anti-PD-(L)1 therapy has provided new therapeutic options for patients with various cancer types, including lung cancer. Limited evidence exists on the incidence of high-grade immune-related adverse events in patients with advanced lung cancer and HBV infection. In clinical trials, the reported incidence of immune-related hepatitis in lung cancer patients ranges from 5–10% with monotherapy to 25–30% with combined chemotherapy (28-30). A case series (31) reported 5 patients developing liver injury, 1 suffering from grade 2 ALT increase and 4 showing grade 1 ALT increase (28). In the retrospective study of Byeon et al., 5 of the 9 NSCLC patients who were treated with PD-1 inhibitors and shown to have severe AST/ALT elevation (grade 3 or higher) were infected with HBV (32). In our study, 6 of 17 patients developed ALT/AST abnormality and 3 experienced severe hepatitis (grade 3). Of the 3 patients, 1 whose HBV-DNA was undetectable and had normal liver function before treatment and had received preventative antiviral drugs. However, that patient showed a higher HBV-DNA copy number and grade 3 liver injury during PD-1 inhibition. Another patient, not receiving antiviral medication, had a HBV DNA copy number of 3.02×102 IU/mL and grade 2 hepatic dysfunction before treatment. After 1 cycle of immunotherapy, ALT elevated to 6-fold from its baseline level while no increase of the DNA load was seen. The remaining 1 patient, whose HBV DNA copy number was 3.26×103 IU/mL and liver metabolism was normal, experienced grade 3 transaminase elevation and higher DNA copy number 2 cycles later. This suggests the incidence of immune-related hepatitis is unpredictable due to different treatment regimens, cycles and individual difference. HBV-positive patients, receiving chemotherapy and immunotherapy, are at high risk of hepatitis reactivation. Antiviral prophylaxis should be considered while HBsAg seropositivity has been confirmed.
It is interesting to note that that 1 patient was newly diagnosed with pulmonary TB during their anti-PD-1 treatment. Previous studies (33,34) have similarly reported patients subsequently infected with active TB, including TB-related fatalities when treated with anti-PD-1 ICIs. As patients with active TB infections are excluded from clinical trials, there is limited research assessing the risk of developing TB secondary to immunotherapy. On this basis, it may be necessary to screen closely for TB infection when initiating immunotherapy. Other possible irAEs, such as fatigue, rash, pruritus and creatinine elevation, appear to be well tolerated and short-lived in duration.
It is currently believed that PD-1 inhibitors exert anti-tumor effects by promoting T-cell activation, rather than through the suppression of T-cells. Patients with HBV infection, characterized by T-cell exhaustion and impaired T-cell function, may theoretically have a poor response to PD-1 blockade therapy. In addition, HBV-positive patients may be even more vulnerable to virus reactivation and immune-mediated liver injury as their baseline liver function is compromised. It should be noted that the anti-tumor efficacy of ICIs is promising with an ORR of 62.5% for lung cancer patients.
As our study included only HBsAg-positive lung cancer patients who received immunotherapy in our center and there is a risk factor for HBV reactivation, the sample sizes were relatively small. Considering this, the included patients may not accurately represent the population of HBV infections and bias may exist in the results of our study. Further, due to the application of multiple kinds of anti-PD-1 in our study, there may be adverse reaction differences caused by differences in the agents.
These results support the clinical observation that lung cancer patients can be treated safely with anti-PD-1 immunotherapy in the context of HBV infection. Treatment-related irAE’s were found to be manageable. Close monitoring for hepatotoxicity, including HBV-DNA is advised and treatment with prophylactic antiviral therapy implemented when indicated. Further studies on the PD-1 ICIs in HBV-infected patients are required.
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group.
Funding: This work was supported by the Science and Technology Program of ZheJiang Province, China (no. q18h160029).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-21-79
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-21-79
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-21-79). SKJ reports grants and personal fees from Merck & Co., outside the submitted work. SW reports personal fees from AstraZeneca, Chugai Pharma, Ono Pharmaceutical, Bristol-Myers, grants and personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, Taiho Pharmaceutical, Pfizer, Novartis, Daiichi Sankyo, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. The privacy of the patients has not been disclosed in our study and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J Thorac Oncol 2019;14:1732-42. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Robinson BW, Rami-Porta R, Call S. Lung cancer staging: a concise update. Eur Respir J 2018;51:1800190 [Crossref] [PubMed]
- Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019;322:764-74. [Crossref] [PubMed]
- Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 2019;12:92. [Crossref] [PubMed]
- Steven A, Fisher SA, Robinson BW. Immunotherapy for lung cancer. Respirology 2016;21:821-33. [Crossref] [PubMed]
- Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019;12:47. [Crossref] [PubMed]
- Vafadar S. Immunotherapy for non-small cell lung cancer. Jaapa 2019;32:37-42. [Crossref] [PubMed]
- Lanini S, Ustianowski A, Pisapia R, et al. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am 2019;33:1045-62. [Crossref] [PubMed]
- Organization. WH. Hepatitis B [Internet] Geneva: World Health Organization; 2019.
- Tao J, Zhang W, Yue H, et al. Prevalence of Hepatitis B Virus Infection in Shenzhen, China, 2015-2018. Sci Rep 2019;9:13948. [Crossref] [PubMed]
- Yang Y, Zhao X, Wang Z, et al. Nuclear Sensor Interferon-Inducible Protein 16 Inhibits the Function of Hepatitis B Virus Covalently Closed Circular DNA by Integrating Innate Immune Activation and Epigenetic Suppression. Hepatology 2020;71:1154-69. [Crossref] [PubMed]
- Wu J, Han M, Li J, et al. Immunopathogenesis of HBV Infection. Adv Exp Med Biol 2020;1179:71-107. [Crossref] [PubMed]
- Huang SC, Yang HC, Kao JH. Hepatitis B reactivation: diagnosis and management. Expert Rev Gastroenterol Hepatol 2020;14:565-78. [Crossref] [PubMed]
- Martín Hidalgo-Barquero MV, Ruiz-Calero Cendrero RM, Castellano BC, et al. Chemotherapy and occult reactivation of HBV in hemodialysis patients. Nefrologia 2020;40:565-566. [Crossref] [PubMed]
- Wei J, Zhu X, Mao X, et al. Severe early hepatitis B reactivation in a patient receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of diffuse large B-cell lymphoma. J Immunother Cancer 2019;7:315. [Crossref] [PubMed]
- Guo L, Wang D, Ouyang X, et al. Recent Advances in HBV Reactivation Research. Biomed Res Int 2018;2018:2931402 [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Chinese Society of Infectious Diseases, Chinese Medical Association. Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi 2019;27:938-61.
- Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med 2017;11:490-501. [Crossref] [PubMed]
- Chen CY, Tien FM, Cheng A, et al. Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol 2018;18:6. [Crossref] [PubMed]
- Marinone C, Mestriner M. HBV disease: HBsAg carrier and occult B infection reactivation in haematological setting. Dig Liver Dis 2011;43:S49-56. [Crossref] [PubMed]
- Qin L, Wang F, Zou BW, et al. Chemotherapy-induced fatal hepatitis B virus reactivation in a small-cell lung cancer patient. Mol Clin Oncol 2016;5:382-4. [Crossref] [PubMed]
- Yao ZH, Liao WY, Ho CC, et al. Incidence of hepatitis B reactivation during epidermal growth factor receptor tyrosine kinase inhibitor treatment in non-small-cell lung cancer patients. Eur J Cancer 2019;117:107-15. [Crossref] [PubMed]
- Reddy HG, Schneider BJ, Tai AW. Immune Checkpoint Inhibitor-Associated Colitis and Hepatitis. Clin Transl Gastroenterol 2018;9:180. [Crossref] [PubMed]
- Jennings JJ, Mandaliya R, Nakshabandi A, et al. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 2019;15:231-44. [Crossref] [PubMed]
- Wang S, Hao J, Wang H, et al. Efficacy and safety of immune checkpoint inhibitors in non-small cell lung cancer. Oncoimmunology 2018;7:e1457600 [Crossref] [PubMed]
- Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res 2018;28:155-8. [Crossref] [PubMed]
- Byeon S, Cho JH, Jung HA, et al. PD-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Med 2020;9:2352-62. [Crossref] [PubMed]
- Fujita K, Terashima T, Mio T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol 2016;11:2238-40. [Crossref] [PubMed]
- Zaemes J, Kim C. Immune checkpoint inhibitor use and tuberculosis: a systematic review of the literature. Eur J Cancer 2020;132:168-75. [Crossref] [PubMed]



