
Tarlatamab: the promising immunotherapy on its way from the lab to the clinic
Small cell lung cancer (SCLC) is a highly aggressive type of lung cancer that typically originates in the bronchi, which are the breathing tubes located in the central area of the chest. It accounts for about 10–15% of all lung cancer cases and tends to spread quickly to other parts of the body (lung, the brain, liver, adrenal glands, and bone) (1). The 5-year survival rate for SCLC is less than 7%, making it one of the deadliest forms of lung cancer (2). Patients with SCLC typically experience respiratory symptoms, such as coughing, difficulty breathing, and coughing up blood. Due to its aggressive nature, most patients are diagnosed with advanced or metastatic disease (3).
For many years, the standard treatment for newly diagnosed metastatic SCLC has been a combination of a platinum-based chemotherapy (cisplatin or carboplatin) and etoposide (1). While recent randomized phase III studies have shown that adding an immune checkpoint inhibitor, such as atezolizumab or durvalumab, to first-line chemotherapy can provide benefits for some patients with extensive-stage SCLC, most patients do not experience long-lasting benefits from this approach (4,5). Therefore, identifying the characteristics of tumors and hosts that are associated with a response to immunotherapy and developing additional anticancer strategies, especially in the second-line setting and beyond, is essential to improve outcomes for SCLC patients. A recent global multi-institutional phase 1 study led by Paz-Ares found that tarlatamab, a novel bispecific T cell engager (BiTE), has a manageable safety profile and provides encouraging response durability in patients with recurrent SCLC who have previously received platinum-based regimens and anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) antibodies (6).
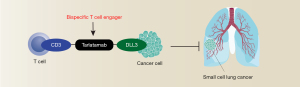
A BiTE is a type of immunotherapy that has shown great promise in treating different types of cancer, including Kirsten rat sarcoma viral oncogene homolog (KRAS)-driven tumors (7). The fundamental concept behind BiTEs is to create an antibody that can bind to both a T cell and a cancer cell simultaneously, bringing the T cell close to the cancer cell and enabling it to recognize and attack it. BiTEs are designed to be very specific, allowing them to target cancer cells while leaving healthy cells unharmed (8). One promising BiTE is tarlatamab, also known as AMG 757 (9). This drug works by binding to delta-like ligand 3 (DLL3) on tumor cells and cluster of differentiation 3 (CD3) on T cells, which triggers T cell-dependent antitumor immunity (Figure 1). DLL3 is an inhibitory Notch ligand that is highly expressed in SCLC and other neuroendocrine tumors, but it is minimally expressed in normal tissues (10,11). As a result, DLL3 is being explored as a potential therapeutic target in SCLC (12).
In a previous preclinical study conducted by Paul E. Hughes’ group, potent and specific killing of SCLC cell lines by AMG 757 with very low DLL3 expression (<1,000 molecules per cell) was observed (9). AMG 757 was also found to effectively engage human T cells when systemically administered, induce T-cell activation, and redirect T cells to kill tumor cells, resulting in significant tumor regression and complete responses in patient derived xenograft models of SCLC, as well as in orthotopic models of established primary lung SCLC and metastatic liver lesions. Furthermore, a 1-month repeat dose nonhuman primate toxicology study revealed that AMG 757 was well tolerated at a dose of 4.5 mg/kg, which provided exposure levels exceeding the mean in vitro cell effective concentration 50% (EC50) values. No AMG 757-related adverse findings were observed in animal. Overall, these preclinical studies indicate that AMG 757 may be a viable option for targeting DLL3-expressing SCLC tumors in the clinical setting.
The current clinical trials conducted by Paz-Ares et al. were sponsored by Amgen Inc. (6). A total of 107 patients were enrolled in the trial, and DLL3 expression (≥1%) was detected in 94% of the evaluable patients. Participants received tarlatamab intravenously every 2 weeks at doses ranging from 0.003 to 100 mg. Patients whose tumors improved received step dosing. The mean terminal elimination half-life of tarlatamab was found to be 5.7 days. Tarlatamab was discontinued in 4 (3.7%) patients, and 25 patients showed a confirmed response (23.4%), including 2 with a complete response. The median duration of response was 12.3 months, and the longest duration of response observed was 14.9 months. However, the drug also showed some toxicity, and 90.7% of the patients experienced side effects. The most common side effects were cytokine release syndrome in 56 patients (52.3%), pyrexia in 43 patients (40.2%), and constipation in 33 patients (30.8%). Cytokine release syndrome is an inflammatory syndrome caused by an aggressive immune response, which was generally grade 1 in this trial. Treatment-related grade 3 adverse events occurred in 33 patients (30.8%), and grade 5 adverse event was reported in one patient (1%). The disease control rate was found to be 51.4%. The median progression-free survival and overall survival were 3.7 and 13.2 months, respectively. As expected, the treatment with tarlatamab resulted in increased T cell infiltration and activation in tumor samples with interferon gamma production.
In summary, this phase 1 human trial demonstrated that tarlatamab as a first-in-class DLL3-targeted BiTE is reasonably effective and has manageable levels of toxicity. The treatment was well-durable in SCLC patients who responded to the drug. However, to better understand the long-term response and potential side effects of tarlatamab, more multicenter evidence is needed, either as monotherapy or in combination therapy. In normal tissues, DLL3 expression is generally high not only in brain tissue but also in the pituitary gland and testis. Further monitoring of the functional effects of tarlatamab on these tissues is required. Successful patient recruitment is a major challenge in conducting clinical trials, and further monitoring of the relationship between underlying disease and response to tarlatamab in SCLC patients could help better define patient stratification of the trial population. Finally, effective biomarkers for clinical evaluation of tarlatamab drug and antitumor immune response are still lacking. Understanding the mechanisms of drug-induced cell death, including tarlatamab, and drug resistance is an important task in basic cancer research (13).
Acknowledgments
Funding: Research by DT and RK was supported by grants from the National Institutes of Health (Nos. R01CA160417, R01CA229275, and R01CA211070).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-115/coif). Research by DT and RK was supported by grants from the National Institutes of Health (Nos. R01CA160417, R01CA229275, and R01CA211070).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725-37. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Paz-Ares L, Champiat S, Lai WV, et al. Tarlatamab, a First-In-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small Cell Lung Cancer: An Open-Label, Phase I Study. J Clin Oncol 2023;41:2893-903. [Crossref] [PubMed]
- Song X, Zhou Z, Kang R, et al. Bispecific T cell engagers kill resistant cells during KRAS-G12C blockade therapy. Oncoimmunology 2022;11:2141978. [Crossref] [PubMed]
- Zhou S, Liu M, Ren F, et al. The landscape of bispecific T cell engager in cancer treatment. Biomark Res 2021;9:38. [Crossref] [PubMed]
- Giffin MJ, Cooke K, Lobenhofer EK, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res 2021;27:1526-37. [Crossref] [PubMed]
- Chou J, Egusa EA, Wang S, et al. Immunotherapeutic Targeting and PET Imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. Cancer Res 2023;83:301-15. [Crossref] [PubMed]
- Kim JW, Ko JH, Sage J. DLL3 regulates Notch signaling in small cell lung cancer. iScience 2022;25:105603. [Crossref] [PubMed]
- Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42-51. [Crossref] [PubMed]
- Tang D, Kang R, Berghe TV, et al. The molecular machinery of regulated cell death. Cell Res 2019;29:347-64. [Crossref] [PubMed]


