
Real-world analysis of first-line afatinib in patients with EGFR-mutant non-small cell lung cancer and brain metastasis: survival and prognostic factors
Highlight box
Key findings
• First-line afatinib in the real-world setting showed clinically meaningful effectiveness in patients with epidermal growth factor receptor (EGFR)-mutant non-small-cell lung cancer (NSCLC) and brain metastases.
• CNS failure was a poor prognostic factor for time in treatment and overall survival (OS) correlating with younger age, poor ECOG performance status, higher metastatic number, advanced disease stage, uncommon EGFR mutations, and baseline liver and/or bone metastases.
What is known and what is new?
• OS in patients with NSCLC and brain metastases is poor.
• We identified prognostic factors and ascertained treatment outcomes of first-line afatinib for patients with EGFR-mutant NSCLC with brain metastases in a real-world setting.
What is the implication, and what should change now?
• Our findings confirm that first-line afatinib in the real-world setting has clinically meaningful effectiveness in patients with EGFR-mutant NSCLC and brain metastases. Prognostic factors identified may support treatment decisions.
Introduction
In 2020, lung cancer was the second most commonly diagnosed cancer globally (behind female breast cancer), with more than 2.2 million new cases diagnosed and accounting for nearly 1.8 million deaths (1). Projections estimated that, during 2021 in Korea, more than 32,000 incident cancer cases and nearly 19,000 cancer deaths due to lung cancer would occur (2). Over the past two decades, 5-year relative survival rates in Korea have improved from 11.3% [1993–1995] to 30.2% [2013–2017], which is probably due to improvements in diagnosis and therapy (3). However, over 40% of patients with non-small cell lung cancer (NSCLC) present with stage IV disease (4).
Targeted therapy based on establishing molecularly distinct driver mutations for NSCLC has increased treatment options and improved clinical outcomes. Targeted agents include tyrosine kinase inhibitors (TKIs), gefitinib, erlotinib, afatinib and osimertinib, which target mutations in epidermal growth factor receptor (EGFR) and the multitarget TKI, crizotinib, for anaplastic lymphoma kinase (ALK)-positive or ROS1-positive NSCLC (5,6). EGFR mutation frequency varies according to ethnicity and, in Asian patients with advanced NSCLC and adenocarcinoma histology, EGFR mutations are found in approximately half of all tumors. The most common EGFR mutations are deletion of exon 19 and a L858R point mutation in exon 21 (7).
Afatinib is a second-generation irreversible ErbB-family TKI which covalently binds to EGFR and HER2/ERBB2 (erb-b2 receptor tyrosine kinase 2). In randomized and open-label clinical trials, first-line afatinib has consistently shown clinical benefit with good tolerability in patients with NSCLC and EGFR mutations achieving median progression-free survival (PFS) of 11.0 to 17.0 months (8-13).
Analysis of large cancer databases in the USA estimated the incidence of brain metastases (BMs) in NSCLC and lung cancer as approximately 10% and 20%, respectively (14,15). Elsewhere, in a population-based cancer registry in The Netherlands (n=938), the estimated cumulative 5-year incidence of BMs in lung cancer was 16.3% (16) and a retrospective analysis of East Asian patients with NSCLC (n=1,127) reported that 23.2% developed BMs (17). Despite improvements in treatment, survival for many NSCLC patients with BMs is poor, ranging from 7 to 47 months (18).
BMs in patients with NSCLC are often accompanied by EGFR mutations (24–40%) or ALK gene rearrangements at diagnosis (17,19,20) and in one study of NSCLC patients with BMs (n=381), the cumulative incidence of EGFR mutations increased over time from 24% at baseline to 34%, 47% and 53% at 1, 3 and 5 years, respectively (19).
Preclinical studies have shown that afatinib penetrates the mouse blood brain barrier (21,22) and, in clinical studies, afatinib is active in patients with central nervous system (CNS) lesions as illustrated following a post hoc analysis of the LUX-Lung 3 (8), LUX-Lung 6 (9) and LUX-Lung 7 (10) randomized controlled trials (23,24), and in a prospective multicenter study in patients with EGFR mutation-positive NSCLC with leptomeningeal carcinomatosis (25). The CNS activity of afatinib, including its intracranial objective response rate and PFS, as well as CNS failure rate, in patients with baseline BMs is supported by data from real-world studies (26-29). However, there are limited reports on CNS failure in patients without baseline BMs during afatinib treatment.
To evaluate the CNS failure rate particularly in patients without baseline BMs as well as effectiveness of first-line afatinib therapy within a large cohort, we conducted a national, multicenter retrospective study in Korean patients with EGFR mutation-positive NSCLC in the real-world setting. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-832/rc).
Methods
Study design
This non-interventional, retrospective observational study reviewed electronic records of patients with EGFR-mutant NSCLC who received first-line afatinib treatment between October 2014 and October 2019 in 16 hospitals across South Korea. No power calculations to determine sample size were required for the study, although the study aimed to include at least 700 eligible patients.
Objectives
The primary objective was to investigate the CNS failure rate of first-line afatinib in NSCLC patients with EGFR mutations in a real-world setting. Secondary objectives were to determine the time on treatment (TOT) of first-line treatment and to assess other real-world effects of afatinib on overall survival (OS).
Ethics and patient confidentiality
The study and protocol were approved by the Institutional Review Board of the Kosin University Gospel Hospital (IRB, KUGH No. 2019-07-038); the other 15 participating hospitals were also informed and agreed with the study. This non-interventional, retrospective chart review study was carried out in compliance with the protocol and principles laid down in the Declaration of Helsinki (as revised in 2013), in accordance with the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Good Clinical Practice and the relevant sponsor’s Standard Operating Procedures. Patient identification code numbers were used to ensure patient confidentiality. As this was a non-interventional, retrospective chart review study based on existing data in general practice, it did not require patient informed consent as per Korean regulations.
Inclusion and exclusion criteria
Patients aged >18 years treated with first-line afatinib for EGFR mutation-positive stage IIIB/IV NSCLC were included in the study. Main exclusion criteria were patients who received first-line drug(s) other than afatinib, and those with insufficient clinical data.
Treatment
Patients with EGFR mutation-positive NSCLC received afatinib therapy, 40, 30 or 20 mg per day, orally.
Endpoints
The primary endpoint was CNS failure rate which was defined as CNS progression, e.g., appearance of new brain or leptomeningeal lesions during afatinib treatment in patients without baseline BMs.
Secondary endpoints included TOT, defined as the length of time from first to last dates of afatinib administration, and OS. Response and progression were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) (30) and tumor response was investigator-assessed.
Statistical analysis
Descriptive statistics for continuous variables were summarized by number, mean, standard deviation, 95% confidence interval (CI), median and range. Categorical and ordinal variables were summarized by frequency and percentage. Chi-squared and Fisher’s exact tests were used to evaluate differences between categorical variables.
TOT and OS were estimated using the Kaplan-Meier method, and the median plus 95% confidence intervals (CI) reported.
Cox proportional hazards (PH) models were used to investigate the effect of independent variables on survival outcomes. Variables with P<0.10 in the univariate Cox PH model were included in the multivariate Cox PH model.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, New York), and R software version 4.0.3 for Windows (R Development Core Team).
Results
Of 703 eligible patients included in the study, 262 (37.3%) had baseline BMs. Baseline characteristics of patients with or without baseline BMs are summarized in Table 1. Compared with patients without BMs at baseline (n=441), the group of patients with baseline BMs (n=262) consisted of fewer males (45.4% vs. 54.9%, P=0.015), and had involvement of more metastatic sites (3–6 sites: 42.4% vs. 12.5%, P<0.001), advanced stage disease (stage IVB: 61.1% vs. 31.5%, P<0.001), increased bone metastatic rates (53.4% vs. 36.3%, P<0.001) and reduced pleural metastatic rates (25.6% vs. 43.3%, P<0.001). The most common EGFR mutations were exon 19 deletion (Del19) and L858R in both the without BM at baseline (58.7% and 31.3%, respectively) and with BM at baseline groups (56.1% and 35.5%, respectively). Uncommon EGFR mutations in patients with or without baseline BMs are summarized in Table S1.
Table 1
| Parameter | No baseline brain metastases | Baseline brain metastases (n=262) | P | |||
|---|---|---|---|---|---|---|
| Total (n=441) | New brain metastases (n=92) | No brain metastases (n=349) | P | |||
| Age (years), mean (SD) | 63.5 (11.2) | 60.9 (10.3) | 64.2 (11.3) | 0.012 | 63.9 (11.0) | 0.652 |
| Sex, n (%) | 0.909 | 0.015 | ||||
| Male | 242 (54.9) | 50 (54.3) | 192 (55.0) | 119 (45.4) | ||
| Female | 199 (45.1) | 42 (45.7) | 157 (45.0) | 143 (54.6) | ||
| Smoking status, n (%) | 0.743 | 0.676 | ||||
| Never | 281 (64.0) | 57 (62.0) | 224 (64.6) | 173 (67.3) | ||
| Former | 119 (27.1) | 25 (27.2) | 94 (27.1) | 63 (24.5) | ||
| Current | 39 (8.9) | 10 (10.9) | 29 (8.4) | 21 (8.2) | ||
| ECOG performance status, n (%) | <0.001 | 0.342 | ||||
| 0–1 | 383 (93.2) | 70 (81.4) | 313 (96.3) | 216 (91.1) | ||
| ≥2 | 28 (6.8) | 16 (18.6) | 12 (3.7) | 21 (8.9) | ||
| EGFR mutation, n (%) | 0.305 | 0.468 | ||||
| Del19 | 259 (58.7) | 53 (57.6) | 206 (59.0) | 147 (56.1) | ||
| L858R | 138 (31.3) | 26 (28.3) | 112 (32.1) | 93 (35.5) | ||
| Other | 44 (10.0) | 13 (14.1) | 31 (8.9) | 22 (8.4) | ||
| No. of metastatic organs, n (%) | <0.001 | <0.001 | ||||
| 0–2 | 386 (87.5) | 62 (67.4) | 324 (92.8) | 151 (57.6) | ||
| 3–6 | 55 (12.5) | 30 (32.6) | 25 (7.2) | 111 (42.4) | ||
| Stage (AJCC 8th edition), n (%) | <0.001 | <0.001 | ||||
| 3–4A | 302 (68.5) | 45 (48.9) | 257 (73.6) | 102 (38.9) | ||
| 4B | 139 (31.5) | 47 (51.1) | 92 (26.4) | 160 (61.1) | ||
| Liver metastasis, n (%) | 37 (8.4) | 14 (15.2) | 23 (6.6) | 0.008 | 33 (12.6) | 0.072 |
| Bone metastasis, n (%) | 160 (36.3) | 48 (52.2) | 112 (32.1) | <0.001 | 140 (53.4) | <0.001 |
| Pleural metastasis, n (%) | 191 (43.3) | 33 (35.9) | 158 (45.3) | 0.105 | 67 (25.6) | <0.001 |
| Type of brain and leptomeningeal metastasis, n (%) | – | – | ||||
| Single | – | 19 (20.7) | – | 45 (17.2) | ||
| Multiple | – | 52 (56.5) | – | 204 (78.2) | ||
| Leptomeningeal | – | 5 (5.4) | – | 3 (1.1) | ||
| Single + Leptomeningeal | – | 1 (1.1) | – | 3 (1.1) | ||
| Multiple + Leptomenigenal | – | 15 (16.3) | – | 6 (2.3) | ||
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; AJCC, American Joint Committee on Cancer.
Of 441 patients with no baseline BM, 92 (20.9%) developed BMs (CNS failure) during afatinib treatment. Compared with 349 patients without CNS failure, patients who developed CNS failure during treatment were younger (mean age: 60.9 vs. 64.2 years, P=0.012), had a higher ECOG performance status (PS) (≥2: 18.6% vs. 3.7%, P<0.001), more metastatic site involvement (3–6 sites: 32.6% vs. 7.2%, P<0.001), advanced stage disease (stage IVB: 51.1% vs. 26.4%, P<0.001), and baseline liver metastasis (15.2% vs. 6.6%, P=0.008) and/or bone metastasis (52.2% vs. 32.1%, P<0.001) (Table 1).
Median duration of CNS failure in patients receiving afatinib (n=92) was 12.2 months (95% CI: 9.9–14.6).
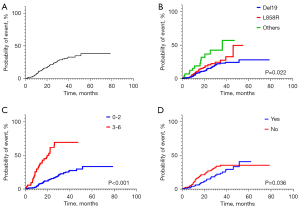
In patients without BM at baseline, the cumulative incidence of CNS failure in years 1, 2 and 3 was 10.1%, 21.5% and 30.0%, respectively. In univariate analysis, cumulative incidence was significantly higher in patients with ECOG PS ≥2 (P<0.001), compound or uncommon (i.e., not Del19 or L858R) EGFR mutations (P=0.022), stage IVB disease (P<0.001), liver metastasis (P=0.002), bone metastasis (P<0.001), and absence of pleural metastasis (P=0.036) (Table 2).
Table 2
| Parameter | Univariate analysis (CI, %) | P | ||
|---|---|---|---|---|
| 1 year | 2 years | 3 years | ||
| Overall (n=441) | 10.1 | 21.5 | 30.0 | |
| Sex | 0.962 | |||
| Male (n=242) | 13.2 | 22.5 | 28.5 | |
| Female (n=199) | 6.5 | 18.5 | 32 | |
| Age (years) | 0.456 | |||
| <65 (n=229) | 12.6 | 24.1 | 31.0 | |
| ≥65 (n=212) | 7.1 | 18.4 | 29.1 | |
| Smoking status | 0.895 | |||
| Never (n=281) | 7.8 | 21.5 | 30.1 | |
| Former (n=119) | 15.5 | 20.9 | 30.9 | |
| Current (n=39) | 11.6 | 25.0 | 29.1 | |
| ECOG performance status | <0.001 | |||
| 0–1 (n=383) | 7.7 | 19.2 | 26.3 | |
| ≥2 (n=28) | 39.9 | 56.8 | 78.4 | |
| EGFR mutation | 0.022 | |||
| Del19 (n=259) | 9.2 | 19.9 | 29.4 | |
| L858R (n=138) | 9.0 | 20.4 | 27.3 | |
| Other2 (n=44) | 18.8 | 36.5 | 42.3 | |
| No. of metastatic organs | <0.001 | |||
| 0–2 (n=386) | 6.6 | 15.4 | 24.0 | |
| 3–6 (n=55) | 33.8 | 61.6 | 69.3 | |
| Stage (AJCC 8th edition) | <0.001 | |||
| 3–4A (n=302) | 6.9 | 14.1 | 19.8 | |
| 4B (n=139) | 16.7 | 37.7 | 53.7 | |
| Liver metastasis | 0.002 | |||
| Yes (n=37) | 18.0 | 41.8 | 53.0 | |
| No (n=404) | 9.3 | 19.6 | 27.8 | |
| Bone metastasis | <0.001 | |||
| Yes (n=160) | 18.7 | 36.5 | 45.3 | |
| No (n=281) | 5.2 | 13.4 | 21.8 | |
| Pleural metastasis | 0.036 | |||
| Yes (n=191) | 5.9 | 14.7 | 22.0 | |
| No (n=290) | 13.3 | 26.9 | 35.1 | |
1, CNS failure was defined as CNS progression e.g., appearance of new brain or leptomeningeal lesions during afatinib treatment; 2, compound plus uncommon mutations. CI, cumulative incidence; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; AJCC, American Joint Committee on Cancer.
In multivariate analysis, the incidence of CNS failure was significantly higher in patients with ECOG PS ≥2 [hazard ratio (HR) = 5.98, 95% CI: 3.26–10.99; P<0.001], uncommon EGFR mutations (HR =3.07, 95% CI: 1.61–5.83; P=0.001), and no baseline pleural metastasis (HR =0.56, 95% CI: 0.35–0.90; P=0.017) (Table 3). These results are illustrated graphically in Figure 1.
Table 3
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | Ref. | – | – |
| Female | 0.83 | 0.47–1.46 | 0.51 |
| Age (years) | |||
| <65 | Ref. | – | – |
| ≥65 | 1.22 | 0.78–1.91 | 0.394 |
| Smoking status | |||
| Never | Ref. | – | – |
| Former | 0.93 | 0.49–1.77 | 0.826 |
| Current | 1.08 | 0.48–2.42 | 0.854 |
| ECOG performance status | |||
| 0–1 | Ref. | – | – |
| ≥2 | 5.98 | 3.26–10.99 | <0.001 |
| EGFR mutation | |||
| Del19 | Ref. | – | – |
| L858R | 1.13 | 0.69–1.86 | 0.63 |
| Other | 3.07 | 1.61–5.83 | 0.001 |
| No. of metastatic organs | |||
| 0–2 | Ref. | – | – |
| 3–6 | 4.65 | 2.56–8.45 | <0.001 |
| Stage (AJCC 8th edition) | |||
| 3–4A | Ref. | – | – |
| 4B | 1.21 | 0.67–2.16 | 0.528 |
| Liver metastasis | |||
| Yes | 1.28 | 0.63–2.62 | 0.502 |
| No | Ref. | – | – |
| Bone metastasis | |||
| Yes | 1.27 | 0.72–2.22 | 0.414 |
| No | Ref. | – | – |
| Pleural metastasis | |||
| Yes | 0.56 | 0.35–0.90 | 0.017 |
| No | Ref. | – | – |
1, CNS failure was defined as CNS progression, e.g., appearance of new brain or leptomeningeal lesions during afatinib treatment. CNS, central nervous system; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; AJCC, American Joint Committee on Cancer.
Median TOT of afatinib in all patients was 16.0 months (95% CI: 14.8–17.2) and was significantly different (P<0.001) between patients with CNS failure (12.2 months, 95% CI: 9.9–14.6), without CNS failure (18.9 months, 95% CI: 16.8–21.0), and with baseline BM (14.1 months, 95% CI: 11.9–16.3) (Table S2; Figure 2). Significant within-category differences in TOT were found for ECOG PS (P=0.001), EGFR mutation status (P=0.049), number of metastatic organs (P<0.001), disease stage (P=0.008), baseline liver metastasis (P=0.001) and baseline bone metastasis (P=0.024) (Table S2).

Median OS in all patients was 52.9 months (95% CI: 45.4–60.3) and was significantly different (P<0.001) between patients with CNS failure (29.1 months, 95% CI: 23.3–35.0), without CNS failure (67.3 months, 95% CI: 43.7–90.9) and with baseline BM (48.5 months, 95% CI: not available) (Table S3; Figure 2). The between-group difference in OS at 10 months was significant (P=0.012). Significant within-category differences in OS were found for ECOG PS (P=0.016), EGFR mutation status (P<0.001), number of metastatic organs (P<0.001), disease stage (P=0.001), baseline liver metastasis (P<0.001) and baseline bone metastasis (P<0.001) (Table S3).
Discussion
This retrospective real-world study investigated the CNS failure rate of first-line afatinib in patients with EGFR-mutant NSCLC with or without BM. Reviews of patient electronic records revealed that, at baseline, BMs were identified in 37.3% of the whole cohort, and a further 20.9% of patients with no BMs at baseline developed CNS failure during afatinib treatment. These results are similar to those from a real-world study of first-line afatinib in Asian patients with EGFR mutation-positive NSCLC (n=422) which reported that 17.7% of patients without baseline BMs subsequently developed BMs after starting afatinib treatment (31). In contrast to real-world studies, the risk of de novo CNS progression with afatinib estimated from analysis of the LUX-Lung 3 and LUX-Lung 6 clinical trials was much lower—6% (24).
Reported rates of CNS failure with other EGFR TKIs were also variable. A retrospective study of patients in the USA with advanced NSCLC treated with the first-generation EGFR TKIs gefitinib or erlotinib (n=100), reported a crude incidence rate for CNS progression of 28%. Twenty of 28 patients developed de novo CNS metastases (32). Continued follow-up of this cohort for a median of 25 months found that a third developed CNS progression including 26 patients (26%) without baseline BMs, and the estimated 6-, 12-, and 24-month cumulative risk of CNS progression in patients without pre-existing BMs (n=77) was 1%, 3%, and 15%, respectively (33). Retrospective analysis of Korean patients with advanced NSCLC (n=232) reported a CNS failure rate of 16% for first-generation EGFR TKIs (gefitinib or erlotinib). Interestingly, isolated CNS failure was significantly more frequent in patients having clinical benefit from TKI treatment (n=127) than those who did not show clinical benefit (13% vs. 1%) (34). A retrospective study of Japanese patients with EGFR-mutated NSCLC treated with first-line gefitinib (n=144) or erlotinib (n=26) the incidence of CNS metastases was lower in the erlotinib group (11.5% vs. 29.9%). In patients with no baseline CNS metastases, CNS failure rates were 4.8% and 24.5%, respectively (35). Post hoc analysis of the Chinese ADJUVANT trial reported that CNS metastasis occurred in 27.4% (29/106) of patients receiving adjuvant gefitinib therapy in resected early-stage EGFR-mutation positive NSCLC (36). The multinational ARCHER 1050 trial, which compared the second-generation irreversible EGFR TKI dacomitinib with gefitinib in patients with advanced NSCLC and activating EGFR mutations, de novo BMs developed in 0.4% (1/227) and 4% (9/225) of patients, respectively (37,38). Similar CNS failure rates were found in Asian patients enrolled in the ARCHER 1050 trial: 0.6% and 4.5%, respectively (39). In contrast to the LUX-Lung 3 and LUX-Lung 6 trials, which included patients with clinically asymptomatic and controlled BMs (8,9), the ARCHER 1050 trial excluded patients with CNS metastases (39). In the multinational FLAURA trial, events of CNS progression in patients with EGFR mutation-positive advanced NSCLC treated with the third-generation, irreversible EGFR TKI, osimertinib or first-generation EGFR TKIs (gefitinib or erlotinib) were observed in 6% and 15%, respectively (40). Preclinical studies show osimertinib has greater penetration of the rodent blood-brain barrier than other EGFR TKIs (21,41), which may explain these results. However, there are no studies which directly compare the incidence of CNS failure in patients with metastatic NSCLC treated with osimertinib or afatinib.
This study demonstrated the clinically meaningful effectiveness of first-line afatinib which produced a median TOT of 16.0 months and median OS of 52.9 months. These effectiveness results are comparable to results from a recent meta-analysis of real-world studies of afatinib treatment for advanced EGFR-mutant NSCLC which calculated a median time to failure (TTF) for first-line afatinib (4 studies) of 15.7 months and, in seven studies of first- and further-line afatinib, median OS was 31.6 months although there was significant heterogeneity between studies (42). Data presented in this study also support those from the LUX-Lung clinical trial program, in particular the LUX-Lung 3 (8), 6 (9) and 7 (10,42) trials which demonstrated the efficacy of afatinib including in patients with asymptomatic BMs at baseline (12–16% of all patients). In the multinational LUX-Lung 3 trial which included both Asian and non-Asian patients, median PFS in afatinib-treated patients with vs. without BMs was 11.1 vs. 13.8 months; in the LUX-Lung 6 study of Asian patients was 8.2 vs. 11.1 months; and in the multinational LUX-Lung 7 study was 7.2 vs. 12.7 months (10,23). In a combined analysis of LUX-Lung 3 and 6 trials, afatinib significantly improved PFS compared with chemotherapy in patients with BMs (8.2 vs. 5.4 months; P=0.0297) (23). In the LUX-Lung 7 trial, there was a significant difference in PFS between afatinib and gefitinib, favoring afatinib, in pre-planned subgroups which included the presence versus absence of baseline BMs (10), although there was no significant difference in OS (43). Results from a recent prospective non-interventional study in Germany of patients with EGFR-mutant NSCLC (including approximately one third with baseline BMs) support clinical trial data for first-line afatinib in routine clinical practice: median PFS and median OS were 12.2 and 30.4 months, respectively (44).
Real-world studies of Asian patients with EGFR mutation-positive advanced NSCLC, including those with BMs, have also demonstrated the effectiveness of first-line afatinib. These include a retrospective review of electronic case reports from patients with advanced EGFR mutation-positive NSCLC (n=422) including 39.8% with BMs at diagnosis in South Korea. Median time to treatment discontinuation was significantly longer in patients without vs. with BMs (22.9 vs. 14.8 months, P=0.001) and OS was also prolonged in patients without BMs (not reached vs. 40.3 months, P=0.0009) (31). In a Taiwanese study, first-line afatinib (n=115) compared to gefitinib (n=116), had superior PFS (12.7 vs. 9.8 months; HR =0.59, P=0.001) and OS (39.1 vs. 22.0 months; HR =0.64, P=0.035) (45). Furthermore, in a Cox model adjusted for possible confounding factors, cumulative incidence of BM was lower for afatinib compared with gefitinib (HR =0.49, 95% CI: 0.34–0.71, P<0.001) although median PFS and median OS were comparable between the two TKIs in patients with baseline BMs (45). In a single center Korean real-world study of patients with EGFR-mutant NSCLC (n=467) including 40% with BMs at baseline, median PFS for first-line afatinib was 19.1 months and was superior to that for first-line gefitinib (13.7 months) and erlotinib (14.0 months) (P=0.001) (46).
Our study showed that patients developing BMs had a poorer outcome than patients without CNS failure or with BMs at baseline, with significantly reduced TOT and OS. In most studies, among patients with EGFR mutation-positive NSCLC, patients with baseline BMs have a worse outcome compared to those without baseline BMs (18,47). However, in the present study, patients who developed de novo BMs showed a worse prognosis than patients with baseline BMs. Several theories have been proposed for the poor outcomes of CNS failures patients without baseline BMs. First, acquired resistance mutations against EGFR TKIs could induce CNS progression and poorer survival. The T790M mutation in the EGFR gene is the most common cause of resistance after first- or second-generation TKI therapy (48,49) and is localized within the ATP-binding pocket of EGFR. The primary cause of TKI resistance mediated by T790M mutant EGFR is its increased affinity for ATP (50). Resistance to EGFR TKIs can also result from mechanisms including transformation into small cell lung cancer (SCLC) and amplification of MET or ERBB2 genes (51-53). Prognosis for newly developed CNS progression by these mechanisms may be worse than for patients with baseline BMs.
In NSCLC, brain is the third most common single metastatic site after bone and lung; and for two metastases, the most common sites are bone plus lung followed by bone plus brain (54). In this study, multivariate analysis of first-line afatinib in patients with EGFR mutation-positive NSCLC without BM revealed that CNS failure was associated with ECOG status, uncommon EGFR mutations, and pleural metastasis status. Retrospective studies of patients with EGFR mutation-positive advanced NSCLC have previously identified younger age (55,56), number of extracranial metastases (56), malignant pleural effusion (56), serum carcinoembryonic antigen (CEA) (55) and point mutations in EGFR exon 21 (55) as independent risk factors for BM. Recent data, from a large real-world cohort of NSCLC patients with common and uncommon EGFR mutations treated with first-line afatinib, showed that EGFR L858R patients had a significantly higher CNS progression; there was a tendency to higher CNS progression in patients with uncommon mutations excluding exon 20 insertion and de novo T790M with high allele frequency (57).
Our analysis showed that pleural metastases were present in 26% of cases with initial BMs and 43% of cases without initial BMs, which differs from the high incidence of metastases to other organs (liver, bone) in the presence of initial BMs. Moreover, the absence of pleural metastases was a risk factor for the development of BMs. There may be differences in the organs that metastasise depending on the characteristics of the patient’s lung cancer, so patients with pleural metastases may have characteristics that make them less likely to develop BMs. Similarly, Li et al. (58), found that patients with BMs from NSCLC had more liver, lymph node, and adrenal metastases, but fewer pleural metastases, compared with patients without BMs. And in both univariate and multivariate analyses, the absence of pleural metastases was a risk factor for brain or leptomeningeal metastases (brain pleural metastases, OR: 0.495, 95% CI: 0.325–0.756; leptomeningeal metastases, OR (0.307, 95% CI: 0.172–0.547). In contrast, Ouyang et al. (56), found that in CNS failure in patients without BMs, pleural metastasis was a risk factor in univariate and multivariate analysis (OR: 5.283, 95% CI: 1.851–15.053). An alternative hypothesis is that afatinib is so effective against pleural metastases that it prevents deterioration that could lead to BMs. Further studies are needed to assess the relationship between pleural effusion and the risk for developing BMs, and the associated underlying mechanism.
Several risk factors for EGFR mutation-positive advanced NSCLC are in common with previously identified risk factors for BM in NSCLC. Factors include EGFR mutation-positive status (59), younger age (60), non-squamous cell carcinoma, especially adenocarcinoma (60-62), advanced stage disease (59), lesion diameter of the primary tumor (63), and elevated serum levels of NSE (60,62), CEA (60,63), CA125 (60) and calcium (63).
Limitations of this non-interventional, retrospective observational study are the potential variable quality and integrity of data, including length of follow-up, in patient electronic records which were reviewed. Importantly, because brain imaging data were not analyzed further in this study, there is a possibility that BMs were missed in patients classified as being without baseline BMs. This may have an impact in assessing the cumulative incidence of CNS failure in patients without baseline BMs. Additionally, although we collected data on the type of BMs, we did not investigate the type of local treatment. Although this is a limitation of our study, the combination of afatinib and local treatment for initial BMs is still controversial and is based on the judgement of medical staff. And if a patient without BMs develops BMs after using afatinib, we assume that the best local treatment would have been chosen based on the patient’s brain lesion status. Finally, the generalizability of our findings is limited by the fact that this real-world analysis only assessed data from patients across Korea.
Conclusions
First-line afatinib in the real-world setting in Korea showed clinically meaningful effectiveness in patients with EGFR-mutant NSCLC and BM. CNS failure during afatinib treatment was a poor prognostic factor for TOT and OS, correlating with younger age, poor ECOG PS, higher metastatic number, advanced disease stage, uncommon EGFR mutations, and baseline liver or bone metastases.
Acknowledgments
Under the guidance of the authors, medical writing assistance was provided by Robert A. Furlong PhD and David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from Boehringer Ingelheim Singapore.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-832/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-832/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-832/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-832/coif). Sung Yong Lee has received honoraria from Boehringer Ingelheim for lectures. YSC is a stockholder of Big Bio Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study and protocol were approved by the Institutional Review Board of the Kosin University Gospel Hospital (IRB, KUGH No. 2019-07-038). The other 15 participating hospitals were also informed and agreed with the study. This non-interventional, retrospective chart review study was carried out in compliance with the protocol and principles laid down in the Declaration of Helsinki (as revised in 2013), in accordance with the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Good Clinical Practice and the relevant sponsor’s Standard Operating Procedures. As this was a non-interventional, retrospective chart review study based on existing data in general practice, it did not require patient informed consent as per Korean regulations. Patient identification code numbers were used to ensure patient confidentiality.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Jung KW, Won YJ, Hong S, et al. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res Treat 2021;53:316-22. [Crossref] [PubMed]
- Lee JG, Kim HC, Choi CM. Recent Trends of Lung Cancer in Korea. Tuberc Respir Dis (Seoul) 2021;84:89-95. [Crossref] [PubMed]
- Choi CM, Kim HC, Jung CY, et al. Report of the Korean Association of Lung Cancer Registry (KALC-R), 2014. Cancer Res Treat 2019;51:1400-10. [Crossref] [PubMed]
- Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36-54. [PubMed]
- Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 2015;20:370-8. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced NSCLC of adenocarcinoma histology. J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Park K, Kim JS, Kim JH, et al. An open-label expanded access program of afatinib in EGFR tyrosine kinase inhibitor-naïve patients. BMC Cancer 2021;21:802. [Crossref] [PubMed]
- de Marinis F, Laktionov KK, Poltoratskiy A, et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer 2021;152:127-34. [Crossref] [PubMed]
- Passaro A, de Marinis F, Tu HY, et al. Afatinib in EGFR TKI-Naïve Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Pooled Analysis of Three Phase IIIb Studies. Front Oncol 2021;11:709877. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Waqar SN, Samson PP, Robinson CG, et al. Non-small-cell Lung Cancer With Brain Metastasis at Presentation. Clin Lung Cancer 2018;19:e373-9. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Sperduto PW, Mesko S, Li J, et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol 2020;38:3773-84. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Ge M, Zhuang Y, Zhou X, et al. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol 2017;135:413-8. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Zhang SR, Zhu LC, Jiang YP, et al. Efficacy of afatinib, an irreversible ErbB family blocker, in the treatment of intracerebral metastases of non-small cell lung cancer in mice. Acta Pharmacol Sin 2017;38:233-40. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol 2018;14:1117-32. [Crossref] [PubMed]
- Tamiya A, Tamiya M, Nishihara T, et al. Cerebrospinal Fluid Penetration Rate and Efficacy of Afatinib in Patients with EGFR Mutation-positive Non-small Cell Lung Cancer with Leptomeningeal Carcinomatosis: A Multicenter Prospective Study. Anticancer Res 2017;37:4177-82. [PubMed]
- Yoon SH, Kim YS, Chung JH, et al. A real-world experience of first-line afatinib in Korean patients with EGFR-mutant non-small cell lung cancer. Ann Oncol 2019;30:IX163. [Crossref]
- Li SH, Liu CY, Hsu PC, et al. Response to afatinib in treatment-naïve patients with advanced mutant epidermal growth factor receptor lung adenocarcinoma with brain metastases. Expert Rev Anticancer Ther 2018;18:81-9. [Crossref] [PubMed]
- Wei YF, Lim CK, Tsai MS, et al. Intracranial Responses to Afatinib at Different Doses in Patients With EGFR-mutated Non-small-cell Lung Carcinoma and Brain Metastases. Clin Lung Cancer 2019;20:e274-83. [Crossref] [PubMed]
- Jung HA, Woo SY, Lee SH, et al. The different central nervous system efficacy among gefitinib, erlotinib and afatinib in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer. Transl Lung Cancer Res 2020;9:1749-58. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Lee SY, Choi CM, Chang YS, et al. Real-world experience of afatinib as first-line therapy for advanced EGFR mutation-positive non-small cell lung cancer in Korea. Transl Lung Cancer Res 2021;10:4353-67. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012;18:4406-14. [Crossref] [PubMed]
- Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 2010;116:1336-43. [Crossref] [PubMed]
- Yoshida K, Kanda S, Shiraishi H, et al. Difference in central nerve system metastasis during gefitinib or erlotinib therapy in patients with EGFR-mutated non-small cell lung cancer: a retrospective study. J Thorac Dis 2019;11:1347-54. [Crossref] [PubMed]
- Xu ST, Xi JJ, Zhong WZ, et al. The Unique Spatial-Temporal Treatment Failure Patterns of Adjuvant Gefitinib Therapy: A Post Hoc Analysis of the ADJUVANT Trial (CTONG 1104). J Thorac Oncol 2019;14:503-12. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244-50. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs 2021;81:257-66. [Crossref] [PubMed]
- Cheng Y, Mok TS, Zhou X, et al. Safety and efficacy of first-line dacomitinib in Asian patients with EGFR mutation-positive non-small cell lung cancer: Results from a randomized, open-label, phase 3 trial (ARCHER 1050). Lung Cancer 2021;154:176-85. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Colclough N, Chen K, Johnström P, et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res 2021;27:189-201. [Crossref] [PubMed]
- Zhang L, Luo Y, Chen J, et al. Efficacy and Safety of Afatinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with EGFR Mutations: A Meta-Analysis of Real-World Evidence. J Oncol 2021;2021:8736288. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Brückl WM, Reck M, Griesinger F, et al. Afatinib as first-line treatment in patients with EGFR-mutated non-small cell lung cancer in routine clinical practice. Ther Adv Med Oncol 2021;13:17588359211012361. [Crossref] [PubMed]
- Su PL, Wu YL, Chang WY, et al. Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer. Ther Adv Med Oncol 2018;10:1758835918797589. [Crossref] [PubMed]
- Kim Y, Lee SH, Ahn JS, et al. Efficacy and Safety of Afatinib for EGFR-mutant NSCLC, Compared with Gefitinib or Erlotinib. Cancer Res Treat 2019;51:502-9. [Crossref] [PubMed]
- Noronha V, Joshi A, Gokarn A, et al. The Importance of Brain Metastasis in EGFR Mutation Positive NSCLC Patients. Chemother Res Pract 2014;2014:856156. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Lee JK, Lee J, Kim S, et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 2017;35:3065-74. [Crossref] [PubMed]
- Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Ther Adv Respir Dis 2015;9:242-50. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Xu Z, Yang Q, Chen X, et al. Clinical associations and prognostic value of site-specific metastases in non-small cell lung cancer. Oncol Lett 2019;17:5590-5600. [Crossref] [PubMed]
- Ma X, Zhu H, Guo H, et al. Risk factors of brain metastasis during the course of EGFR-TKIs therapy for patients with EGFR-mutated advanced lung adenocarcinoma. Oncotarget 2016;7:81906-17. [Crossref] [PubMed]
- Ouyang W, Yu J, Zhou Y, et al. Risk factors of metachronous brain metastasis in patients with EGFR-mutated advanced non-small cell lung cancer. BMC Cancer 2020;20:699. [Crossref] [PubMed]
- Huang CH, Ju JS, Chiu TH, et al. Afatinib treatment in a large real-world cohort of nonsmall cell lung cancer patients with common and uncommon epidermal growth factor receptor mutation. Int J Cancer 2022;150:626-35. [Crossref] [PubMed]
- Li Q, Lin Z, Hong Y, et al. Brain parenchymal and leptomeningeal metastasis in non-small cell lung cancer. Sci Rep 2022;12:22372. [Crossref] [PubMed]
- Kim M, Suh CH, Lee SM, et al. Development of Brain Metastases in Patients With Non-Small Cell Lung Cancer and No Brain Metastases at Initial Staging Evaluation: Cumulative Incidence and Risk Factor Analysis. AJR Am J Roentgenol 2021;217:1184-93. [Crossref] [PubMed]
- Ji Z, Bi N, Wang J, et al. Risk factors for brain metastases in locally advanced non‐small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys 2014;89:330-7. [Crossref] [PubMed]
- Sun DS, Hu LK, Cai Y, et al. A systematic review of risk factors for brain metastases and value of prophylactic cranial irradiation in non-small cell lung cancer. Asian Pac J Cancer Prev 2014;15:1233-9. [Crossref] [PubMed]
- An N, Jing W, Wang H, et al. Risk factors for brain metastases in patients with non-small-cell lung cancer. Cancer Med 2018;7:6357-64. [Crossref] [PubMed]
- He J, Wang X, Xiao R, et al. Risk factors for brain metastases from non-small-cell lung cancer: A protocol for observational study. Medicine (Baltimore) 2021;100:e24724. [Crossref] [PubMed]


