
Concordance between cryobiopsy and forceps biopsy specimens in assessment of immunohistochemistry staining for non-small cell lung carcinoma
Highlight box
Key findings
• Freezing and thawing associated with cryobiopsy had little influence on immunohistochemistry (IHC) results.
What is known and what is new?
• Cryobiopsy allows for the collection of larger volume and higher quality of tissue samples than conventional forceps biopsy, but knowledge of the influence on IHC due to its freezing and thawing process is limited. In the present study, we focused particularly on HER2 and HER3, which are the major targets of antibody-drug conjugates (ADCs), and revealed that the influence on these IHC was little.
What is the implication, and what should change now?
• Cryobiopsy specimens would be potentially ideal for precision medicine and translational research.
Introduction
In the past, cytotoxic chemotherapy was the only treatment for advanced non-small cell lung carcinoma (NSCLC). Now, various kinds of molecular targeted drugs are used in the presence of driver mutations such as epidermal growth factor receptor (EGFR) (1-4), anaplastic lymphoma kinase (ALK) (5-7), ROS proto-oncogene 1 (ROS1) (8), b-raf proto-oncogene (BRAF) (9), mesenchymal epithelial transition factor (MET) (10), ret proto-oncogene (RET) (11), Kirsten rat sarcoma viral oncogene homologue (KRAS) (12), and tumor-agnostic neurotrophic tyrosine receptor kinase (NTRK) (13). In the absence of driver mutations, the first choice of treatment is a single agent immune checkpoint inhibitor (ICI) or its combination of chemotherapy. The expression of programmed death-ligand 1 (PD-L1) in tumors, as assessed by tumor proportion score (TPS), is important as a predictor of the effect of ICIs (14-16). Thus, there is a need for the comprehensive genetic mutation analysis of tumors and assessments of the immune profile to determine the indications for appropriate treatments.
Tissue is essential not only for definitive diagnosis but also for determining the treatment strategy for advanced NSCLC, and bronchoscopy plays an important role in obtaining tissues. Although forceps is the most frequently used biopsy device in bronchoscopy, it has the following problems: (I) only anteriorly located components can be obtained, (II) tissue destruction is likely to occur during pinching, and (III) sample volume is small.
In contrast, recently introduced cryobiopsy has the potential to facilitate the collection of larger volume and higher quality of tissue samples (17). Cryobiopsy requires a cryoprobe, which uses compressed gas that is decompressed within its metallic tip, causing a cooling (Joule-Thomson) effect. As a result, tissue surrounding the tip is frozen and can be extracted. In addition, cryobiopsy allows bronchoscopists to perform an entirely circumferential biopsy of areas in contact with its tip. It has already been reported to be useful for diagnosis of endobronchial tumors and interstitial lung diseases (ILDs) (18,19). Cryobiopsy is also being actively promoted for biopsy of tumors in the lung periphery, and the obtained tissue samples have been reported to be more useful compared to those obtained using forceps in precision medicine for lung cancer (20).
The development and introduction of antibody-drug conjugates (ADCs) have been focused on as a new drug therapy. ADCs consist of three elements: an antibody, a cytotoxic payload, and a linker that connects them. ADCs form complexes with antigens on the cell membrane and are then internalized and translocated to lysosomes, where the ADCs are digested, thereby releasing their payload. The payload is usually a cytotoxic compound such as emtansine, which inhibits tubulin polymerization. In the early stages of their development, chimeric antibodies were used, which were unstable in the blood, resulting in toxicity and low antitumor effects. Thereafter, the use of human monoclonal antibodies has allowed the drug to reach tumor cells more safely and effectively by extending its half-life in the blood and increasing its stability. The anti-human epidermal growth factor receptor 2 (HER2) antibodies, CD30 and CD22 are examples of such antibodies that have been used (21).
Alterations in HER2 gene, including mutation, amplification, and HER2 overexpression, are known to be associated with poor prognoses in lung cancer along with other cancers. In breast and gastrointestinal cancers, HER2-targeted therapies, such as tyrosine kinase inhibitors (TKIs) that bind to intracellular domains and ADCs that bind to extracellular domains, are in clinical use (22). In NSCLC, there have been clinical trials of molecularly targeted drugs for HER2 amplification/mutation, but all of them ended in negative studies (23). Meanwhile, the development of HER2-targeted ADCs is now underway. Phase II clinical trials of trastuzumab deruxtecan, one of the ADCs, showed high efficacy in patients with HER2 mutants and poor efficacy in those with its overexpression evaluated by immunohistochemistry (IHC) 2+/3+ (24,25).
In addition, another target for ADCs is human epidermal growth factor receptor 3 (HER3). HER3 is aberrantly expressed in many cancers, including NSCLC. Accumulated evidences indicate that HER3 plays a crucial role in survival of cancer cells and drug resistances. HER3 expression has been reported to contribute to the TKI resistance in EGFR-mutated NSCLC by maintaining anti-apoptotic HER3/PI3K/AKT signaling (21,26). A phase I clinical trial of patritumab deruxtecan, one of the HER3-targeted ADCs, in patients with or without EGFR-mutated NSCLC demonstrated a good response rate and a disease control rate (27,28).
As aforementioned, to select appropriate drug therapies for NSCLC, it is necessary to investigate various tumor characteristics for each case; there are high expectations for cryobiopsy specimens, which are larger in volume and have minimal tissue destruction. However, the influence of freezing and thawing of tissues when performing cryobiopsy on IHC is still not well understood. Accordingly, cryobiopsy specimens are not currently recognized as a standard for translational research. We therefore aimed to compare the results of IHC assessment in samples obtained by cryobiopsy versus those obtained by conventional forceps biopsy from the same site and in the same procedure. We selected PD-L1 and HER2, which are well-established in NSCLC, as well as HER3 as an exploratory target, for IHC assessment in this study. All procedures were performed by or under the supervision of bronchoscopist experts. We present this article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/rc).
Methods
Patients
This study was approved by the National Cancer Center Institutional Review Board (No. 2019-123). Written informed consent for the clinical procedure and for comprehensive researches using residual specimens were obtained from all eligible patients. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Consecutive patients who underwent diagnostic bronchoscopy with cryobiopsy for peripheral pulmonary lesions (PPLs) at our institution between June 2017 and November 2021 were reviewed retrospectively. Among them, cases of diagnosed unresectable or recurrent NSCLC were selected for the study. Finally, cases were included in the analysis if a sufficient amount of tumor cells, i.e., more than 100 cells, without crush artifacts, were seen in slides prepared from both cryobiopsy and forceps biopsy specimens.
Tissue sampling
The diagnostic bronchoscopy with cryobiopsy was performed as previously described by us (17). All patients underwent bronchoscopy under moderate to deep sedation with a combination of opioids and sedatives compliant with general anesthesia.
One of the following bronchoscopes (P260F, P290, 1T260, or 1TQ290; Olympus, Tokyo, Japan) was inserted through the intubated tracheal tube (Portex® Uncuffed Ivory PVC, Oral/Nasal Tracheal Tube; Smiths Medical, Minneapolis, MN, USA) and deeply wedged near the relevant bronchus, navigated by virtual bronchoscopy. The location of the target lesion was estimated by a radial endobronchial ultrasound under X-ray fluoroscopic guidance, and biopsies were performed 4–6 times using a forceps (1.8-mm FB-15C-1 or 1.9-mm FB-231D; Olympus, Tokyo, Japan). Next, a 1.9-mm flexible cryoprobe was inserted into the same position in combination with ERBECRYO® 2 (Erbe Elektromedizin GmbH, Tübingen, Germany). The sampling position was confirmed by matching the X-ray fluoroscopic image with the virtual fluoroscopic image in up to three directions.
The tip of the cryoprobe was then frozen to approximately −42 ℃ for 3–6 seconds, and the frozen tissue was pulled out along with the bronchoscope. The tip was then thawed in saline solution, and the tissue was detached and fixed in individual formalin bottles. If the bleeding was well controlled, cryobiopsy was performed up to two times.
Tissue staining
Tissue samples were fixed in 10% neutral buffered formalin, and after 24–48 hours, paraffin-embedded blocks were prepared in an ISO15189-certified laboratory. After hematoxylin and eosin (HE) staining, IHC staining was performed on a new set of slides containing 4-µm thick sections using primary antibodies targeting the following proteins: PD-L1 (PD-L1 IHC 22C3 pharmDx, Dako/Agilent, Tokyo, Japan), HER2 [Ventana I-VEW PATHWAY anti-HER-2/new (4B5), Roche, Basel, Switzerland], and HER3 (HER3/ErbB3 (D22C5) XP Rabbit mAb, Cell Signaling Technology, Danvers, the United States). The PD-L1 and HER3 IHC staining were conducted on the Autostainer Link 48 platform (Dako/Agilent, Tokyo, Japan) according to manufacturer’s protocol. The HER2 IHC staining was conducted on VENTANA BENCHMARK XT (Roche, Basel, Switzerland).
Tissue assessment
The following clinicopathological features of the patients were collected from clinical records and pathological reports: age, sex, histology, and TNM stage. The histology of lung cancer was classified according to the 2021 WHO classification for lung tumors.
The protein expression of PD-L1 was measured using a TPS based on the percentage of viable tumor cells exhibiting partial or complete membrane IHC staining on the slide, following the manufacturer’s protocol. The TPS was evaluated in 5% increments and scored at three levels: namely, TPS <1%, TPS 1% to 49%, and TPS ≥50%.
The IHC score for HER2 was determined according to the scoring guidelines for breast cancer. Although there is no established method for assessing HER3, we followed the scoring of HER2 in breast cancer. The expression levels were categorized as 0, 1+, 2+, and 3+, respectively, according to the degree of IHC staining on the membranes of tumor cells.
Tumor cells that were no longer present after thin sectioning were considered for evaluation, in which case the score was set to 0.
The TPS (%) for PD-L1 and the IHC scores (0–3+) for HER2 and HER3 were assessed by a medical doctor (KN) and the results were confirmed by a pathologist (YY). Clinical information was blinded during the assessments.
Statistical analysis
The Pearson’s correlation coefficients were calculated to evaluate the agreement and inconsistency between the TPS results of each biopsy specimen. The closer the value of coefficient of determination (R2) is to 1, the more it is interpreted as a match. The κ coefficient and weighted κ coefficient were used to evaluate the agreement and inconsistency between the IHC results of each biopsy specimen. The κ coefficient of 0.75 or less indicates poor to fair agreement, and the value of greater than 0.75 indicates almost perfect agreement. Statistical significances were set at P values <0.05. Statistical analyses were performed using an SPSS® 28.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
During the study period, there were 603 patients who underwent cryobiopsy following forceps biopsy for PPLs (Figure 1). Of the 487 patients who consented to comprehensive research using residual specimens, 62 were diagnosed with unresectable or recurrent NSCLC. Of these, we excluded cases in which no or few tumor cells were detected, as well as cases in which tumor cells were crushed either in cryobiopsy or forceps biopsy specimens. Three cases were excluded due to few tumor cells in forceps specimens and three cases in cryobiopsy specimens, respectively. Eight cases were excluded due to crush for forceps specimens and no case for cryobiopsy specimens. Finally, 40 patients were eligible for the study.
The patient characteristics and histology are summarized in Table 1. Twenty-four of 40 patients were male (60%). Six patients had stage III disease, 33 had stage IV, and one had recurrent disease. The most frequent histologic type was adenocarcinoma (n=31, 77.5%), followed by NSCLC, not otherwise specified (n=4, 10%), squamous cell carcinoma (n=3, 7.5%), and others (n=2, 5%).
Table 1
| Variable | Value |
|---|---|
| Age in years, median [range] | 64 [39–85] |
| Sex, n (%) | |
| Male | 24 (60.0%) |
| Female | 16 (40.0%) |
| Histology, n (%) | |
| Adenocarcinoma | 29 (72.5%) |
| Squamous cell carcinoma | 3 (7.5%) |
| Non-small cell carcinoma, not otherwise specified | 4 (10.0%) |
| Others | 4 (10.0%) |
| TNM stage, n (%) | |
| III | 6 (15%) |
| IV | 33 (82.5%) |
| Recurrence | 1 (2.5%) |
IHC assessment
The TPS for PD-L1 showed strong correlations between the cryobiopsy and forceps biopsy specimens (R2=0.831, Figure 2). Thirty-four of 40 samples (85%) produced the same results between the two sampling methods (weighted kappa value: 0.835, P<0.001, Table 2). Only six samples produced different results, four scored higher with cryobiopsy and two scored higher with forceps biopsy.
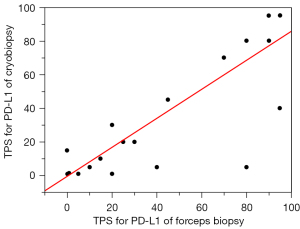
Table 2
| PD-L1 | Cryobiopsy | Weighted kappa value (P value) | |||
|---|---|---|---|---|---|
| <1% | 1–49% | 50–100% | Total | ||
| Forceps biopsy | 0.835 (<0.001) | ||||
| <1% | 13 | 4 | 0 | 17 | |
| 1–49% | 0 | 13 | 0 | 13 | |
| 50–100% | 0 | 2 | 8 | 10 | |
| Total | 13 | 19 | 8 | 40 | |
PD-L1, programmed death-ligand 1.
For HER2, 29 samples (72.5%) produced the same results between the cryobiopsy and forceps biopsy specimens (weighted kappa value: 0.637, P<0.001) (Table 3). Nine samples scored higher with cryobiopsy and two scored higher with forceps biopsy. When the IHC scores were binarized to 0/1+ and 2+/3+, 39 samples (97.5%) showed the same results (weighted kappa value: 0.850, P<0.001), almost a perfect match (Table 4).
Table 3
| HER2 | Cryobiopsy | Weighted kappa value (P value) | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | Total | ||
| Forceps biopsy | 0.637 (<0.001) | |||||
| 0 | 17 | 7 | 0 | 0 | 24 | |
| 1+ | 2 | 12 | 1 | 0 | 15 | |
| 2+ | 0 | 0 | 0 | 1 | 1 | |
| 3+ | 0 | 0 | 0 | 0 | 0 | |
| Total | 19 | 19 | 1 | 1 | 40 | |
HER2, human epidermal growth factor receptor 2.
Table 4
| HER2 | Cryobiopsy | Weighted kappa value (P value) | ||
|---|---|---|---|---|
| 0/1+ | 2+/3+ | Total | ||
| Forceps biopsy | 0.850 (<0.001) | |||
| 0/1+ | 38 | 1 | 39 | |
| 2+/3+ | 0 | 1 | 1 | |
| Total | 38 | 2 | 40 | |
HER2, human epidermal growth factor receptor 2.
For HER3, 30 samples (75%) produced the same results between the cryobiopsy and forceps biopsy specimens (weighted kappa value: 0.697, P<0.001) (Table 5). Six samples scored higher with cryobiopsy and four scored higher with forceps biopsy. When binarized as in HER3, 32 samples (80%) showed the same results (weighted kappa value: 0.545, P<0.001) (Table 6). A representative case with completely concordant results for each IHC is shown in Figure 3.
Table 5
| HER3 | Cryobiopsy | Weighted kappa value (P value) | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | Total | ||
| Forceps biopsy | 0.697 (<0.001) | |||||
| 0 | 2 | 0 | 1 | 0 | 3 | |
| 1+ | 1 | 6 | 4 | 0 | 11 | |
| 2+ | 0 | 3 | 17 | 1 | 21 | |
| 3+ | 0 | 0 | 0 | 5 | 5 | |
| Total | 3 | 9 | 22 | 6 | 40 | |
HER3, human epidermal growth factor receptor 3.
Table 6
| HER3 | Cryobiopsy | Weighted kappa value (P value) | ||
|---|---|---|---|---|
| 0/1+ | 2+/3+ | Total | ||
| Forceps biopsy | 0.545 (<0.001) | |||
| 0/1+ | 9 | 5 | 14 | |
| 2+/3+ | 3 | 23 | 26 | |
| Total | 12 | 28 | 40 | |
HER3, human epidermal growth factor receptor 3.

Discussion
In the present study, we compared the IHC expressions of PD-L1, HER2, and HER3 in cryobiopsy specimens with those in conventional forceps biopsy specimens. We observed fine agreements for PD-L1 and HER2, and a reasonable agreement for HER3, suggesting that the effect of freezing and thawing during cryobiopsy is minimal. Tumors are collections of tumor tissues with heterogeneous characteristics, and they can change over time. While it is easier to use surgical specimens from the same case for comparison, we considered that it was important to particularly compare specimens taken from the same location at the same time in order to derive correct results. Therefore, comparisons between biopsy specimens obtained during the same procedure and from the same site are valuable; although limited number of cases (n=40) were analyzed herein, we were able to draw valuable comparisons. Some previous reports have shown that the IHC expressions of TTF-1, p40, and PD-L1 in cryobiopsy specimens were approximately the same as those in forceps biopsy specimens (20,29). In this study, the results of the TPS for PD-L1 were similar in line these previous studies. These aforementioned results and our findings combined suggest that the effect of freezing during cryobiopsy on the IHC results of PD-L1 is insignificant. However, no study till date has evaluated the IHC results for the expression of HER2 and HER3 in cryobiopsy specimens.
In breast cancer, HER2 gene amplification or HER2 protein overexpression is observed in 15–25% of invasive breast cancers and is evaluated by IHC and/or in situ hybridization (ISH) methods. Among the four expression levels of IHC (i.e., 0, 1+, 2+, and 3+), 0/1+ is considered HER2-negative, 2+ is equivocal, and 3+ is HER2-positive (30). The results of IHC 0/1+ and 3+ are reported to be in good agreement with those of ISH (31). In contrast, for cases of IHC 2+, the frequency of HER2 gene amplification by ISH ranges from 17% to 81%, and trastuzumab is less effective in cases without HER2 gene amplification (31,32). Therefore, ISH is recommended to be performed on the same specimen in cases of IHC 2+. In gastric cancer, IHC 0/1+ is considered HER2-negative, 2+ is equivocal, and 3+ is HER2-positive, as in breast cancer. In cases of IHC 2+, further ISH is performed, and the cases are considered positive if there is HER2 gene amplification (HER2/CEP17 ≥2.0) (33). Meanwhile, it has been reported that there is no correlation between HER2 protein overexpression and HER2 gene mutation/amplification in NSCLC (34). Therefore, the overexpression needs to be assessed separately from the gene alterations, and consequently IHC is expected to play an important role in precision medicine also.
In addition, as aforementioned, HER3 is attracting attention as an acquired resistance mechanism for EGFR-TKIs, and the development of drugs targeting HER3 is underway (35,36). Since there are no clear criteria for assessing the IHC score for HER3 in NSCLC and other carcinomas, we conducted an exploratory evaluation and found concordant results between the cryobiopsy and forceps biopsy specimens. HER3 has been reported to be overexpressed in a wide range of NSCLC, ranging from 7% to 86% (37,38). The HER3 protein overexpression in NSCLC, as well as HER2, is not necessarily caused by HER3 gene amplification (39). Regardless of gene alterations, the expression of HER3 is one of the key targets for ADCs. In the phase I study of patritumab deruxtecan (HER3-DXd, anti-HER3 ADC), patients with locally advanced or metastatic EGFR-mutated NSCLC with prior EGFR-TKI therapy were eligible. Responses were observed in patients with known and unknown EGFR-TKI resistance mechanisms, and it suggested that HER3-DXd could be an approach to a broad range of drug-resistant cancers (40). Therefore, IHC for HER3 may play a key role in screening for those targeted drugs.
One common feature of IHC for PD-L1, HER2, and HER3 is that it assesses the staining on the cell membranes. Of course, accurate assessment requires preservation of cellular structure, and the presence of crush artifacts in tumor cells can have a significant impact on IHC results. In this study, cases that did not contain more than 100 evaluable tumor cells in each specimen were excluded beforehand, and forceps biopsy specimens had to be excluded more often than cryobiopsy specimens, mainly due to crush artifacts. In particular, HER3 can be stained not only in the cell membrane but also in the cytoplasm, but the presence of crush artifacts obscures the boundary between the two, making the determination of IHC staining susceptible. In fact, in the present study, there was a case in which the HER3 results were incorrect owing to a decrease in the number of tumor cells in the forceps biopsy specimen during additional thin sectioning, combined with crush artifacts (Figure 4). Therefore, the results of cryobiopsy specimens might more accurately reflect the characteristics of the tumor in each case.

Furthermore, cryobiopsy, when performed using a 1.9-mm cryoprobe, results in difficulty in maneuverability owing to the thickness and rigidity of the probe. Specifically, there were some cases that had to be excluded because the cryobiopsy probe did not reach the lesion, and the consequent specimen did not contain any tumor cells. Recently, new cryoprobes with smaller diameters of 1.1 and 1.7 mm have been clinically introduced to improve the maneuverability. Despite the smaller diameters, it has been reported that the sample size collected was comparable to that with conventional cryoprobes (41). The improved induction is especially important for PPLs, and the chances of diagnosis of NSCLC made using cryobiopsy specimens is expected to increase (42).
There are several limitations to our study. First, this is a single-center retrospective study with a relatively small number of cases. However, for an accurate comparison of IHC, this small number of cases is still considered to be worthwhile because only a limited number of cases can be obtained from the same location at the same time with a sufficient amount of tumor cells by both cryobiopsy and forceps biopsy. Second, we investigated staining comparability for cryobiopsy versus conventional biopsy specimens against only three different antibodies. The more the types of antibodies to be examined, the greater the volume of tissue samples are needed for the study, but it is often difficult to secure larger samples, especially with conventional forceps biopsy. Meanwhile, the effects of freezing and thawing are expected to remain basically the same even if the type of antibody is changed, and thus the results could be extrapolated to other antibodies.
Conclusions
We found that freezing and thawing associated with cryobiopsy had little influence on IHC results. Cryobiopsy specimens would be potentially ideal for precision medicine and translational research.
Acknowledgments
We thank Toshiko Sakaguchi, Chizu Kina, and Sachiko Miura of Department of Diagnostic Pathology, National Cancer Center Hospital for performing immunohistochemistry staining.
Funding: This work was supported by the National Cancer Center Research and Development Fund (Nos. 29-A-13 and 2020-A-12).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/coif). YO serves as an unpaid editorial board member of Translational Lung Cancer Research from January 2020 to December 2023. YM reports research fund from Hitachi and honoraria from Olympus, AstraZeneca, NOVARTIS, COOK, AMCO, Thermo Fisher Scientific, Erbe Elektromedizin GmbH, Fujifilm, Chugai, Eli Lilly, Merck and Takeda. TI reports grants from Hitachi High-Tech Corporation and honoraria from COOK, Chugai pharma, Eli Lilly, Olympus, Novartis pharma, Fujifilm and Thermo Fisher Scientific K.K. KU reports grants from Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Nos. JP22K15698 JP19K16966), and receives payments for lectures from Novartis, Thermo Fisher Scientific, AstraZeneca and Chugai. TY reports lecture fees from AstraZeneca, Chugai, Ono, Eli Lilly, and Takeda, and research funds from AMGEN, AstraZeneca, Takeda, Daiichi-Sankyo, Ono, MSD, AbbVie, Novartis, Chugai, Novartis, Chugai, Merck, Blueprint, and BMS. YG reports grants from AZK, Pfizer, Abbvie, Eli Lilly, Pfizer, Bristol Myers Squibb, Ono, Novartis, Kyorin, DaiichiSankyo, Novartis, and Prefered Networ, honoraria from Eli Lilly, Chugai,Taiho, Boehringer Ingelheim, Ono, Bristol Myers Squibb, Pfizer, MSD, Novartis. Merck, and Thermo Fischer, participation on an advisory board from AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho, Pfizer, Novartis, Guardant Health Inc., Illumina, DaiichiSankyo, Ono Pharmaceutical, Bristol Myers Squibb, and MSD, and leadership in Cancer Net Japan, JAMT. HH serves as a consultant to AstraZeneca, Eli Lilly, Chugai, Roche, Ono, BMS, and MSD, and receives lecture fees from AstraZeneca, MSD, Eli Lilly Ono, BMS, Chugai, Roche, Kyowa-Kirin, and Novartis, and research funds from MSD, AstraZeneca, BMS, Ono, Merck Biopharma, Daiichi-Sankyo, Janssen, Genomic Health, Chugai, Roche, and Novartis. Yuichiro Ohe receives lecture fees from AstraZeneca and Chugai, and research funds from AstraZeneca, Chugai, Eli Lilly, Ono, BMS, Kyorin, Dainippon-Sumitomo, Pfizer, Taiho, Novartis, Takeda, Kissei, Daiichi-Sankyo, Janssen, and LOXO. YY reports honoraria for lectures from MDS, Chugai-pharma, AstraZeneca, Novartis, Pfizer, Thermo Fisher Science, and Amgen. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by The National Cancer Center’s Institutional Review Board (No. 2019-123). Written informed consent for the clinical procedure and for comprehensive researches using residual specimens were obtained from all eligible patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019;30:1121-6. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET fusion–positive Non–small-cell lung cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–small-cell lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Matsumoto Y, Nakai T, Tanaka M, et al. Diagnostic Outcomes and Safety of Cryobiopsy Added to Conventional Sampling Methods: An Observational Study. Chest 2021;160:1890-901. [Crossref] [PubMed]
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012;39:685-90. [Crossref] [PubMed]
- Maldonado F, Danoff SK, Wells AU, et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases: CHEST Guideline and Expert Panel Report. Chest 2020;157:1030-42. [Crossref] [PubMed]
- Udagawa H, Kirita K, Naito T, et al. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: Prospective single-arm study. Cancer Sci 2020;111:2488-98. [Crossref] [PubMed]
- Yonesaka K. HER2-/HER3-Targeting Antibody—Drug Conjugates for Treating Lung and Colorectal Cancers Resistant to EGFR Inhibitors. Cancers (Basel) 2021;13:1047. [Crossref] [PubMed]
- Wu HX, Zhuo KQ, Wang K. Efficacy of targeted therapy in patients with HER2-positive non-small cell lung cancer: A systematic review and meta-analysis. Br J Clin Pharmacol 2022;88:2019-34. [Crossref] [PubMed]
- Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol 2019;14:1086-94. [Crossref] [PubMed]
- Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2022;386:241-51. [Crossref] [PubMed]
- Nakagawa K, Nagasaka M, Felip E, et al. OA04.05 trastuzumab Deruxtecan in HER2-overexpressing metastatic non-small cell lung cancer: interim results of DESTINY-Lung01. J Thorac Oncol 2021;16:S109-10. [Crossref]
- Yonesaka K, Tanizaki J, Maenishi O, et al. HER3 Augmentation via Blockade of EGFR/AKT Signaling Enhances Anticancer Activity of HER3-Targeting Patritumab Deruxtecan in EGFR-Mutated Non-Small Cell Lung Cancer. Clin Cancer Res 2022;28:390-403. [Crossref] [PubMed]
- Yu HA, Baik CS, Gold K. LBA62 Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER3 directed antibody drug conjugate, in patients (pts) with EGFR-mutated (EGFRm) NSCLC. Ann Oncol 2020;31:S1189-90. [Crossref]
- Uliano J, Corvaja C, Curigliano G, et al. Targeting HER3 for cancer treatment: a new horizon for an old target. ESMO Open 2023;8:100790. [Crossref] [PubMed]
- Nishida T, Matsumoto Y, Sasada S, et al. Feasibility study of cryobiopsy for practical pathological diagnosis of primary lung cancer including immunohistochemical assessment. Jpn J Clin Oncol 2021;51:271-8. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 2004;291:1972-7. [Crossref] [PubMed]
- Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 2005;11:6598-607. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 2014;85:373-8. [Crossref] [PubMed]
- Sequist LV, Gray JE, Harb WA, et al. Randomized Phase II Trial of Seribantumab in Combination with Erlotinib in Patients with EGFR Wild-Type Non-Small Cell Lung Cancer. Oncologist 2019;24:1095-102. [Crossref] [PubMed]
- Meulendijks D, Jacob W, Martinez-Garcia M, et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clin Cancer Res 2016;22:877-85. [Crossref] [PubMed]
- Koutsopoulos AV, Mavroudis D, Dambaki KI, et al. Simultaneous expression of c-erbB-1, c-erbB-2, c-erbB-3 and c-erbB-4 receptors in non-small-cell lung carcinomas: correlation with clinical outcome. Lung Cancer 2007;57:193-200. [Crossref] [PubMed]
- Kumagai T, Tomita Y, Nakatsuka SI, et al. HER3 expression is enhanced during progression of lung adenocarcinoma without EGFR mutation from stage 0 to IA1. Thorac Cancer 2018;9:466-71. [Crossref] [PubMed]
- Manickavasagar T, Yuan W, Carreira S, et al. HER3 expression and MEK activation in non-small-cell lung carcinoma. Lung Cancer Manag 2021;10:LMT48. [Crossref] [PubMed]
- Jänne PA, Baik C, Su WC, et al. Efficacy and safety of Patritumab Deruxtecan (HER3-DXd) in EGFR inhibitor–resistant, EGFR-mutated Non–small cell lung cancer. Cancer Discov 2022;12:74-89. [Crossref] [PubMed]
- Hetzel J, Linzenbold W, Boesmueller H, et al. Evaluation of Efficacy of a New Cryoprobe for Transbronchial Cryobiopsy: A Randomized, Controlled in vivo Animal Study. Respiration 2020;99:248-56. [Crossref] [PubMed]
- Tanaka M, Matsumoto Y, Imabayashi T, et al. Diagnostic value of a new cryoprobe for peripheral pulmonary lesions: a prospective study. BMC Pulm Med 2022;22:226. [Crossref] [PubMed]



