
Patient-led advocacy in ALK-positive lung cancer
Introduction
Background
ALK-positive cancer, an oncogene-driven disease amenable to targeted therapies, remains incurable in many cases
Activation of anaplastic lymphoma kinase (ALK) activity in normally proliferating lung cells is observed in approximately 4–7% of newly diagnosed non-small cell lung cancer (NSCLC) patients annually (1,2). Analogous activation of ALK kinase activity occurs in many other cancers (3-6). Patients with ALK-positive (ALK+) cases of NSCLC often benefit from effective treatments with multiple lines of ALK-targeted tyrosine kinase inhibitors (ALK TKIs) that, in many cases, lead to several years of progression-free survival (PFS) and overall survival (OS) (7,8). Implementation of this targeted precision medicine approach to treating ALK+ oncogene-driven NSCLC is enabled by the diagnosis of tumor-biopsied samples as exhibiting ALK rearrangements (9,10).
In 2005, the five-year survival for lung cancer stood at 16% in the United States (11). The OS for ALK+ patients at that time may have been even lower than that, as retrospective analysis suggests that ALK+ lung cancer is one of the more aggressive forms of LC. The first treatment for advanced ALK+ NSCLC with an ALK TKI began in December 2007 (12). By 2018, the deployment of multiple generations of ALK-targeted TKIs has led to median OS for ALK+ Stage IV LC patients greater than seven years (13). Despite these substantial therapeutic benefits of ALK TKIs for many patients, they do not cure this cancer, and ultimately the ALK TKI therapies become ineffective at inhibiting the ALK+ NSCLC.
To date, personalized tumor vaccines, chemotherapy, radiation, and checkpoint inhibitors have proven to be relatively ineffective treatments for ALK+ cancer (14-17). The tumor microenvironment is thought to contribute to low levels of immune infiltration observed for ALK+ NSCLC (“immune-cold status”) (14). As a result, small molecule ALK TKIs are currently the only category of highly effective therapies for this type of cancer.
Unfortunately, in many instances, oncologists do not implement genomic assessments that would identify ALK-expressing gene fusions in all non-smokers and smokers diagnosed with lung cancer. Such delayed diagnosis of ALK+ cancer often leads to premature administration of chemotherapy treatments, which are less effective than ALK-targeted therapies (8). For example, a recent Cochrane Database review of randomized clinical trials reported that ALK-targeted therapies led to a large increase in progression-free survival compared to chemotherapy, with a hazard ratio (HR) of 0.45 for ALK-targeted therapies vs. chemotherapy (7). Many patients describing their ALK+ diagnosis and treatment histories within the ALK Positive Facebook Support Group have written that delayed diagnosis resulted in premature administration of non-targeted therapies (17), sometimes resulting in adverse consequences such as checkpoint inhibitor-related pneumonitis or radiation pneumonitis (18). Delay in the prescription of ALK-targeted TKIs can be even more consequential for ALK+ patients because initial diagnosis of ALK+ NSCLC usually occurs at Stage IV, based on undetected pre-diagnostic disease spread from the lung into the lymphatics, lung pleura, pericardium, brain, liver, bone and other tissues (19,20).
Despite the transformative benefit of ALK-targeted TKIs in lung cancer, TKI on-target resistance (21-23) and/or TKI-bypass resistance (24,25) eventually eliminate efficacy of all available TKIs, so nearly every patient eventually runs out of TKI options. These inevitable clinical response patterns, including the eventual failure of all available TKI therapies, are motivating efforts to further develop therapeutic options, including successive generation TKI therapies, combination therapies that inhibit TKI-bypass signaling, and immune mobilization therapies that stimulate tumor cell killing, reduction in tumor volumes and elimination of minimal residual disease.
Objectives
Extended TKI-enabled patient survival post-diagnosis, combined with the urgent need for further improvements in quality of life (QOL) and OS for ALK+ LC patients, has enabled and motivated the formation of ALK Positive as a worldwide, patient-led group that advocates for improved treatments, diagnosis, care, and support for ALK+ cancer patients, and their care-partners, as described in the remainder of this historical review. We outlined many of the services and opportunities that our collaborative community has created to improve the quality and quantity of life for ALK+ cancer patients, and to enable and advance the work of care-partners, researchers and clinicians who seek to develop new therapies for ALK+ cancer patients, and to improve and extend the lives of these patients.
Origins and history of ALK Positive
In 2015, a nurse diagnosed with ALK+ lung cancer, and her husband, started a private page on the Facebook platform, named ALK Information Empathy and Support (now named ALK Positive Support Group) (Figure 1). Admission is generally granted by the administrators only to ALK+ cancer patients and their care-partners, conditional upon providing a brief autobiographical introduction.
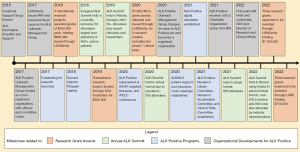
In 2017, some members of that Support Group formed a public-facing organization named the ALK Positive Outreach Management Group (OMG), with the intention of selecting and funding ALK-specific research, as well as advocating for increases in funding for lung cancer research by other organizations, commensurate with deaths per capita funding compared to other types of cancer. Due to stigma connecting lung cancer with smoking (26), as well as a lack of progress in improving patient outcomes for lung cancer for many decades, there have been notable discrepancies in research funding for lung cancer in comparison with other types of cancer (27).
The OMG members elected Officers and selected Committee Chairs for Medical, Legal, Finance, Advocacy and other Committees. OMG was not a registered organization, and could not accept donations that would be allowable as charitable donations. The members of the Board of Directors were all patients or care-partners, as were all the Committee Chairs and volunteers. The board was a “working board”. LUNGevity Foundation (LUNGevity) acted as the fiscal sponsor for the OMG, keeping separate accounts for donations received for ALK Positive, and earmarking funds for grant awards, the Second Opinion Program and other general expenses.
Fundraising was started in late 2017. The Chair of the Medical Committee (MC) provided ongoing promotion within the Facebook Support Group, a large matching donation, and a personal guarantee to LUNGevity, which all helped to boost fundraising to the extent that by the time the Research Review Panel (RRP) was making final selections for the 2018 grant awards, the organization was able to fund the best three proposals submitted, rather than just one. A section on the continued success in awarding research grants can be found below.
The OMG was a virtual organization, and its first in-person gathering was the ALK Summit held in 2018, the largest ever gathering of ALK+ cancer patients up until that time. A section on the history and growth of the summit can be found within this chapter.
In 2018, the Second Opinion Program was started, with the goal of helping patients get advice from doctors familiar with the best and latest treatments for ALK+ patients, because most doctors were, and still are, unfamiliar with treating this less frequent cancer. There is a separate section on this program, below.
The advocacy efforts of the OMG with the American Cancer Society (ACS), culminating in the in-person attendance of the largest ever gathering of lung cancer patients (all ALK patients and care-partners) at the ACS headquarters in Atlanta in 2019, helped lead to a changed stance by the ACS. It shifted its emphasis on lung cancer from “anti-smoking campaigns” to “better treatments” as their organization realized that enormous numbers of lung cancer patients had done nothing to cause their lung cancer and their anti-smoking campaigns led to continued public misconceptions.
ALK Positive has been represented by its board members at, and has presented at, numerous conferences, including the International Association for the Study of Lung Cancer (IASLC) Targeted Therapies of Lung Cancer Meeting, the American Association for Cancer Research (AACR) International Joint Conference, the IASLC World Conference on Lung Cancer, the American Society of Clinical Oncology (ASCO) Annual Meeting, and others.
In 2020, the OMG changed its name to ALK Positive. and became a registered organization with by-laws. It shifted its scope to include all ALK+ cancers, not just lung cancers, and its mission was established “To improve the life expectancy and quality of life for ALK-positive cancer patients worldwide”. New communications avenues were opened with an e-newsletter and Zoom-based ALKtALK webinars.
In 2021, the registered organization received 501(c)(3) status from the IRS (Figure 1). This allowed the organization to seek donations directly rather than through the fiscal sponsorship of other NPOs. The MC created several new subcommittees and greatly expanded its activities and number of volunteers.
In 2022, the organization hired a Chief Executive Officer (CEO) as its first paid employee/contractor to provide strategic direction and manage organizational functions. It then hired a Director of Research and Clinical Affairs (DoRCA) to lead the greatly expanded MC activities. Further revenue growth to expand the activities and achievements of the organization can be expected with the next hire, an Executive Director who will serve also as Development Officer. These hires will also facilitate the gradual transition of our Board of Directors from a “working board” to a “managing board”.
The first audit of ALK Positive has been conducted, and revenues are growing each year, as is interest from academics and industry in working with the organization. Board members and Committee members are from multiple countries around the globe, and “internationalization” of our organization will be a future priority.
There are many other ALK advocacy organizations and support groups forming in many countries and regions around the world [e.g., United Kingdom (28), France, Germany, Italy, Denmark, Australia/New Zealand, India]. These organizations have a more local focus. The leaderships of these organizations work closely with ALK Positive. ALK Positive, although registered in the USA, is the only one of these organizations that we know of that has, at present, worldwide reach and mission.
The ALK Positive organization (Figure 2) and programs have served as an inspiration for other patient-led organizations battling oncogene-driven cancers, for ALK and for other genetic drivers. ALK Positive is happy to share its experiences and knowledge with those organizations, and to collaborate with them where mutually beneficial, to help improve patient outcomes for other cancer populations.
Throughout this chapter we address the evolution of ALK Positive, in order to help other, more newly evolving groups understand our growth process. For clarity, the ALK Positive NPO is an entity entirely separate from the ALK Positive Worldwide Support Group, although they remain very closely affiliated.
The ALK Positive organization continues to be a “virtual organization”, and its future is bright as its many dozens of volunteers work with a growing cadre of compensated professionals to further its mission.
Overview of current ALK Positive organizational structure, committees, and programs
Organizational structure
The ALK Positive bylaws restrict the number of board members to not fewer than eight, and not more than 21, members. A majority of board members must be either an ALK+ patient or care-partner, ensuring the unique qualities and urgency of mission that only a patient-driven organization can generate in such situations. At this time, all board members are volunteers and almost all are ALK+ patients or care-partners (Figure 3).
The board is currently striving to transition from a “working board” into a “managing board”, as board members struggle to balance family time, personal time, careers, health challenges, and a “shortened but unknown life expectancy”, against the burning desire to improve outcomes for ALK+ cancer patients. Volunteering by patients and care-partners, whether it be for the board or a committee, is a difficult balance for members living with (and sometimes dying from) a “terminal cancer”—two young board members have passed away during the last 12 months.
The future will likely see new board members added who are not directly touched by ALK+ cancer. It is hoped these new board members can add new perspectives and expertise in taking the organization into the future, and helping to make the mission ever more achievable.
The board plans to add an Executive Director, in the near future, in addition to the CEO and DoRCA. It is expected that consultants will be used as required, for example for writing grant applications, auditing, payroll, and specialist legal advice.
Committees and programs
A few of the ALK Positive programs and committees are described in the following sections of this chapter.
Much, if not all, of what ALK Positive achieves is through ongoing collaborations with clinicians, researchers, other advocacy organizations, biotech companies and pharma companies. These collaborations have been incredibly helpful in the journey of ALK Positive as it strives to further its impact on patient outcomes.
Programs and research will continue to be the mainstay of ALK Positive, and the more funding ALK Positive can raise for these purposes, the more patient outcomes can be improved. The organization’s goal is to improve patient outcomes as rapidly as possible, which may require tens of millions of dollars. The groundwork is being laid for such expansion. The objective is to remain an organization whose every decision will be guided by the question, “How will this help accelerate our mission to improve life expectancy and quality of life for ALK-positive cancer patients worldwide?”
ALK Positive Medical Committee
In 2017 the ALK Positive OMG created the Medical and Pharmaceutical Advocacy Committee (MPAC). The MPAC was tasked with overseeing ALK Positive initiatives related to patient care, medical research and research funding, and it was renamed the Medical Committee when ALK Positive became registered in 2020.
In 2017, ALK Positive partnered with the lung cancer advocacy organization LUNGevity Foundation to fund and help select a $200,000 grant for research on ALK+ lung cancer.
Volunteer members of the Support Group were selected to serve on a patient/care-partner review panel named the Research Review Panel (RRP), which is discussed later in this chapter.
Evolution
Since its formation in 2017, the MPAC/MC has created, and managed, multiple subcommittees and Patient Support programs, including the ALK Positive Oncologist Registry, ALK Positive Second Opinion Program, the ALK Positive Registry Committee, the Research Acceleration Committee, the Clinical Trials Committee, and the Research Library Committee, which are all described below.
The MPAC, when first formed, consisted of just the five members of the RRP. There are now over 50 volunteers in the MC, with several serving on more than one of the programs and committees, ensuring that each of these are not operating in isolation. Many of the members have had careers in the medical sciences and health industry, while most of the others have had careers in business and entrepreneurism. Interactions and synergies of these committees with academia, industry, other non-profit organizations and patients/care-partners are the foundations of their accomplishments.
Accomplishments, insights, and learnings
The accomplishments of these programs and committees continue to expand rapidly. New programs and committees will undoubtedly be developed over the coming years.
There are limits to the amount of time and expertise that the volunteers possess, especially with the difficulties of surviving with ALK+ cancer. As ALK Positive transitions from being an entirely volunteer-based organization to an organization led by highly qualified professionals, the impact of these programs and subcommittees will expand, and we expect that new doors will open to help achieve ALK Positive’s mission more rapidly. Due to the generosity of donors, ALK Positive has the financial strength to now support employment of a full-time DoRCA, who recently joined ALK Positive. The DoRCA leads the MC, and all its programs and committees.
There have been internal and external discussions about how best to fund, create, or participate in a venture capital fund, or other entrepreneurial enterprise, to rapidly accelerate research advancing the development of improved therapies with the potential to transform ALK+ cancer into a chronic, or even curable, disease.
Oncologist Registry
The Oncologist Registry is a program provided by the MC. Because ALK+ cancer is a less frequent disease with specific therapeutic options, it is important to know where medical teams with expertise in this type of cancer are located. Teams that are well-versed in ALK+ cancer treatment are best positioned to define optimal therapeutic approaches for those patients.
However, the community of oncologists with sufficient expertise in treating ALK+ cancers is relatively small, and often it is not readily accessible for many ALK+ patients. While some patients are willing and able to travel once or twice for a second opinion, many patients cannot travel long distances for regular oncology care. Consequently, the name of a local physician with experience working with ALK+ cancer patients is one of the most requested pieces of information sought by ALK+ LC patients.
As a result of this need, a MC member created a list of providers who have already treated several ALK+ patients and thus have familiarity and expertise with this oncogene-driven cancer—enabling ALK+ patients to transfer their care to one of those providers, or seek a second opinion from them. The provider list will be available to patients/care-partners on the ALK Positive website (Figure 2), and is shared within the ALK Positive Worldwide Support Group.
The list has grown to include over 300 health care providers in more than 25 countries. It is searchable by country, state, city and zip codes, and is updated quarterly. By providing this list, ALK Positive helps ease some of the burden and anxiety that plagues most patients who seek expert care, especially in the early days of treatment planning following the typically unexpected diagnosis of ALK+ LC. Having the care of a team experienced with this cancer, which is sometimes defined as a rare disease, can help to optimize treatment outcomes.
Second Opinion Program (SOP)
The ALK Positive SOP Committee is a subcommittee of the MC that was established in 2018 to administer the SOP. The program is intended to help those patients with ALK+ cancers who do not have the financial resources to obtain a second opinion from leading ALK+ cancer experts. The program was created through the philanthropic support of an ALK Positive member, and the efforts of the ALK Positive MC. The program covers costs for a consultation with an ALK+ cancer expert, so the patient does not need to make a payment for that service (Figure 4).
Patient application forms are available on the ALK Positive website (Figure 2), and are reviewed by the SOP team. As of September 2022, 86 applications have been approved and 39 patients (37 individuals, 2 follow-up requests) have benefited from second opinions through this program, with nearly $30,000 in consultations paid for through the program, to date.
The program is now supported by the generous donations of patients, care-partners, family members, foundations and corporations. The future goals of the program are to increase the number of ALK expert doctors participating in the SOP, both within and outside the United States, and to enable more remote consultations, in order to provide more opportunities for ALK+ cancer patients to personally benefit from the expertise of leading doctors in the field.
Scholarship Fund
The ALK Positive Cancer Scholarship was established in 2020. The husband of a patient who passed from ALK+ cancer learned there were scholarships for children with cancer, but fewer for supporting the children of parent(s) living with, or deceased from, cancer, and that patients and care-partners worry not only about cancer care, but also family finances, as a result of the added costs of their cancer.
He donated $10,000 to launch the ALK Positive Cancer Scholarship Fund. For the 2020–2021 school year, the scholarship fund provided two $5,000 scholarship awards to help student applicants meeting the following criteria:
- A parent (living or deceased) who has been diagnosed with ALK+ lung cancer.
- A currently enrolled student, accepted, or is currently awaiting acceptance into any trade school, two-year college program, or four-year undergraduate college program (graduate programs do not qualify).
Research Library Committee (RLC)
The ALK Positive RLC was created in 2021. Its primary aim has been to create the ALK Positive Research Library, a comprehensive, open, and accessible repository of items related to ALK+ cancer research. The audience includes patients, care-partners, health care professionals, and researchers, and others with an interest in ALK+ cancer.
The Library can be accessed at https://research.alkpositive.org/ on the www.alkpositive.org website (Figure 2). The Library provides a summary of each research item (articles, presentations, videos), along with a link to its original source. To date, there are 1,015 research items “stocking the shelves” of the Library. Typically research from prior to 2019 is not included, with exceptions for seminal material (Figure 5).
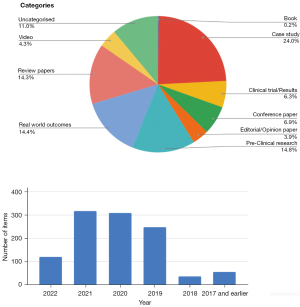
Visitors can search the Library for relevant content using a search box, or by filtering by category (Book; Case Study; Conference Paper; Clinical Trial/Results; Editorial/Opinion; Pre-Clinical; Real World Outcomes and Population Survey; Review Paper; Video) (Figure 5).
The RLC has volunteers residing in countries across the globe. The RLC, and the Library, enable: identification of potential partnership leads for the Research Acceleration Committee; creation of in-house media related to ALK+ cancer research; sharing of research with the ALK+ community through the ALK Positive Newsletter and the ALK Positive Facebook Support Group page, provided with commentary rendering the research more comprehensible to non-expert audiences; sharing the latest ALK+ cancer research on social media to expand the impact of ALK Positive in the wider international community of cancer patients, researchers, and policy makers; creation of a Twitter account that has shared over 220 research items which receive mentions from healthcare professionals and researchers; contribution to the growth in traffic to the ALK Positive website (Figure 2); support of the growth of the international reach of ALK Positive; augmentation of the profile of ALK Positive as an exemplar of patient advocacy among health care professionals and researchers; and development by ALK Positive of a research-focused brand.
A process of searching, recording, reviewing, publishing, and promoting research items is followed to add content to the Library. The RLC does not judge, evaluate, or review research items. Each research item is presented using the information provided by the authors/creators.
The Library content offers hope to patients and care-partners that ALK Positive cancer research will one day transform ALK+ cancer into a chronic disease, or even a curable one, and also offers materials to aid patients in self-advocating for optimal care.
A goal of the RLC is for the Library to provide a “go-to platform of engagement” amongst healthcare professionals, researchers, and ALK+ cancer patients and care-partners.
ALKtALK Education Program and ALKtALK Healing Arts Program
These programs engage ALK+ patients, care-partners and invited speakers from around the globe within a platform that enables participants to interact with each other—to educate, inform, and make connections through Zoom “webinars” (Figure 6).
The ALKtALK Education Program is a series of interactive 60-minute webinars held on Sunday evenings, providing information to ALK+ patients and care-partners about ALK treatment and research. Speakers have included leading health care clinicians and researchers, providing current content in a timely fashion. The ALK+ community is grateful to the experts who share an hour of their time on a Sunday night with as many as 350 live attendees. The ALKtALK meetings are recorded (29).
A typical ALKtALK Education webinar begins with interactive polling questions, proceeds to an expert-led presentation, and concludes with a live chat Q&A session. Attendees often use the information gained from ALKtALK to discuss treatment plans with their own doctors. In its first 18 months, there have been 33 ALKtALKs, with 5,200 live viewers and 15,600 YouTube viewers.
The Healing Arts Program has recruited volunteers to conduct weekly and monthly workshops on several days every week. The goal of the Program is to connect ALK+ patients seeking to balance a productive life, treatment for side effects, and the mental stress of having an ALK+ diagnosis. These classes are free of charge to the ALK+ community and allow patients/care-partners to form friendships and create bonds with others.
Annual ALK Summit
The first ALK Positive Summit was an in-person meeting held in Louisville, Kentucky, in 2018 with about 50 attendees. It was focused on bringing together ALK+ cancer patients who would otherwise typically never meet another ALK+ cancer patient (Figure 7).
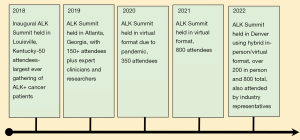
The 2019 Summit was in-person in Atlanta, Georgia, with over 130 attendees. The Summit expanded from simply connecting patients and care-partners with one another, to connecting them with clinicians, researchers, and advocates expert in ALK+ cancer. The agenda expanded to include informational presentations on clinical care and research, as well as breakout sessions for further education and support, and sharing patient stories, while continuing to provide support and community for patients and care-partners worldwide. The overarching theme was that there is hope for ALK+ cancer patients.
The 2022 Summit was convened in Denver, Colorado, as a hybrid conference hosting over 200 in-person attendees, 800+ virtual attendees, and many more viewing recorded sessions on the ALK Positive YouTube Channel. The sessions included a Friday night welcoming reception, a keynote speech on “Hope” by Dr. Ross Camidge; a multi-disciplinary panel with physicians discussing their different specialties and how they work collaboratively; a session on clinical trials; a panel of patients sharing their stories; candid videos that added personal and emotional touches; updates on each of the ALK Positive-funded research projects; a panel discussion by leading researchers sharing possible exciting treatments for the future; education about The White Ribbon Project; sessions with industry partners to answer patient and care-partner questions; an inspiring presentation by Dr. David Carbone on the enormous strides being made in lung cancer treatment; and breakout sessions allowing attendees to speak one-on-one with leading ALK specialists or small group sessions with other patients or care-partners.
The success and growing enthusiasm for this event are due to the hard work of many ALK Positive volunteers and the generous support of industry sponsors, clinicians, researchers, and other advocacy organizations.
Advocacy in the future tense
Patient Registry Committee (RC)
The ALK Positive RC was formed to create an IRB-approved longitudinal survey, and a registry, of ALK+ cancer patients. The RC members are ALK+ patients or care-partners, including MDs, assisted by professionals in registry and survey creation and analysis. The RC will be involved in analysis and dissemination of the data collected, to provide real world evidence (RWE) to support the development of interventions and policy/practice changes to improve patient outcomes. The survey was launched in October 2022.
The goals of this registry are to: follow patients over time to examine factors associated with patient-centered outcomes; understand previous experiences of diagnosis, treatment, and survival of lung cancer; and identify and recruit patients for future research studies.
The data collected include the patient experience at the following timepoints: just before first symptoms; time of LC diagnosis; time of answering the initial survey; every six months thereafter. Information collected will be made available as analyzed data, or de-identified for others’ analysis and use.
Patients and care-partners in the ALK+ cancer community can participate in the Registry. A link enabling ALK+ patients and care-partners to participate in the survey is shared with patients, care-partners, oncologists who treat ALK+ cancer patients, researchers in academic and non-academic institutions, cancer advocacy organizations, and others. A diverse representation of the ALK+ community comprises the RC Advisory Group, who will guide, monitor and support the survey creating the registry.
The RC-created survey instruments address patients’ real-world experiences with ALK+ cancer over time. Patients are asked about their diagnosis, treatment and survival experiences. The information collected is comprehensive and detailed, including scan details, on-target and off-target mutations reported in biopsy results, adverse effects, environmental exposures, pregnancies, and more. Standardized instruments are utilized to gather data about diet, physical activity, and quality of life.
A patient-led survey registry is important for the perception of ownership, and it can encourage members to share their information.
Research Review Committee (RRC)
The ALK Positive RRC is a subcommittee of the MC that makes recommendations to the ALK Positive Board of Directors (BOD) regarding how funds raised by the organization are spent on research (Figure 8). The RRC works with the BOD to form grant-awarding partnerships with other established lung cancer non-profit organizations. It makes recommendations as to what types of research to fund, at what funding levels. Additionally, RRC members form a Research Review Panel (RRP) that participates in the grant review process and recommends to the BOD which specific projects to fund, and to continue funding, if progress is deemed satisfactory.

Funded projects have ranged from early pre-clinical research to clinical trials. ALK Positive research grants thus far have been two-year awards of amounts ranging from $200,000 to $750,000, with a total near $6,000,000 by the end of 2022, at which time there will have been 12 awards made, from a total of about 55 applications from close to 10 countries.
The first RRP was established in early 2018. Five volunteers (all ALK+ patients or care-partners) with scientific grant-review or medical experience were selected by ALK Positive to comprise that RRP. Since then, four more volunteers with career expertise in medicine, oncology research and the pharmaceutical industry have been added to the RRC.
ALK Positive has established research funding partnerships with three well-established non-profit organizations (NPOs) with expertise in reviewing, selecting and funding research: LUNGevity Foundation, GO2 for Lung Cancer, and Lung Cancer Research Foundation (LCRF).
Before ALK Positive became an independent 501(c)(3) non-profit in 2021, these organizations acted as fiscal agents for ALK Positive, accepting fundraising donations specifically for ALK research projects. Now that ALK Positive is a registered non-profit organization, that aspect of the partnerships is no longer necessary, but these organizations continue to provide their valuable expertise in both grant review and grants administration while ALK Positive provides the research grant funds.
The ALK Positive-LCRF partnership differs in that LCRF is doing its own fundraising for ALK-specific projects, and will contribute a portion of the grant funds, with ALK Positive providing the rest.
The RRC now follows a similar process for each round of funding:
- The RRC works with the NPO partner to craft a request for applications (RFA) reflecting the current research priorities of ALK Positive, as determined by the RRC and the BOD. Before the RFA is published, ALK-expert researchers from the NPO scientific advisory board review it for clarity and relevance.
- Each grant applicant submits a letter of intent (LOI) in response to the RFA, with short descriptions of their proposed project aims. The LOIs are reviewed by a panel convened by the non-profit partner, consisting of ALK experts and the RRP members, and the writers of the most promising LOIs are then asked to submit full proposals.
- The full proposals are in an NIH grant application format, and contain much more detail. The same review panel then scores and prepares written reviews of the full proposals, which are collected and collated by the non-profit partner, who also convenes a study section-style online meeting to discuss the reviews.
- After grants are awarded, the NPO receives a written progress report from each grant recipient semiannually. All reports are reviewed by the RRP, as well as by the research leaders at the NPO, and the 12- and 24-month reports are also read by the scientific reviewers. Satisfactory progress triggers the release of the next installment of grant funding to each awardee, after approval by the RRP and the BOD.
- The RRP now has online face-to-face progress report meetings with the funded researchers semiannually. These meetings aid the RRP in understanding the grantees’ research, and have even led to ALK Positive connecting the awardees with biotech companies, or other researchers, to form collaborations to accelerate their progress.
- The awardees present periodic research updates to the ALK+ patients at the ALK Summit and ALKtALKs. The researchers find it meaningful to present directly to the patients impacted by their work, and patients derive hope from learning about research that is progressing and may lead to improved treatments for them in the future.
This process would not be possible without the collaborative efforts of the ALK-expert scientists and biostatisticians, the expert guidance of the research leaders at the LUNGevity, GO2 Foundation, and LCRF, the brilliant researchers submitting the proposals, the efforts of the volunteers in the RRC, and the donors raising the required funds.
ALK Positive has funded, along with partners, 12 scientific projects to date, including three clinical trials. The translational research projects funded thus far range over areas including new and redeployed small molecule therapies, adaptive and innate immunity, immunological therapies, genetics and epigenetics of therapeutic resistance and metabolomics. The funded clinical trials are either yet to commence, or still in progress, so we do not yet know whether the therapies they address will have beneficial impacts on patient outcomes. Information and videos about the projects that have been funded are available on the ALK Positive website (Figure 2), under the Research-Research Grants tab.
Research Acceleration Committee (RAC)
The RAC is a subgroup of the ALK Positive MC, formed in 2021, and tasked with identifying the most promising ALK research and accelerating its journey to the clinic, with a focus on second- and later-line therapies (Figure 9).
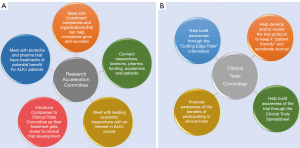
The RAC works with Key Opinion Leaders (KOLs), academic centers, pharma and biotechnology companies to advocate for the advancement of promising pre-clinical research and help unblock impediments to progress. The RAC leverages its network of academic institutions, KOLs, biotechnology companies, and pharmaceutical companies to make valuable connections between parties that share common research interests. The RAC also interfaces frequently with biotechnology and pharmaceutical companies to understand whether therapeutics approved for other indications may be suitable for ALK+ cancers, and advocates for further inclusivity of ALK+ cancer as a focus area for upcoming trials and pre-clinical research.
The RAC was created with the recognition of the need for greater investments in new approaches that have the potential to be more truly transformational for ALK+ patients than those ALK Positive could fund directly. While ALK+ lung cancers have several very good first-line therapeutic options available, none are yet considered curative, which creates a large opportunity for meaningful therapeutic progress.
The committee now has over 15 members, all patients or care-partners. The diverse group is composed of entrepreneurs, investors in biotechnology, and several PhDs in relevant fields such as immunology, biochemistry, and molecular biology, all of whom provide crucial expertise when vetting research and opportunities.
The RAC initially focused on developing relationships with KOLs, hosting several roundtables to understand their perspectives on the most promising therapeutic options that were underexplored. With an understanding of how to prioritize, the RAC then set about connecting with over 50 different biotechnology and pharmaceutical companies to facilitate and enable collaborative research leading to novel clinical trials. To date, the RAC has facilitated several important collaborations between academic researchers, and biotechnology companies. The RAC has also formed collaborative relationships with key academic research institutions studying ALK+ cancers, and has helped several institutions connect with biotechnology and pharmaceutical companies that have the capacity to provide support, such as drugs for preclinical testing or potential investigator-initiated trials.
The MC has several other subcommittees, described in other sections. Once the RAC identifies initiatives that are ready for a clinical trial, the Clinical Trials Committee will step in and provide suggestions for trial design based on their expertise. The RAC also interfaces with the RRP once they have made their project selections for grant funding. If a particular project or KOL requires assistance, the RAC is often able to lend support in the form of connections with other domain experts, materials, or introductions to biotechnology companies that can serve as manufacturing partners or investment partners.
The RAC is exploring innovative models for helping accelerate by many multiples the amount of research that can benefit ALK+ cancer patients.
Clinical Trials Committee (CTC)
The CTC was established as a subcommittee of the MC in early 2021 (Figure 9). The purpose of the CTC is to facilitate accrual of ALK+ cancer patients in clinical trials that may benefit them, which in turn can accelerate approval of new treatments for ALK+ cancer patients by the FDA and other regulatory bodies.
The CTC functions to achieve these purposes in multiple ways:
- Ideally the members of the CTC meet with the company executives or Principal Investigators before the trial protocol is developed. A major obstacle for many trials is lack of timely enrollment, i.e., participant accrual. The CTC has the experience to review the trial protocol to ensure that ALK+ patients are not excluded unnecessarily, and to help ensure other obstacles to patient participation are avoided, such as inconvenient locations, challenging washout periods, excessive requirements for biopsies, blood tests, and office visits, and added costs on top of normal treatment. The CTC has met with innumerable companies and has always been well-received and had productive meetings.
- Promote awareness of the benefits of participating in clinical trials to ALK+ patients. This is done through presentations within ALKtALKs, in the ALK Positive Newsletter, and in articles on the ALK Positive website (Figure 2).
- Provide a comprehensive and searchable listing of almost all clinical trials that allow participation by ALK+ cancer patients. This listing is available on the ALK Positive website under “Clinical Trials” in the Research tab (30).
- Provide patient-friendly information about “cutting-edge trials” that involve new treatment modalities such as oncolytic viruses, TIL therapy, fourth generation ALK TKIs, antibody-drug conjugates, combining ALK TKI with inhibitors of other pathways, tumor-treating fields, and others. There are now more than 10 such articles available, under “Cutting Edge Trials” under the Research tab (31).
The goal is to help, not hinder, the process of getting new treatments through to regulatory approval, and also to improve access for ALK+ patients to trials at any stage of their treatment, whether it be early during their treatment journey, or after “standard options” are exhausted.
The members of the CTC are all volunteers, and include a retired genetics and biochemistry professor, an immunology PhD, a clinical oncologist, successful entrepreneurs, senior veterans of “big pharma” in oncology, MDs, and passionate advocates.
If a company or institution is considering conducting a clinical trial that does not exclude ALK+ cancer patients, it could benefit by seeking the input from the CTC team, and the increased awareness of its trial that the CTC can provide to the ALK+ community. The CTC focuses on working with industry and principal investigators (PIs) to assist their trials in making it through the registration processes of the relevant regulatory bodies, when appropriate, because their success in developing better treatments enhances survival of ALK+ cancer patients.
Conclusions
Patient advocacy in support of improved patient outcomes and therapeutic advances
ALK Positive has grown rapidly into a 501(c)(3) organization dedicated to improving outcomes for ALK+ cancer patients worldwide. Collaborations among patients, care-partners, clinicians and researchers have led to the creation of numerous programs and opportunities for education, community-building, and interpersonal, clinical and therapeutic support for ALK+ patients, their families and friends, and the clinicians who meet and treat these patients. Our aspirations include improving quality of life and care for ALK+ cancer patients through patient support and education programs, and the broader application of existing effective treatments through more widespread and earlier diagnosis, and improved clinician awareness. We intend to expand our impact on the development of new therapies for ALK+ cancers through increased funding of research, development and early-stage clinical trials that enable registration of new TKIs, new treatments for bypass resistance and immunotherapies that reduce and ideally eliminate primary, metastatic and minimal residual disease—with the hope of transitioning ALK+ cancers into chronic and ultimately curable diseases. We intend to evolve ALK Positive into a global organization with more effective worldwide impact by continuing and expanding our outreach to and collaboration with patients, care-partners, researchers and clinicians around the world.
Notable challenges
Our review is a historical narrative as remembered and provided by the authors. As such, our review summarizes a history that could be subject to recall bias and presentation bias. Nonetheless, we have striven to be accurate and objective in reporting our collective history. We anticipate the Patient Registry project will provide more comprehensive RWE regarding challenges in diagnosis and treatment for ALK+ cancers and enable further improvements in patient quality of life. The research projects we and other agencies fund will expand therapeutic options, including expansion of TKI options and treatment of TKI resistance, and we anticipate that immunotherapies will be required to ultimately convert ALK+ cancers into chronic and then curable diseases through elimination of minimal residual disease. Finally, ALK Positive is committed to defining strategies to increase the reach and impact of our work to an even more robust global scale in the future.
Acknowledgments
We have benefited from the thoughtful comments on the manuscript provided by Dr. Ken Culver, Director of Research and Clinical Affairs, ALK Positive. We express our appreciation to all the patients, care-partners, clinicians, clinician-scientists, and members of the lung cancer advocacy organizations and biotechnology and pharmaceutical communities who have worked continuously to improve the life expectancy and quality of life for ALK+ cancer patients worldwide. We acknowledge the many contributions of time, talent, enthusiasm and financial resources by the volunteers and donors who have contributed to the evolution, growth and work of ALK Positive, since its beginning as the ALK Information Empathy and Support Facebook platform. We gratefully acknowledge the lives and contributions of ALK+ cancer patients who are still striving to live with the disease, as well as patients who have passed away from a disease we hope to transform, someday, into one that is chronic, and ultimately curable.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jessica J. Lin and Justin F. Gainor) for the series “ALK-Positive NSCLC” published in Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-713/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-713/coif). The series “ALK-Positive NSCLC” was commissioned by the editorial office without any funding or sponsorship. CB reports payments received from Genentech and Pfizer, and participates on advisory boards of University of Michigan, Judy Tam ALK Lung Cancer Initiative and ALK Positive Medical Committee. SHF reports payments or honoraria received from Nuvalent, Daiichi Sankyo, Inc. and ASCO Advantage, support from American Lung Association, Lung Force Advocacy Day Hero, and participates on board for ALK Positive, and University of Pennsylvania Telehealth Research Center of Excellence-Penn Trace Advisory Board. AMK is the founding member of and participates on board of ALK Positive Europe. DM participates as Accounting, Fundraising and Second Opinion Program roles for ALK Positive, and holds stock or stock options of Johnson & Johnson. NP participates on Board of Directors for ALK Positive. CV received monetary support for attending ASCO 2022 conference as a patient advocate, and participates on Research Acceleration Committee of ALK Positive. ESV is a member of the ALK Survey Working Group of the Lung Cancer Registry, created by GO2 for Lung Cancer, and is a member of the ALK Positive. Medical Committee. MATM received consulting fees from Summation Bio Inc., Black Diamond Therapeutics, and Civetta Therapeutics, and reports pending patent applications (Epigenetics and protein production, and Sequences and gene therapy), participated on the Scientific Advisory Board of Summation Bio Inc. and participates on the Medical Committee and the Board of Directors of ALK Positive. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaw AT, Engelman JA. ALK in lung cancer: Past, present and future. J Clin Oncol 2013;31:1105-11. [Crossref] [PubMed]
- Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613-38. [Crossref] [PubMed]
- Ross JS, Ali SM, Fasan O, et al. ALK Fusions in a Wide Variety of Tumor Types Respond to Anti-ALK Targeted Therapy. Oncologist 2017;22:1444-50. [Crossref] [PubMed]
- Lin E, Li L, Guan Y, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res 2009;7:1466-76. [Crossref] [PubMed]
- Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007;110:2259-67. [Crossref] [PubMed]
- Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 1999;59:2776-80. [PubMed]
- Cameron LB, Hitchen N, Chandran E, et al. Targeted therapy for advanced anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. Cochrane Database Syst Rev 2022;1:CD013453. [PubMed]
- Chazan G, Solomon BJ. Optimal first-line treatment for metastatic ALK+ non-small cell lung cancer. Transl Lung Cancer Res 2023;12:369-78. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- McLeer-Florin A, Duruisseaux M, Pinsolle J, et al. ALK fusion variants detection by targeted RNA-next generation sequencing and clinical responses to crizotinib in ALK-positive non-small cell lung cancer. Lung Cancer 2018;116:15-24. [Crossref] [PubMed]
- Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med 2005;172:523-9. [Crossref] [PubMed]
- Sahu A, Prabhash K, Noronha V, et al. Crizotinib: A comprehensive review. South Asian J Cancer 2013;2:91-7. [Crossref] [PubMed]
- Peled N, Gillis R, Kilickap S, et al. GLASS: Global Lorlatinib for ALK(+) and ROS1(+) retrospective Study: real world data of 123 NSCLC patients. Lung Cancer 2020;148:48-54. [Crossref] [PubMed]
- Voena C, Menotti M, Mastini C, et al. Efficacy of a Cancer Vaccine against ALK-Rearranged Lung Tumors. Cancer Immunol Res 2015;3:1333-43. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Schenk EL. Narrative review: immunotherapy in anaplastic lymphoma kinase (ALK)+ lung cancer-current status and future directions. Transl Lung Cancer Res 2023;12:322-36. [Crossref] [PubMed]
- Sadik H, Pritchard D, Keeling DM, et al. Impact of Clinical Practice Gaps on the Implementation of Personalized Medicine in Advanced Non-Small-Cell Lung Cancer. JCO Precis Oncol 2022;6:e2200246. [Crossref] [PubMed]
- Cheng J, Pan Y, Huang W, et al. Differentiation between immune checkpoint inhibitor-related and radiation pneumonitis in lung cancer by CT radiomics and machine learning. Med Phys 2022;49:1547-58. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Kimura H, Nakajima T, Takeuchi K, et al. ALK fusion gene positive lung cancer and 3 cases treated with an inhibitor for ALK kinase activity. Lung Cancer 2012;75:66-72. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Patcas A, Chis AF, Militaru CF, et al. An insight into lung cancer: a comprehensive review exploring ALK TKI and mechanisms of resistance. Bosn J Basic Med Sci 2022;22:1-13. [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Fukui T, Tachihara M, Nagano T, et al. Review of Therapeutic Strategies for Anaplastic Lymphoma Kinase-Rearranged Non-Small Cell Lung Cancer. Cancers (Basel) 2022;14:1184. [Crossref] [PubMed]
- Kamath SD, Kircher SM, Benson AB. Comparison of Cancer Burden and Nonprofit Organization Funding Reveals Disparities in Funding Across Cancer Types. J Natl Compr Canc Netw 2019;17:849-54. [Crossref] [PubMed]
- Trasta A. Where does public funding for cancer research go: Allocation of research funding for cancer and COPD is not always proportional to disease burden. EMBO Rep 2018;19:e45859. [Crossref] [PubMed]
- Abbott J, Beattie K, Montague D. The Role of UK Oncogene-Focussed Patient Groups in Supporting and Educating Patients with Oncogene-Driven NSCLC: Results from a Patient-Devised Survey. Oncol Ther 2021;9:187-93. [Crossref] [PubMed]
- Available online: https://www.youtube.com/channel/ UCZPMeuzQGj-9uATmotZ5b8w
- Available online: https://docs.google.com/ spreadsheets/d/1Tk2Q8Mygxi4jBnp7V1__f4HBKMFEkfy/edit?fbclid=IwAR1wnjCWnAxAwV lk01R-lx7bFhbWcCDgbjOaZK_0Man4FLf_ vpgtwGQ8gVg#gid=660468034
- Available online: https://www.alkpositive.org/cuttingedge-clinical-trials




