
Five-year follow-up after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer: a multicenter study
Highlight box
Key findings
• SBRT achieved excellent LC and minimal toxicity in patients with early-stage NSCLC.
What is known and what is new?
• SBRT achieved high rates of long-term tumor control and low toxicity for patients with early-stage NSCLC.
• This study offered robust long-term outcome data of SBRT in a Chinese population, which was very rarely reported in China before.
What is the implication, and what should change now?
• Long-term data regarding the outcomes of SABR are needed to strengthen confidence in its popularization and application.
Introduction
Lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer death around the world (1). Non-small cell lung cancer (NSCLC) represents approximately 85% of all lung cancer cases (2). For patients with medically inoperable early-stage NSCLC, stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy (SABR), has been the standard treatment (3,4). Many prospective and retrospective studies have shown that SBRT provides promising clinical outcomes for patients with inoperable and operable early-stage NSCLC (5-8). For example, the landmark Radiation Therapy Oncology Group (RTOG) 0236 trial (9,10) reported 3- and 5-year local control (LC) rates of 91% and 80%, respectively. The rapid and widespread adoption of SBRT in clinical practice has been driven by its scheduling convenience, tolerability, and high rates of primary tumor control. As the incidence of lung cancer is still high worldwide and SBRT has proven to be effective, understanding the long-term clinical outcomes for SBRT is becoming more imperative as well.
With a robust median follow-up of more than 5 years’ period, we conducted a retrospective study to examine the effectiveness and adverse effects of SBRT for patients with medically inoperable NSCLC being treated in three major Chinese hospital systems. This is one of the most comprehensive reviews to date of the clinical outcome, failure pattern, and adverse effects of SBRT in a Chinese cohort. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-180/rc).
Methods
Patients
A total of 145 early-stage NSCLC patients who received SBRT at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Shandong Cancer Hospital and Institute, and Shanghai Pulmonary Hospital between October 26, 2012, and March 14, 2019 were included in this analysis. Patients who were unable or refused to undergo a surgical operation after multidisciplinary discussion received SBRT. Patients who had American Joint Committee on Cancer (AJCC) clinical stage I or II T1–3N0M0 NSCLC, based on either radiographic evaluation or biopsy confirmed by pathology, were included. Patients who did not have biopsy results ought to fulfill these criteria: (I) patients should have positive radiological features of malignant tumor. For instance, the lesions enlarged progressively, the ground-glass opacity (GGO) increased in the density or proportion, or the vascular perforation and spiculation signs appeared at the edge in contrast-enhanced CT or 1–3 mm thin-section CT; (II) patients had positive signs in positron emission tomography/computed tomography (PET/CT); (III) multidisciplinary team of surgeons, oncologists and radiologists agreed therapy reached an agreement. Table 1 provides the specific information about the enrolled patients. The study was performed following the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Shanghai Pulmonary Hospital (No. L20-320Y) and the other hospitals were informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Table 1
| Characteristics | Value |
|---|---|
| Age (years) | |
| Mean | 72 |
| Median [range] | 74 [50–88] |
| Sex, n (%) | |
| Male | 107 (73.8) |
| Female | 38 (26.2) |
| ECOG performance status, n (%) | |
| 0 | 33 (22.8) |
| 1 | 105 (72.4) |
| 2 | 7 (4.8) |
| Lesion size (cm) | |
| Mean | 2.2 |
| Median [range] | 2.00 [0.50–5.16] |
| Tumor location, n (%) | |
| Peripheral | 140 (96.6) |
| Central | 5 (3.4) |
| Biopsy of primary lesion, n (%) | 94 (64.8) |
| Histologic diagnosis, n (%) | |
| Adenocarcinoma | 49 (52.1) |
| Squamous cell carcinoma | 28 (29.8) |
| NSCLC NOS | 17 (18.1) |
| Undifferentiated | 1 (1.1) |
| Smoking status, n (%) | |
| Past or current smoker | 88 (60.7) |
| Never smoker | 57 (39.3) |
| SBRT dose (BED), n (%) | |
| 50 Gy/5 fractions (100.0 Gy) | 71 (49.0) |
| 50 Gy/4 fractions (112.5 Gy) | 26 (17.9) |
| 60 Gy/10 fractions (96.0 Gy) | 19 (13.1) |
| 60 Gy/8 fractions (105.0 Gy) | 8 (5.5) |
| 70 Gy/10 fractions (119.0 Gy) | 6 (4.1) |
| 55 Gy/5 fractions (115.5 Gy) | 3 (2.1) |
| 60 Gy/6 fractions (120.0 Gy) | 3 (2.1) |
| 48 Gy/4 fractions (105.6 Gy) | 1 (0.7) |
| Median follow-up (95% CI) (months) | 65.6 (63.0–68.2) |
| GOLD, n (%) | |
| 0 | 56 (38.6) |
| I | 21 (14.5) |
| II | 54 (37.2) |
| III | 13 (9.0) |
| IV | 1 (0.7) |
| Median FEV1 [range] (%predicted) | 61 [24–139] |
| Median FEV1 [range] (L) | 1.19 [0.50–3.27] |
| Charlson comorbidity index ≥6, n (%) | 4 (2.8) |
| Chronic pulmonary disease, n (%) | |
| Yes | 41 (28.3) |
| No | 104 (71.7) |
| LR, n (%) | 9 (6.2) |
| RR, n (%) | 12 (8.3) |
| DM, n (%) | 21 (14.5) |
| Disease progression or death, n (%) | 53 (36.6) |
| Death, n (%) | 40 (27.6) |
| Fractions, n (%) | |
| <8 | 111 (76.6) |
| ≥8 | 34 (23.4) |
ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; SBRT, stereotactic body radiotherapy; BED, biologically effective dose; CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Diseases stage; FEV1, forced expiratory volume in 1 second; LR, local recurrence; RR, regional recurrence; DM, distant metastasis.
Treatment methods
Patient immobilization was achieved with a vacuum cushion device. Four-dimensional CT (4D-CT) simulations were performed on all patients. The internal target volume (ITV) was generated by depicting the gross tumor volume (GTV) on the maximum intensity projection (MIP) images and consistently modified according to ten phases of the respiratory cycle to take account of the trajectory of tumor motion. Planning target volume (PTV) was generated by adding a circumferential margin around the ITV of about 5 mm. Dose calculation was performed on the average intensity projection. According to institutional practices, treatment plans were optimized to limit high doses and protect organs at risk, such as the healthy lung, trachea, proximal bronchial tree, spinal cord, esophagus, great vessels, heart, and chest wall. The prescribed isodose line met the condition of a >95% coverage of the PTV and 100% of the ITV. Cone beam CT was used immediately before each treatment fraction.
Follow-up
Regular follow-up examinations at our institutions were encouraged for all patients. The follow-up frequency and the patient visits were scheduled as follows: the patient was followed up approximately every 3 months for 2 years after SBRT, then every 6 months for 3 years, and every 12 months thereafter. Follow-up was measured from the first day of diagnosis until the last follow-up death. Local recurrence (LR) was defined as a recurrence or new lesion confirmed by PET/CT or biopsy in the same lobe on CT. LR was classified as an in-field recurrence (occurring within the area inside the PTV), involved lobe failure (out-of-field relapse in the same lobe), or marginal failure (disease recurrence occurring within 1 cm of the PTV in any direction). Regional recurrence (RR) was defined as any intrathoracic lymph node relapse outside of the PTV. Distant metastasis (DM) was defined as any failure in a different lobe or any disease recurrence outside the chest.
Statistical analysis
Progression-free survival (PFS) as the primary endpoint was defined as the time from the beginning date of SBRT treatment to the date of any new recurrence or death. Secondary endpoints included overall survival (OS) and treatment-related toxic effects. OS was defined as the time from the beginning date of SBRT treatment to the date of death from any cause or last follow-up. The National Cancer Institute’s Common Toxicity Criteria, version 3.0, was used for grading of adverse events (AEs). Survival was analyzed by the Kaplan-Meier method. Univariate and multivariate Cox regression proportional hazards models were employed, with hazard ratios (HRs) and 95% confidence intervals (CIs), to assess potential correlations between the observed factors and OS. A P value of <0.05 was regarded as statistically significant. Statistical analyses were carried out with SPSS software (version 26.0, IBM Corp., Armonk, NY, USA) and GraphPad Prism software (version 8.4.0, GraphPad Software, Inc., San Diego, CA, USA).
Results
Patient characteristics
A total of 145 patients were deemed eligible for analysis. The patients and treatment characteristics are shown in Table 1. The median age of the patient group was 74 (range, 50–88) years, with 107 (73.8%) patients being men. The average tumor size was 2.0 (range, 0.5–5.2) cm. Among the 94 (64.8%) patients who underwent biopsy, most lesions 49/94 (52%) were adenocarcinoma, 28/94 (30%) were squamous cell carcinoma, and 17/94 (18%) were not otherwise specified (NOS). Additionally, 138 (95.2%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 at the time of SBRT treatments. After the first SBRT treatment, the median follow-up period was 65.6 (range, 2.4–99.2) months.
Patterns of failure and survival
Table 2 summarizes the clinical outcomes of the patients. A total of 35 (24.1%) patients developed disease progression of any type, and 9 (6.2%), 12 (8.3%), and 21 (14.5%) patients experienced local, regional, and distant recurrences, respectively. Cumulative rates of LR, RR, and DM over a 3-year period were 5.1%, 7.4%, and 13.2%, respectively. The corresponding rates of incidence over a 5-year period were 9.6%, 9.8%, and 15.8%, respectively (Figure 1A, Table 2). The estimated 3- and 5-year PFS rates were 69.2% and 60.5%, respectively, while the estimated 3- and 5-year OS rates were 78.1% and 70.1%, respectively (Figure 1B, Table 2). As shown in Tables 3,4, in multivariate analyses with the Cox proportional hazards models, age, ECOG status, pulmonary function, and chronic pulmonary disease status were not associated with LC or OS.
Table 2
| Event | Estimated cumulative incidence (95% CI) (%) | ||
|---|---|---|---|
| 1-year | 3-year | 5-year | |
| LR | 1.5 (0.0–3.6) | 5.1 (1.3–8.8) | 9.6 (3.7–15.5) |
| RR | 5.0 (1.1–8.9) | 7.4 (3.5–11.3) | 9.8 (3.9–15.7) |
| DM | 7.9 (4.0–11.8) | 13.2 (9.2–14.8) | 15.8 (9.9–21.7) |
| PFS | 82.8 (76.9–88.6) | 69.2 (61.4–77.1) | 60.5 (50.7–70.3) |
| OS | 88.3 (82.4–94.2) | 78.1 (72.2–84.0) | 70.1 (62.3–78.0) |
CI, confidence interval; LR, local recurrence; RR, regional recurrence; DM, distant metastasis; PFS, progression-free survival; OS, overall survival.
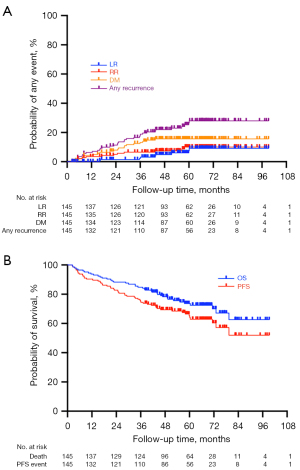
Table 3
| Clinical factors | UVA | MVA | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 1.574 (1.045–2.369) | 0.030 | 1.107 (0.630–1.946) | 0.722 | |
| T size (cm; continuous) | 0.854 (0.679–1.074) | 0.177 | 0.851 (0.676–1.071) | 0.169 | |
| Age (continuous) | 0.998 (0.978–1.019) | 0.871 | – | – | |
| Smoking status (never vs. past or current) | 1.529 (1.071–2.183) | 0.020 | 1.463 (0.901–2.375) | 0.124 | |
| Chronic pulmonary disease (no vs. yes) | 1.094 (0.747–1.601) | 0.644 | – | – | |
| BED (<110 vs. ≥110) | 0.972 (0.673–1.405) | 0.882 | – | – | |
| Fractions (<8 vs. ≥8) | 1.029 (0.677–1.564) | 0.894 | – | – | |
| ECOG performance score (continuous) | 1.245 (0.869–1.786) | 0.233 | – | – | |
| DLCO% (continuous) | 1.000 (0.997–1.003) | 0.865 | – | – | |
| FEV1% (continuous) | 0.996 (0.991–1.001) | 0.126 | 0.996 (0.991–1.001) | 0.164 | |
UVA, univariate analysis; MVA, multivariate analysis; HR, hazard ratio; CI, confidence interval; BED, biologically effective dose; ECOG, Eastern Cooperative Oncology Group; DLCO, diffusion lung capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second.
Table 4
| Clinical factors | UVA | MVA | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 2.340 (0.981–5.583) | 0.055 | 0.990 (0.293–3.345) | 0.987 | |
| T size (cm; continuous) | 1.081 (0.754–1.552) | 0.671 | – | – | |
| Age (continuous) | 1.033 (0.993–1.075) | 0.108 | 1.026 (0.984–1.070) | 0.227 | |
| Smoking status (never vs. past or current) | 3.412 (1.444–6.838) | 0.004 | 2.842 (0.957–8.444) | 0.060 | |
| Chronic pulmonary disease (no vs. yes) | 0.730 (0.347–1.534) | 0.406 | – | – | |
| BED (<110 vs. ≥110) | 0.463 (0.205–1.048) | 0.065 | 0.449 (0.197–1.020) | 0.056 | |
| Fractions (<8 vs. ≥8) | 1.713 (0.883–3.324) | 0.111 | 1.428 (0.732–2.788) | 0.296 | |
| ECOG performance score (continuous) | 1.660 (0.868–3.176) | 0.125 | 1.472 (0.689–3.145) | 0.319 | |
| DLCO% (continuous) | 1.002 (0.997–1.008) | 0.391 | – | – | |
| FEV1% (continuous) | 0.998 (0.990–1.007) | 0.725 | – | – | |
UVA, univariate analysis; MVA, multivariate analysis; OS, overall survival; HR, hazard ratio; CI, confidence interval; BED, biologically effective dose; ECOG, Eastern Cooperative Oncology Group; DLCO, diffusion lung capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second.
Toxicity
Toxicity was generally mild in these patients after SBRT. The most common side effects were pneumonitis, cough, and fatigue. Majority of patients had asymptomatic changes in follow-up imaging. Grade 3 or greater adverse effects are displayed in Table 5. Five patients (3.4%) experienced grade 3 treatment-related AEs (two patients with radiation pneumonitis, one with cough, and two with chest wall pain). No patient experienced grade 4 or 5 toxicity.
Table 5
| Adverse effect | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) |
|---|---|---|---|
| Pneumonitis/pulmonary fibrosis | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Fatigue | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chest wall pain | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| Fracture | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Esophagitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
SBRT, stereotactic body radiotherapy.
Discussion
In this large retrospective cohort of inoperable early NSCLC treated with SBRT, we found excellent benefit with low incidences of LR, RR, and DM at 5 years (9.6%, 9.8%, and 15.8%, respectively) with tolerable levels of toxicity. In multivariate analysis, no significant association between any variable and OS was observed.
In our study, the median PFS of patients was 65.6 (95% CI: 61.2–70.0) months, and the 3- and 5-year PFS rates were 69.2% and 60.5%, respectively. The median OS was 65.6 (95% CI: 63.0–68.2) months, and the 3- and 5-year OS rates were 78.1% and 70.1%, respectively. The clinical outcomes in our study are comparable to those reported after surgical treatment (11,12). A pooled analysis conducted by Chang et al. (12), of 2 randomized studies (that closed early due to poor accrual), evaluate 31 and 27 patients with medically operable early-stage NSCLC and randomly assigned to SBRT and lobectomy, respectively. The pooled results suggested that SBRT was comparable to lobectomy in terms of PFS, with improved OS at 3 years, and a lower toxicity profile. The lower rates of toxicity profiles by SBRT, compared to surgery, may contribute to the prolonged survival observed in the SBRT group. The STARS investigators extended the trial to a single-arm SBRT trial and compared it with published longitudinal follow-up data for patients with stage IA NSCLC after lobectomy and mediastinal lymph node dissection. They found long-term survival after SABR is non-inferior to lobectomy combined with mediastinal lymph node dissection (6). Currently, large randomized clinical trials with long-term follow-up are needed to determine whether SBRT should replace lobectomy in operable patients. Besides, SBRT is a recognized treatment option for older patients with physically reduced lung function or cardiac comorbidities. In our multivariate analysis, age, ECOG performance status, pulmonary function, and chronic pulmonary disease were not associated with LC and OS. Thus, these finding support the use of SBRT in such older population.
The ideal dosing regimen to optimize LC and minimize toxicity has also been an area of research focus. RTOG 0915 was a completed phase 2 trial that compared 48 Gy/4 fractions vs. 34 Gy/1 fraction for peripheral lesions in patients with medically inoperable NSCLC; the results showed similar primary tumor control rates at 5 years and a median survival time of 4 years for each arm, but a lower rate of ≥ grade 3 toxicity and distant failure in the 34/1 arm (13). In our study using a multi fractionated regimen, the 3-year LR of 5.1% is consistent with the results of previous prospective and retrospective studies, which range from 4% to 14% (Table 6) (8-10,12,14-17). We found that, through administration of a biologically effective dose (BED) of 96–120 Gy, a different dose does not affect the clinical efficacy; moreover, the fraction of treatment was not significantly correlated with LC and OS (Tables 3,4). In our study, the incidence of ≥ grade 3 radiational pneumonitis was 3.4% at the end of follow-up; in comparison, RTOG 0236 reported incidences of adverse effects ≥ grade 3 at 3 and 5 years of 16.3% and 30.9%, respectively. In the RTOG 0236 and RTOG 0618 trials (7,9,10), no grade 5 treatment-related AEs were reported. According to our findings and those published in the literature, the adverse effects after SBRT treatment appear tolerable. Moreover, the question if SBRT should be delivered in a single or multi fractionation remains unanswered.
Table 6
| Study | Total no. of patients/lesions | Median follow-up (months) | Prescribed (dose/fractions) | BED | LR (%) | RR (%) | DM (%) | PFS (%) | OS (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | |||||||||
| Retrospective series | ||||||||||||||||||
| von Reibnitz, 2018 (14) | 398 | 24.4 | 45–60 Gy/3–5 F | 112.5–132.0 | 13.6 | – | – | – | – | – | – | – | 53.1 | – | ||||
| Karasawa, 2018 (15) | 56 | – | 48 Gy/4 F | 105.6 | – | 24.6 | – | – | – | – | – | – | – | 44.6 | ||||
| Prospective series | – | – | – | – | – | – | – | – | – | – | ||||||||
| Timmerman, 2010 (9) | 59 | 34.4 | 54 Gy/3 F | 151.2 | 9.4 | – | 12.8† | – | 22.1 | – | – | – | 55.8 | – | ||||
| Timmerman, 2018 (10) | 55 | 48.0 | 54 Gy/3 F | 151.2 | – | 20 | – | 10.9 | – | 23.6 | – | – | – | 40.0 | ||||
| Chang, 2015 (12) | 31 | 40.2 | 54 Gy/3 F, 50 Gy/4 F, 60 Gy/5 F | 112.5–151.2 | 4.0 | – | 10.0 | – | 3.0 | – | 86.0 | – | 95.0 | – | ||||
| Sun, 2017 (16) | 65 | 86.2 | 50 Gy/4 F | 112.5 | 4.6 | 8.1 | 9.2 | 10.9 | 9.2 | 11.0 | 64.6 | 49.5 | 70.8 | – | ||||
| Ball, 2019 (8) | 66 | – | 54 Gy/3 F, 48 Gy/4 F | 105.6–151.2 | – | – | – | – | – | – | – | – | – | – | ||||
| Nyman, 2016 (17) | 49 | 37.0 | 66 Gy/3 F | 211.2 | – | – | – | – | – | – | 53.0 | 42.0 | 68.0 | 54.0 | ||||
†, locoregional failure rate. NSCLC, non-small cell lung cancer; SBRT, stereotactic body radiotherapy; BED, biologically effective dose; LR, local recurrence; RR, regional recurrence; DM, distant metastasis; PFS, progression-free survival; OS, overall survival; F, fraction.
In line with Timmerman et al. (10) and others (18,19), DM and RR was the predominant type of recurrence. Sun et al. (16) reported that about one-third of the patients who developed DM or regional nodal recurrence within 6 months that was thought to potentially have arisen from an occult tumor. The adjuvant treatment of chemotherapy, or especially immunotherapy, might effectively manage occult metastases and therefore improve patients’ prognoses. This is currently being investigated in ongoing prospective clinical trials such as PACIFIC4. Emerging clinical evidence suggests that there might be a synergistic effect of SBRT and immunotherapy (20), though better data are needed. Recently, a combination of SABR and an immunotherapy (referred to as “ISABR”) has been proposed that may be a promising approach to manage both overt and occult disease and further improve the benefit of SABR in patients with early-stage NSCLC (16,21-23). Moreover, when combined with concurrent or following immune-checkpoint inhibitors, SBRT may exert systemic antitumor activity beyond the target volume in metastatic NSCLC, which is known as the “abscopal effect” (21,24), though this remains controversial.
This study, reporting that SBRT achieved high rates of tumor control and low toxicity for patients with early-stage NSCLC, offered robust long-term outcome data of SBRT in a Chinese population, which was rarely reported in China before. Indeed, the report of long-term data regarding the outcomes of SABR in our study would help to strengthen to some degree the confidence in its popularization and application in China.
Although the SBRT produced satisfactory oncological outcomes and minimal toxicity in a large cohort study, it is important to highlight some study limitations related to the retrospective design. First, selection bias could have been present in the enrollment of patients. Second, a typical design for follow-up frequency with chest CT is 3 months after completion of treatment, but because this was not a prospective protocol, the actual follow-up schedule and studies varied across patients. Third, due to the relatively small sample size, we did not analyze the differences of failure patterns based on the histology subtype. We will collect a larger cohort of patients than in this study to broaden this analysis in the future. Finally, the SBRT schedule was used according to the radiation oncologist’s discretion. But even with these problems, the results seen here are consistent and give us important information about how well SBRT works on the Chinese population.
Conclusions
Our report represents a big data multicenter study using SBRT for early-stage NSCLC in a Chinese context. Our study revealed excellent outcomes for SBRT with high OS and PFS, along with low rates of LR, RR, and DM at 5 years. Moreover, the toxicity rates were low and highly tolerable, with most of the patients developing late radiographic changes but without serious impairments of lung function over time.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by the Start-Up Fund for Talent Introduction of Shanghai Pulmonary Hospital (No. 201801 to YX) and the Science Research Foundation of China Ministry of Health-Zhejiang Medicine & Health Key Research Fund (No. 201339868 to YX).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-180/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-180/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-180/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-180/coif). MTM reports that he received a speaker fee from Astra Zeneca and royalties from Wolters Kluwer. TTS reports that he provides strategic and scientific recommendations as a member of the advisory board and speaker for Novocure, Inc. and also as a member of the advisory board to Galera Therapeutics and Catalyst Pharmaceuticals, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed following the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Shanghai Pulmonary Hospital (No. L20-320Y) and the other hospitals were informed and agreed with this study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol 2019;37:1316-25. [Crossref] [PubMed]
- Chang JY, Mehran RJ, Feng L, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol 2021;22:1448-57. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Yuan XS, Chen WC, Lin QR, et al. A propensity-matched analysis of stereotactic body radiotherapy and sublobar resection for stage I non-small cell lung cancer in patients at high risk for lobectomy: the results in a Chinese population. J Thorac Dis 2021;13:1822-32. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Videtic GM, Paulus R, Singh AK, et al. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2019;103:1077-84. [Crossref] [PubMed]
- von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC). Acta Oncol 2018;57:1567-73. [Crossref] [PubMed]
- Karasawa K, Hayakawa S, Machitori Y, et al. Accelerated Hypofractionated Radiotherapy Versus Stereotactic Body Radiotherapy for the Treatment of Stage I Nonsmall Cell Lung Cancer-A Single Institution Experience With Long-Term Follow-Up. Technol Cancer Res Treat 2018;17:1533033818806318. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031-9. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JÅ, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Andratschke N, Zimmermann F, Boehm E, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol 2011;101:245-9. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 2015;21:3727-39. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516-24. [Crossref] [PubMed]
- Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm 2012;27:12-22. [Crossref] [PubMed]
(English Language Editor: J. Gray)


