
Anatomic and clinical implications of venous drainage variations in superior segment resections for clinical T1N0 non-small cell lung cancer
Highlight box
Key findings
• V6b primarily consists of critical intersegmental veins such as V6b2, located between the S6 and S9 segments, and V6b3, located between the S6 and S8 segments. Prioritizing V6b for adequate surgical margins and reducing aggressiveness through early detection may enhance outcomes in superior segmentectomies for clinical T1N0 non-small cell lung cancer (NSCLC).
What is known and what is new?
• Challenges persist in patients undergoing superior segmentectomies for cT1N0 NSCLC despite preoperative planning with three-dimensional computed tomography bronchography and angiography (3D-CTBA) and margin simulation techniques. Anatomy and drainage patterns of the complexly distributed V6b have been seldom focused upon.
• V6b was observed to have a high prevalence with minimal variation in drainage patterns. Focusing on V6b can lead to optimal surgical margins and outcomes.
What is the implication, and what should change now?
• When determining the surgical strategy for superior segmentectomy, we should emphasize anatomy and drainage patterns of V6b by utilizing 3D-CTBA to ensure sufficient surgical margins and favorable surgical outcomes.
Introduction
Lung cancer remains the most frequently diagnosed cancer worldwide and the leading cause of cancer-related mortality (1). The widespread adoption of low-dose chest computed tomography (CT) has significantly enhanced the detection rates of early-stage non-small cell lung cancer (NSCLC) (2,3). Segmentectomy, which conserves more pulmonary tissue and offers outcomes comparable to lobectomy, has emerged as a vital surgical option for treating early NSCLC (4,5).
The application of three-dimensional computed tomography bronchography and angiography (3D-CTBA), along with advancements in surgical techniques, has enabled surgeons to perform segmentectomies efficiently with favorable outcomes (6-8). However, challenges persist, particularly insufficient margins, poorer disease-free survival (DFS) and overall survival (OS) in patients undergoing superior segmentectomies for cT1N0 NSCLC, despite preoperative planning with 3D-CTBA and margin simulation techniques (9,10). Studies by Jones et al. have indicated that the margin/diameter ratio consistently falls below 100% in bilateral superior segmentectomies, which is less favorable compared to other segmentectomies involving the right upper lung, left upper division, or basal segments (9).
The inflation-deflation method and indocyanine green (ICG) fluorescence imaging-guided technique are commonly employed to identify the intersegmental plane (ISP) (11). However, their application is limited due to the time-consuming nature and high demands on surgeons and anesthesiologists. Furthermore, the ISP between the superior segment (S6) and the basal segment is non-linear, resulting in potential residual S6 parenchyma or injury to the basal segment if a linear resection is attempted using staplers guided by anatomical landmarks (12). Moreover, Sarsam et al. reported that the segmental venous volume of S6 exceeds the segmental bronchial and arterial volumes, suggesting that using the bronchus rather than veins to determine the ISP may lead to insufficient margins and venous congestion in the border zone (13). Nonetheless, anatomy and drainage patterns of the complexly distributed superior segmental veins have been seldom focused upon.
In clinical practice, we have observed that among the numerous venous branches responsible for drainage in the superior segment area, those originating from superior segmental vein (V6) are particularly complex and cover a broad drainage area. Drawing on prior literature (14,15) and our center’s clinical experience (16), we have made the initial attempt to define V6b as a type of intrasegmental vein running along the intersubsegmental plane between S6b and S6c (V6b1), and as intersegmental veins running along the ISP between S6b and S9a (V6b2) and between S6b and S8a (V6b3). V6b2 and V6b3 extend transversely across multiple adjacent segments, serving as intersegmental boundaries between S6 and S9, and S6 and S8, respectively (16). The limited research into their anatomical features and clinical implications has attracted our interest. Thus, the term “V6b” in this study refers specifically to V6b2 and V6b3, unless otherwise specified. Both preoperative planning and intraoperative procedures at our center emphasize the significance of V6b. Preoperatively, when a 2-cm simulative cutting margin involves V6b, selecting an extended segmentectomy or combined segmentectomy is deemed more feasible. Intraoperatively, dissecting V6b2 and V6b3 assists in accurately identifying the ISP, ensuring the complete excision of the target segment.
Therefore, the objective of this project is to explore the prevalence and drainage patterns of V6b utilizing 3D-CTBA, and to assess the surgical margins and outcomes specifically for S6 resections performed according to the established protocol of our center. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-807/rc).
Methods
Study cohort
The study, conducted in line with the Declaration of Helsinki (as revised in 2013), received approval from the Ethics Review Board of Jiangsu Province Hospital and The First Affiliated Hospital of Nanjing Medical University (No. 2019-SR-450). Consent was waived for this retrospective analysis.
Our surgical team at Jiangsu Province Hospital and The First Affiliated Hospital of Nanjing Medical University now performs over 2000 pulmonary operations annually. All surgical procedures are collaboratively planned by two senior thoracic surgeons using clinical data and 3D-CTBA imaging before surgery. In principle, all preoperatively planned segmentectomies must ensure that the surgical margin—the distance from the lesion to the intersegmental boundaries defined by the segmental veins—is at least
2 cm. Additionally, the assessment of V6b anatomical features within this study was also performed by two experienced surgeons, ensuring a consistent and expert evaluation of crucial surgical details. The high volume of surgeries and uniform standards for surgical planning provide a robust foundation for our research.
Clinical data of patients with cT1N0 NSCLC who underwent video-assisted thoracic surgery (VATS) from August 2020 to August 2021 were retrospectively analyzed. These patients met the following inclusion criteria: (I) possession of complete DICOM data of chest CT before hospitalization; (II) diagnosis of cT1N0 NSCLC; (III) undergoing lobectomy or sublobectomy.
To calculate the prevalence and drainage patterns of V6b, the exclusion criteria for the primary cohort included: (I) patients with unsatisfactory 2D- or 3D-CTBA imaging; (II) patients with incomplete clinical information. Thus, the primary cohort includes 521 patients (Figure 1).
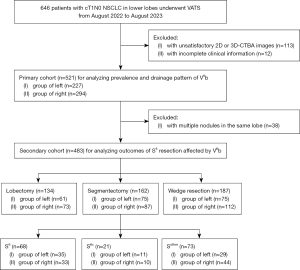
To analyze the outcomes of superior segmentectomies, the exclusion criteria for the secondary cohort included: (I) patients with multiple lesions in the same lobe; (II) the same exclusion criteria as the primary cohort. Consequently, the secondary cohort consists of 483 patients (Figure 1).
3D reconstruction
The CT images were transformed into 3D-CTBA images using the “InferVision” software (17). The software automatically generated the lesion and its associated 2 cm simulative cutting margin. This margin took the form of a quasi-sphere, resembling the lesion’s shape, and was uniformly expanded by 2 cm from the lesion’s outer surface.
Specific measurement method of consolidation-to-tumor ratio (CTR)
The CTR of lesions, as a predictor of postoperative stage and outcomes in cT1N0 NSCLC, was determined using thin-slice CT in the lung window (18). Consolidation was identified as an area of increased opacification that fully obscured underlying vascular structures, whereas ground-glass opacity (GGO) was recognized by a hazy density that preserved the visibility of the vascular structure beneath. The CTR was calculated as the ratio of the maximum consolidation size to the maximum tumor dimension (19).
Statistical analysis
Statistical analysis was conducted using the R platform (Version 4.3.3). Continuous variables were reported as either mean ± standard deviation (SD) or medians with interquartile ranges (IQRs), and categorical variables were presented as number (%). A two-sided P value of less than 0.05 was considered statistically significant.
Differences in probabilities were evaluated using the Z-test. Categorical variables were analyzed with the Chi-squared test or Fisher’s exact test, depending on the appropriateness for the sample size. Differences in continuous variables between groups were assessed using Student’s t-test, while disparities across multiple groups were examined using the Kruskal-Wallis H-test.
DFS was defined as the time from surgery to recurrence or death, and OS was the time from surgery to death. Both were estimated using the Kaplan-Meier method, and differences between segmentectomy locations were analyzed with the log-rank test.
Results
Prevalence and branching patterns of V6b of the primary cohort
A total of 521 patients were included in the primary cohort (Figure 1).
In the bilateral lower lobes, the prevalence of V6b1 was uniformly 100% (521/521). For V6b2, the prevalence was 91.2% (475/521) overall, varying significantly between the right (94.9%, 279/294) and left (86.3%, 196/227) lower lobes (P<0.001). Similarly, V6b3 showed an overall prevalence of 66.2% (345/521), with higher prevalence on the right (78.6%, 231/294) compared to the left (50.2%, 114/227) (P<0.001) (Table 1).
Table 1
| Branches | Prevalence | branching patterns | |||||
|---|---|---|---|---|---|---|---|
| Bilateral | Right | Left | V6a/c | CBV | IPV | ||
| V6b1 | 100% (521/521) | 100% (294/294) | 100% (227/227) | 100% (521/521) | 0% (0/521) | 0% (0/521) | |
| V6b2 | 91.2% (475/521) | 94.9% (279/294) | 86.3% (196/227) | 91.8% (436/475) | 7.6% (36/475) | 0.6% (3/475) | |
| V6b3 | 66.2% (345/521) | 78.6% (231/294) | 50.2% (114/227) | 93.0% (321/345) | 6.4% (22/345) | 0.6% (2/345) | |
V6, superior segmental vein; CBV, common basal vein; IPV, inferior pulmonary vein.
There was little variation in the drainage patterns of V6b into the inferior pulmonary vein (IPV). All V6b1 converged with other branches of V6 before draining into the IPV. For V6b2, in 436 cases, it converged with other branches of V6; in 36 cases, it converged with the common basal vein (CBV); and in 3 cases, it drained directly into the IPV. Similarly, V6b3 converged with other branches of V6 in 321 cases, with the CBV in 22 cases, and directly into the IPV in 2 cases (Table 1) (Figure 2A-2C).

The factor influencing the prevalence of V6b: the relative sizes among the segments
Based on extensive analysis of 3D-CTBA images, we observed that the relative interlobar distribution sizes of the segments within the V6b drainage areas, specifically S6, lateral basal segment (S9), and anterior basal segment (S8), differ among patients. This variability correlates significantly with the prevalence of V6b (P<0.001), as confirmed by Chi-Square testing (Table 2). The typical 3D-CTBA diagrams are shown in Figure 2D-2H. Our findings indicate that variations in the sizes of S6, S9, and S8 significantly influence the prevalence of V6b:
- In S8 size-dominant (S8+) and S6 size-dominant (S6+) type, S6b connects continuously with S9a and S8a, facilitating the presence of intersegmental veins V6b2 and V6b3 (Figure 2D,2G). In the S8+ type, the prevalence rates of V6b2 and V6b3 are both 95.2%. Similarly, in the S6+ type, the prevalence rates for V6b2 and V6b3 are 92.2% and 70.1%, respectively.
- In S9 size-dominant (S9+) type, a discontinuity exists between S6b and S8a, with S6b only connecting with S9a, which is typically associated with the presence of V6b2 alone (100% vs. 28.1%) (Figure 2E).
- S8 size-disadvantaged (S8−) type, often resulting from varying degrees of compression by S10 on S8 and S9, lead to possible continuity or discontinuity between S6b with S9a and S8a. Given S8’s more medial position, both the presence and absence of V6b2 and V6b3 are plausible, with a greater likelihood of V6b3 absence (86.8% vs. 38.6%) (Figure 2H).
- In S9 size-disadvantaged (S9−) type, where S9 is compressed by S8 and S10, appearing smaller and lower, S6b connects only with S8a, often resulting in the presence of V6b3 while V6b2 is absent (100% vs. 17.6%) (Figure 2F).
Table 2
| Branches | The relative sizes among the segments of the lower lobes (n=521) | χ2 | P | ||||
|---|---|---|---|---|---|---|---|
| S9+ (n=96) | S9− (n=17) | S8+ (n=210) | S8− (n=121) | S6+ (n=77) | |||
| V6b2 | 96 (100%) | 3 (17.6%) | 200 (95.2%) | 105 (86.8%) | 71 (92.2%) | 53.69 | <0.001 |
| V6b3 | 27 (28.1%) | 17 (100%) | 200 (95.2%) | 47 (38.8%) | 54 (70.1%) | ||
V6, superior segmental vein; S9+, S9 size-dominant; S9−, S9 size-disadvantaged; S8+, S8 size-dominant; S8−, S8 size-disadvantaged; S6+, S6 size-dominant.
Patients and clinical characteristics of the secondary cohort
A total of 483 patients were included in the secondary cohort (Figure 1), of whom 134 underwent lobectomy, 162 underwent segmentectomy, and 187 underwent wedge resection. The segmentectomy group was further categorized into 3 subgroups, including 68 patients underwent resection of mono S6 (S6 group), 21 patients underwent combined resection of S6 or mono subsegment of S6 plus adjacent segment or subsegment (S6c group, such as resection of S6+8a or S6b+8a), and 73 patients underwent other segmentectomies and subsegmentectomies not involving S6 (Sother group, such as resection of S8 or basal segment). The demographic and clinical characteristics of these patients are detailed in Table 3.
Table 3
| Variables | Left surgery location | Right surgery location | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S6 | S6c | Sother | Llobe | Lwedge | S6 | S6c | Sother | Llobe | Lwedge | |||
| Number | 35 | 11 | 29 | 61 | 75 | 33 | 10 | 44 | 73 | 112 | ||
| Age, years | 61.5±6.8 | 58.3±8.1 | 59.2±6.8 | 61.2±8.5 | 62.1±8.4 | 60.4±7.5 | 61.5±9.8 | 62.9±8.6 | 62.7±8.0 | 60.6±7.5 | 0.27 | |
| Sex | 0.97 | |||||||||||
| Male | 11 (31.4) | 4 (36.4) | 10 (34.5) | 25 (41.0) | 24 (32.0) | 12 (36.4) | 3 (30.0) | 15 (34.1) | 29 (39.7) | 36 (32.1) | ||
| Female | 24 (68.6) | 7 (63.6) | 19 (65.5) | 36 (59.0) | 51 (68.0) | 21 (63.6) | 7 (70.0) | 29 (65.9) | 44 (60.3) | 76 (67.9) | ||
| Smoking history | 0.81 | |||||||||||
| Never | 19 (54.3) | 6 (54.5) | 15 (51.7) | 29 (47.5) | 47 (62.7) | 18 (54.5) | 5 (50.0) | 25 (56.8) | 35 (47.9) | 65 (58.0) | ||
| Ever | 16 (45.7) | 5 (45.5) | 14 (48.3) | 32 (52.5) | 28 (37.3) | 15 (45.5) | 5 (50.0) | 19 (43.2) | 38 (52.1) | 47 (42.0) | ||
| Pulmonary comorbidity | 0.49 | |||||||||||
| Never | 25 (71.4) | 7 (63.6) | 22 (75.9) | 37 (60.7) | 56 (74.7) | 24 (72.7) | 8 (80.0) | 34 (77.3) | 45 (61.6) | 81 (72.3) | ||
| Ever | 10 (28.6) | 4 (36.4) | 7 (24.1) | 24 (39.3) | 19 (25.3) | 9 (27.3) | 2 (20.0) | 10 (22.7) | 28 (38.4) | 31 (27.7) | ||
| Cardiac comorbidity | 0.08 | |||||||||||
| Never | 15 (42.9) | 4 (36.4) | 11 (37.9) | 20 (32.8) | 39 (52.0) | 12 (36.4) | 4 (40.0) | 17 (38.6) | 23 (31.5) | 60 (53.6) | ||
| Ever | 20 (57.1) | 7 (63.6) | 18 (62.1) | 41 (67.2) | 36 (48.0) | 21 (63.6) | 6 (60.0) | 27 (61.4) | 50 (68.5) | 52 (46.4) | ||
| Tumor size, mm | 11.18±3.58 | 10.78±2.09 | 11.95±4.32 | 19.94±6.18 | 9.65±4.61 | 13.25±5.20 | 11.20±3.08 | 11.53±3.14 | 19.42±6.10 | 9.33±3.90 | <0.001 | |
| CTR | <0.001 | |||||||||||
| ≤50% | 25 (71.4) | 8 (72.7) | 20 (69.0) | 10 (16.4) | 62 (82.7) | 23 (69.7) | 7 (70.0) | 34 (77.3) | 19 (26.0) | 75 (67.0) | ||
| >50% | 10 (28.6) | 3 (27.3) | 9 (31.0) | 51 (83.6) | 13 (17.3) | 10 (30.3) | 3 (30.0) | 10 (22.7) | 54 (74.0) | 37 (33.0) | ||
Data are presented as mean ± standard deviation or n (%). S6, resection of mono S6; S6c, combined resection of S6 or mono subsegment of S6 plus adjacent segment or subsegment, such as resection of S6+8a or S6b+8a; Sother, other segmentectomies and subsegmentectomies not involving S6, such as resection of S8 or basal segment; Llobe, lobectomy of lower lobes; Lwedge, wedge resection of lower lobes; CTR, consolidation tumor ratio.
Among these groups subdivided by bilateral lower lobes, variables such as age, sex, smoking history, pulmonary comorbidity, and cardiac comorbidity did not demonstrate statistical significance (P=0.27; P=0.97; P=0.81; P=0.49; P=0.08). The largest tumor sizes were observed in the lobectomy group (19.94±6.18 mm on the left and 19.42±6.10 mm on the right), whereas the smallest were noted in the wedge resection group (9.65±4.61 mm on the left and 9.33±3.90 mm on the right) (P<0.001). Similarly, within the lobectomy group, lesions with a CTR exceeding 50% were significantly more prevalent than those in the other sublobar resection groups, suggesting a higher degree of lesion invasiveness (P<0.001).
Potential factors influencing the outcomes of superior segmentectomies: tumor size, pathology, stage, and surgical margin
Within the segmentectomy group, lesion diameters did not vary significantly among the S6 group (11.18±3.58 mm on the left and 13.25±5.20 mm on the right), the S6c group (10.78±2.09 mm on the left and 11.20±3.08 mm on the right), and the Sother group (11.95±4.32 mm on the left and 11.53±3.14 mm on the right) (P=0.50) (Table 4). Consistently, no significant differences were observed in the CTR between the 3 subgroups within the segmentectomy group (P=0.97) (Table 4).
Table 4
| Variables | Left segmentectomy location | Right segmentectomy location | P | |||||
|---|---|---|---|---|---|---|---|---|
| S6 | S6c | Sother | S6 | S6c | Sother | |||
| Number | 35 | 11 | 29 | 33 | 10 | 44 | ||
| Histology | 0.17 | |||||||
| Adenocarcinoma | 33 (94.3) | 11 (100) | 21 (72.4) | 29 (87.9) | 8 (80.0) | 41 (93.2) | ||
| Squamous cell carcinoma | 0 (0) | 0 (0) | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Other | 2 (5.7) | 0 (0) | 7 (24.1) | 4 (12.1) | 2 (20.0) | 3 (6.8) | ||
| LUAD predominant subtype | 0.24 | |||||||
| Solid/micropapillary | 2 (6.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Other | 31 (93.9) | 11 (100) | 21 (100) | 29 (100) | 8 (100) | 41 (100) | ||
| Pathologic nodal status | >0.99 | |||||||
| N0 | 35 (100) | 11 (100) | 29 (100) | 33 (100) | 10 (100) | 44 (100) | ||
| N1/2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Pathologic TNM stage (IASLC9th) | 0.81 | |||||||
| 0 (AIS) | 9 (27.3) | 2 (18.2) | 5 (22.7) | 5 (17.2) | 1 (12.5) | 6 (14.6) | ||
| I | 24 (72.7) | 9 (81.8) | 17 (77.3) | 24 (82.8) | 7 (87.5) | 35 (85.4) | ||
| II–IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| CTR | 0.97 | |||||||
| ≤50% | 25 (71.4) | 8 (72.7) | 20 (69.0) | 23 (69.7) | 7 (70.0) | 34 (77.3) | ||
| >50% | 10 (28.6) | 3 (27.3) | 9 (31.0) | 10 (30.3) | 3 (30.0) | 10 (22.7) | ||
| Tumor size, mm | 11.18±3.58 | 10.78±2.09 | 11.95±4.32 | 13.25±5.20 | 11.20±3.08 | 11.53±3.14 | 0.50 | |
| Margin distance, mm | 23.83±6.52 | 21.36±5.95 | 21.55±3.30 | 25.21±7.95 | 22.50±4.25 | 21.02±2.97 | 0.11 | |
| Margin distance ≥20 mm | 0.24 | |||||||
| Yes | 33 (94.3) | 10 (90.9) | 29 (100) | 29 (87.9) | 10 (100) | 43 (97.7) | ||
| No | 2 (5.7) | 1 (9.1) | 0 (0) | 4 (12.1) | 0 (0) | 1 (2.3) | ||
| Margin/diameter ratio, % | 236.87±99.33 | 202.85±54.56 | 203.07±77.10 | 224.98±129.14 | 214.75±67.12 | 195.15±56.20 | 0.58 | |
| Margin/diameter ratio ≥100% | 0.33 | |||||||
| Yes | 34 (97.1) | 10 (90.9) | 28 (96.6) | 30 (90.9) | 10 (100) | 44 (100) | ||
| No | 1 (2.9) | 1 (9.1) | 1 (3.4) | 3 (9.1) | 0 (0) | 0 (0) | ||
| Postoperative hospital stay, days | 3.26±0.74 | 3.18±0.40 | 3.34±0.77 | 3.24±0.66 | 3.30±0.67 | 3.36±1.24 | 0.99 | |
| Duration of drainage, days | 2.29±0.71 | 2.18±0.40 | 2.31±0.54 | 2.30±0.59 | 2.30±0.67 | 2.36±1.04 | 0.96 | |
Data are presented as mean ± standard deviation or n (%). S6, resection of mono S6; S6c, combined resection of S6 or mono subsegment of S6 plus adjacent segment or subsegment, such as resection of S6+8a or S6b+8a; Sother, other segmentectomies and subsegmentectomies not involving S6, such as resection of S8 or basal segment; LUAD, lung adenocarcinoma; IASLC9th, International Association for Study of Lung Cancer, 9th Edition; AIS, adenocarcinoma in situ; CTR, consolidation tumor ratio.
Patients in the Sother group on the right demonstrated the highest incidence of adenocarcinoma pathology at 93.2% (41/44). Meanwhile, those in the S6 group on the left also showed a highest rate, at 94.3% (33/35). However, statistical analysis revealed no significant differences across the groups (P=0.17). Only 2 patients of the S6 group on the left with adenocarcinoma exhibited the solid/micropapillary subtype, no patients were found to have lymph node metastases, and all were at TNM stages 0 or 1 (Table 4).
No significant differences were observed in margin distance and margin/diameter ratio among the 6 groups (P=0.11; P=0.58). The majority of these patients exhibited a margin distance of at least 20 mm and a margin/diameter ratio of at least 100%. Specifically, 94.3% (33/35) of patients in the left S6 group had a margin distance ≥ 20 mm, and 87.9% (29/33) of patients in the right S6 group achieved the same threshold. Furthermore, 97.1% (34/35) of patients in the left S6 group and 90.9% (30/33) of patients in the right S6 group had a margin/diameter ratio ≥ 100% (Table 4).
The outcomes of superior segmentectomies
In a manner similar to the absence of significant differences observed among the six groups concerning potential factors influencing the outcomes of superior segmentectomies, no significant differences were noted in the short-term or long-term outcomes of patients undergoing various segmentectomies.
The postoperative hospital stays of patients underwent S6 resection were recorded as 3.26±0.74 days on the left and 3.24±0.66 days on the right. Similarly, the duration of drainage was 2.29±0.71 days on the left and 2.30±0.59 days on the right. These perioperative outcomes were not inferior to those observed in patients undergoing other segmentectomies (P=0.99; P=0.96) (Table 4).
Median follow-up for the entire cohort of segmentectomy was 3.23 years (IQR, 2.99–3.61 years). During the follow-up period, no deaths were reported, and 2 tumor relapses occurred within the segmentectomy group. Specifically, both tumor relapses were observed in the left S6 group. Upon reviewing the clinical data of the 2 patients, it was noted that one experienced a locoregional relapse with a tumor size of 14 mm and a margin of 10 mm, resulting in a margin/diameter ratio of 71.43%. The other patient characterized pathologically as adenocarcinoma with a predominant solid/micropapillary subtype.
In the right S6 group, both the OS rate and the DFS rate were 100% (33/33). In the left S6 group, the OS rate was 100% (35/35) and the DFS rate was 94.3% (33/35). The DFS rate of the bilateral S6 groups (97.1%, 66/68) was comparable to that of the S6c plus Sother groups (100%, 94/94), with a P value of 0.10 by log-rank test (Figure S1).
In the study conducted by Jones et al., at a follow-up duration of 2 years, the OS rate and DFS rate were 69.3% (54/78) and 64.1% (50/78) in the right S6 group, and 66.1% (39/59) and 62.7% (37/59) in the left S6 group (9). Utilizing Z-test, we found that both the OS rate and DFS rate in our cohort were significantly higher than those reported by Jones et al. (P<0.001 for all comparisons), indicating a substantial improvement in long-term outcomes of patients underwent S6 resection in our study (Table S1).
Discussion
The anatomy of superior segmental veins has been extensively studied. Yamashita identified these veins as intrasegmental, running along the intersubsegmental plane between S6a and S6b (V6a), and S6b and S6c (V6b1), and as intersegmental, along the ISP between S6b and S9a (V6b2), and S6b and S10a (V6b3) (15). Yamashita also observed diverse drainage patterns in V6a and V6c but noted limited variation in V6b. However, with the aid of 3D-CTBA, we observed that there are usually branches of superior segmental veins typically running transversely along the ISP between S6b and S9a (V6b2) and between S6b and S8a (V6b3). Given their high prevalence and potential clinical significance, previously underreported, we analyzed the prevalence and branching patterns of V6b and initiated a preliminary investigation into the significant impacts of the relative sizes of lower lobe segments on its prevalence. To our knowledge, this study is the first systematic exploration of the anatomical characteristics of V6b using 3D-CTBA images. This work potentially offers a valuable addition to the existing knowledge of lung anatomy.
In recent years, sublobectomy has emerged as a crucial option for early-stage NSCLC, offering comparable oncological outcomes to lobectomy while providing better OS and postoperative respiratory function (20-22). Notably, studies such as JCOG0802 and CALGB140503 have provided robust evidence supporting sublobectomy for lesions smaller than 2 cm (4,5). This suggests a shift towards more precise thoracic surgeries, positioning segmentectomy as the likely preferred technique for peripheral NSCLC lesions.
However, not all outcomes of segmentectomies are satisfactory. Jones et al. reported that long-term outcomes following RS6 resection in patients with cT1N0 NSCLC undergoing intentional segmentectomy are notably worse, with a five-year DFS rate of 57.6% and an OS rate of 66.3% (9). Additionally, RS6 resection was independently associated with higher recurrence and mortality risks compared to basal segment resection, likely due to aggressive tumor biology, extensive lymph node metastasis, and inadequate surgical margins. In their study, the median margin distance after RS6 resection was 1.0 cm (IQR, 0.5–2.5 cm), with a margin/diameter ratio of 0.7 (IQR, 0.3–1.8). Furthermore, 21% of patients in the RS6 group had tumors with micropapillary/solid subtype and 10% had lymph node metastasis. Although the malignancy levels and prognosis in the LS6 group were not as adverse, the surgical margins were similarly concerning, with a median margin distance of 1.2 cm (IQR, 0.5–2.2 cm) and a margin/diameter ratio of 0.8 (IQR, 0.3–1.7).
The findings reported by Jones et al. raised concerns, as they did not fully align with our center’s clinical experience (9). Consequently, we conducted this retrospective analysis. In our study, most patients who underwent S6 resection achieved favorable margin distances. Notably, only two left S6 group patients with adenocarcinoma exhibited the solid/micropapillary subtype, and no lymph node metastases were detected (Table 4), contrasting sharply with the results of Jones et al.
We speculate that the non-significant malignancy and few lymph node metastases in our cohort may be attributed to advances in early diagnosis. Jones et al. conducted the retrospective analysis of patients who underwent intentional segmentectomy for cT1N0 NSCLC from 2000 to 2018, a period during which diagnostic techniques and standards were updated. This might explain why some cases included in their study, which exhibited local metastasis, were not detected preoperatively. For instance, 10% of cases in the RS6 group of their cohort had lymph node metastases discovered postoperatively, compared to none in our study. Jones et al. suggested that mediastinal lymph node sampling might be insufficient for patients with RS6 tumors, recommending a complete mediastinal lymphadenectomy instead (9). Given the low rate of lymph node metastasis after S6 resection at our center, we remain cautious about this recommendation. In our study, both the RS6 and LS6 groups exhibited satisfactory surgical margins, likely attributable to our rigorous preoperative planning and intraoperative techniques. Following the recommendation that a margin/diameter ratio of 150% in RS6 resections may be associated with improved survival, we achieved these criteria. Preoperative planning with 3D-CTBA was essential to ensure a 2 cm simulated margin free of V6b involvement, a prerequisite for performing S6 resection. However, in many cases where the lesion appeared to be located in S6 but was actually near the ISP, 3D-CTBA indicated that the simulated cutting margin involved V6b, extending beyond the ISP of S6. As a result, complete S6 resection could not ensure adequate margins, which might explain the poor outcomes reported by Jones et al. (9). At our center, these patients underwent either a combined segmentectomy (S6c group in our study) or lobectomy to maintain sufficient margins. Intraoperatively, a part of V6b is routinely isolated, leveraging its inherent characteristics as an ISP to facilitate complete resection of the S6.
Our study has several limitations. First, it is worth noting that although we explain the absence of V6b in some cases by analyzing the relative sizes among the pulmonary segments, in reality, the drainage function of the typical V6b drainage areas cannot be absent. We speculate that some atypical V6b branches do not drain back into V6, CBV, or IPV, but instead into V8a or V9a, and we propose naming these branches VX6b2 and VX6b3. Second, as a single-center retrospective study, it is subject to inevitable selection bias, and its generalizability to other regions and populations remains uncertain; thus, multi-center prospective studies are needed to validate our findings. Third, the follow-up period of less than five years necessitates longer observation to fully assess outcomes.
Conclusions
Our study demonstrates both high prevalence and minimal variation in the branching patterns of V6b. By emphasizing V6b to ensure sufficient surgical margins and potentially benefiting from reduced aggressiveness due to early detection, the outcomes of superior segmentectomies are not only comparable to other segmentectomies within our study but also superior to those reported in previous studies.
Acknowledgments
The authors thank all the study participants, research staff, and students who participated in this study and greatly appreciate the assistance of the AME Thoracic Surgery Collaborative Group and associated organizations.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-807/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-807/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-807/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-807/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Jiangsu Province Hospital and The First Affiliated Hospital of Nanjing Medical University Ethics Review Board (No. 2019-SR-450). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Adams SJ, Stone E, Baldwin DR, et al. Lung cancer screening. Lancet 2023;401:390-408. [Crossref] [PubMed]
- The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Zhang M, Mao N, Zhang K, et al. Analysis of the variation pattern in left upper division veins and establishment of simplified vein models for anatomical segmentectomy. Ann Transl Med 2020;8:1515. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Jones GD, Caso R, Choe G, et al. Intentional Segmentectomy for Clinical T1 N0 Non-small Cell Lung Cancer: Survival Differs by Segment. Ann Thorac Surg 2021;111:1028-35. [Crossref] [PubMed]
- Watanabe S, Suzuki K, Asamura H. Superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways and prognosis. Ann Thorac Surg 2008;85:1026-31. [Crossref] [PubMed]
- Motono N, Iwai S, Funasaki A, et al. Low-dose indocyanine green fluorescence-navigated segmentectomy: prospective analysis of 20 cases and review of previous reports. J Thorac Dis 2019;11:702-7. [Crossref] [PubMed]
- Nakazawa S, Yajima T, Shirabe K. Superior S(6) Segment, a Wolf in Sheep's Clothing? Ann Thorac Surg 2021;112:686-7. [Crossref] [PubMed]
- Sarsam M, Glorion M, de Wolf J, et al. The role of three-dimensional reconstructions in understanding the intersegmental plane: an anatomical study of segment 6. Eur J Cardiothorac Surg 2020;58:763-7. [Crossref] [PubMed]
- Boyden EA. Segmental anatomy of the lungs. A Study of the Patterns of the Segmental Bronchi and Related Pulmonary Vessels. New York: McGrawHill; 1955.
- Yamashita H. Roentgenologic anatomy of the lung. New York: IgakuShoin Medical Publisher; 1978.
- Chen L, Zhu Q, Wu W. Atlas of thoracoscopic anatomical pulmonary subsegmentectomy. Amsterdam: Elsevier; 2023.
- Chen X, Wang Z, Qi Q, et al. A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac Cancer 2022;13:795-803. [Crossref] [PubMed]
- Wu Y, Song W, Wang D, et al. Prognostic value of consolidation-to-tumor ratio on computed tomography in NSCLC: a meta-analysis. World J Surg Oncol 2023;21:190. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Longo JM, Lopez-Rasines G, Ortega E, et al. CT demonstration of an aortoesophageal fistula. Cardiovasc Intervent Radiol 1987;10:84-5. [Crossref] [PubMed]
- Ito H, Suzuki K, Mizutani T, et al. Long-term survival outcome after lobectomy in patients with clinical T1 N0 lung cancer. J Thorac Cardiovasc Surg 2020;S0022-5223(20)30054-4.
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]


