
Spontaneous ventilation video-assisted thoracoscopic surgery for octogenarian non-small cell lung cancer patients: a non-inferiority study
Highlight box
Key findings
• Spontaneous ventilation video-assisted thoracoscopic surgery (SV-VATS) is demonstrated to be a safe and feasible option for octogenarian patients with non-small-cell lung cancer (NSCLC).
• Compared to mechanical ventilation VATS (MV-VATS), SV-VATS significantly reduces the duration of post-anesthesia care unit (PACU) stay and resuscitation time.
• SV-VATS yields comparable surgical outcomes, postoperative recovery, and long-term survival—measured by overall survival (OS) and disease-free survival (DFS) to MV-VATS.
What is known and what is new?
• MV-VATS is the standard surgical procedure for NSCLC but poses certain limitations, particularly for elderly patients.
• Although prior studies have demonstrated benefits of SV-VATS in general patient populations, evidence specific to octogenarian patients remains limited.
What is the implication, and what should change now?
• The findings suggest that SV-VATS should be considered as an alternative to MV-VATS for octogenarian patients with NSCLC, with potential improvements in postoperative recovery.
• Clinicians are encouraged to assess the suitability of SV-VATS for elderly patients to optimize surgical outcomes and enhance patient care.
Introduction
Surgical intervention remains the primary method to treat early-stage non-small cell lung cancer (NSCLC) (1). In recent decades, video-assisted thoracoscopic surgery (VATS) with mechanical ventilation (MV) has become the standard surgical procedure to treat patients with NSCLC (2). However, MV-VATS is associated with several potential limitations and has a certain incidence, including dependent lung atelectasis, airway trauma related to intubation, compromised cardiac function, and residual neuromuscular blockade, which could introduce indeterminacy risks and impact prognosis (3-6).
Recent advancements in spontaneous ventilation (SV)-VATS have demonstrated promising outcomes, particularly in geriatric patients with NSCLC (7). An observation by Wang et al. highlighted the efficacy of SV-VATS in this demographic, showing comparable, if not superior, perioperative outcomes when compared to traditional MV-VATS. This finding underlines the potential of SV-VATS as a less invasive and equally effective option for elderly NSCLC patients (8). SV-VATS has been shown to mitigate unfavourable consequences associated with tracheal intubation and general anaesthesia (9,10). Furthermore, emerging evidence suggests that SV-VATS facilitates rapid postoperative recovery while reducing the duration of anaesthesia, hospital stay, and postoperative complications compared with MV-VATS (11,12).
Given the demonstrated efficacy of SV-VATS in enhancing postoperative outcomes, its application becomes particularly pertinent in the context of lung cancer, which represents the most commonly diagnosed malignancy among individuals aged 85 years and older (13). However, the availability of evidence to inform appropriate treatment decisions for this age group is limited, leaving uncertainty regarding the safety and effectiveness of SV-VATS. In clinical practice, advanced age, particularly in individuals over 80 years, has traditionally been perceived as a contraindication for thoracic surgery (14). However, increasing evidence is now demonstrating the feasibility of thoracic surgeries in individuals of such age groups (15,16).
Considering the above context, this study focused on evaluating the short- and long-term outcomes in patients with NSCLC aged >80 years who proceeded primary lung cancer resection undergoing SV-VATS versus MV-VATS. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-725/rc).
Methods
Patients selections
In this research, a retrospective study design was selected for its practicality in harnessing extensive historical data, essential for assessing long-term outcomes in octogenarian NSCLC patients. This approach, focusing on data from cases treated from January 1, 2017 to December 31, 2022 at the First Affiliated Hospital of Guangzhou Medical University, aptly aligns with our objective of comparing SV-VATS and MV-VATS efficacy and safety in patients over 80 years. Notably, the period encapsulated by our study is characterized by a remarkable stability in thoracic surgical protocols for lung cancer, specifically in the realm of VATS. This stability is corroborated by extant clinical guidelines pertinent to lung cancer surgery (1), which have evidenced minimal deviation in recommended procedures during this timeframe. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. ES-2024-018-01), and waived the requirement for informed consent owing to its retrospective characteristics.
Patients meeting the following criteria were included: (I) age >80 years; (II) confirmed diagnosis of NSCLC based on postoperative pathology; (III) tumour size ≤5 cm; (IV) Eastern Cooperative Oncology Group (ECOG) score ≤1; (V) absence of serious arrhythmias, such as frequent premature beats or atrial fibrillation. Patients who met the following exclusion criteria would be excluded: (I) incomplete data; (II) lost to follow-up in the postoperative 90 days; (III) history of infectious diseases, such as pulmonary infection and tuberculosis; (IV) history of thoracic surgery; and (V) thoracotomy. The selection criteria for SV-VATS is based on the description in the Cohen’s Comprehensive Thoracic Anesthesia (17).
Surgical procedures
SV-VATS group
Anaesthesia induction involved propofol with target-controlled infusion (TCI) at a plasma concentration between 2.0 and 4.0 µg/mL, midazolam at 0.05 mg/kg, and sufentanil at 0.01–0.02 µg/kg. When the value of bispectral index (BIS) descending beneath 60, insertion of a laryngeal mask airway into the patient’s mouth was performed. For anaesthesia maintenance, we continued propofol infusion, targeting at 1.5–3.5 mg/mL. We also administered remifentanil infusion at 0.01–0.1 µg/kg/min and dexmedetomidine at a rate of 0.2–0.8 µg/kg/hr. We facilitated SV by connecting the laryngeal mask airway to the ventilator machine and employed synchronised intermittent mandatory ventilation (SIMV) when the oxygen saturation was below 90% or the end expiratory carbon dioxide partial pressure was above 60 mmHg. We supplemented intravenous anaesthesia with a local nerve block. This included block for the visceral pleural surface, intercostal nerve, and thoracic vagus nerve.
MV-VATS group
Propofol was administered with a concentration of 2–4 µg/mL. Additionally, an infusion of sufentanil was given at a dosage of 0.3–0.6 µg/kg, along with cisatracurium at a rate of 0.2 mg/kg. A double-lumen endotracheal tube was inserted into the trachea. During maintenance, anaesthesia was sustained with propofol at a concentration of 1.5–3 µg/mL, remifentanil at a rate of 0.05–0.15 µg/kg/min, dexmedetomidine at 0.2–0.8 µg/kg/hr, and cisatracurium at 0.06–0.12 mg/kg/hr. We maintained the BIS between 40 and 60 during the operation. After complete recovery from anaesthesia, patients were transferred to the ward. Typically, they are able to walk and resume eating within 4–6 hours postoperatively.
VATS process
The VATS procedure for both groups was standardized, following contemporary thoracic surgery guidelines (18). The procedures, conducted through a 1-port or 2-port method, strictly adhered to these guidelines. Thoracoscopic lung resections, including lobectomies, segmentectomies, and wedge resections, were performed as indicated. This adherence to standardized guidelines aimed to minimize variations attributable to individual surgeon practices and techniques.
Data collection
Baseline, intraoperative, and postoperative data were collected from medical records. Long-term postoperative outcomes, including disease-free survival (DFS) and overall survival (OS), were evaluated through regular computed tomography (CT) scans and supplemented by interviews or telephone follow-ups. Patients included in the study were required to undergo CT scans every 3 months for the first two years and every 6 months starting from the third year. If patients were unable to return to the hospital for follow-up, they were required to upload their CT results to the hospital’s follow-up information system. If the patients did not upload the information on time, the researchers contacted the patients or their immediate family members by phone or conducted in-person interviews to ensure the accuracy and completeness of the results. The platelet-tolymphocyte ratio (PLR) ratio, systemic immune-inflammation index (SII), and neutrophil-to-lymphocyte ratio (NLR) were calculated based on the most recent data available before and after surgery. PLR, SII, and NLR were determined by the following formulae:
Statistical analysis
The age-adjusted Charlson Comorbidity Index (ACCI) was used to evaluate the impact of comorbidities. Preoperative comorbidities were assessed, and the ACCI was calculated. A propensity score matching (PSM) of 1:2 ratio was performed by the nearest-neighbour method on a logit scale with calliper restrictions of 0.2. The age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) status, tumour position, and surgical type were included in PSM. Confounding variables were deemed comparable when the standardized mean difference fell below 0.10 and the P value exceeded 0.05.
Descriptive data were presented as means and standard deviation, and compared using paired Student’s t-tests or Wilcoxon signed-rank tests for continuous variables. Data in categories were displayed in percentage terms and compared through either the paired Chi-squared test or McNemar’s test. Survival analysis was performed using the Kaplan-Meier method, and the log-rank test was stratified by matched triplets.
Statistical analysis was performed using R 4.2.2 (The R Core Team, R Foundation for Statistical Computing, Vienna, Austria) running on R Studio 2022.12.0 (R Studio Team, Posit Software Inc. Boston, MA, USA). A P<0.05 was considered statistically significant.
Results
Patient demographics and baseline characteristics
From January 2017 to December 2022, 251 patients aged ≥80 years with NSCLC who underwent primary lung cancer resection were initially included. After excluding 6 patients with missing information and 14 lost to follow-up, the study included 24 patients in the SV-VATS group and 207 in the MV-VATS group (Figure 1). A total of 22 patients in the SV-VATS group and 44 in the MV-VATS group were successfully matched after PSM (Table 1). Pathological tumor characteristics and intraoperative lymph node dissection results of 66 patients after PSM are presented in Table S1.
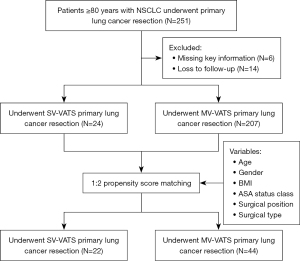
Table 1
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| MV-VATS (n=207) | SV-VATS (n=24) | P value | MV-VATS (n=44) | SV-VATS (n=22) | P value | ||
| Age, mean (SD) (years) | 82.5 (2.49) | 82.5 (1.89) | 0.63 | 82.5 (2.12) | 82.5 (1.95) | 0.89 | |
| Gender, n (%) | 0.95 | 0.38 | |||||
| Male | 128 (61.8) | 15 (62.5) | 23 (52.3) | 14 (63.6) | |||
| Female | 79 (38.2) | 9 (37.5) | 21 (47.7) | 8 (36.4) | |||
| BMI, mean (SD) (kg/m2) | 23.6 (3.82) | 22.3 (2.42) | 0.11 | 21.8 (3.35) | 22.5 (2.39) | 0.37 | |
| ASA status class, n (%) | 0.69 | >0.99 | |||||
| I | 3 (1.4) | 0 | 1 (2.3) | 0 (0) | |||
| II | 106 (51.2) | 15 (62.5) | 25 (56.8) | 13 (59.1) | |||
| III | 95 (45.9) | 9 (37.5) | 18 (40.9) | 9 (40.9) | |||
| IV | 3 (1.4) | 0 (0) | 0 (0) | 0 (0) | |||
| Tumor position, n (%) | 0.03 | 0.45 | |||||
| LUL | 48 (23.2) | 8 (33.3) | 12 (27.3) | 7 (31.8) | |||
| LLL | 21 (10.1) | 6 (25.0) | 7 (15.9) | 6 (27.3) | |||
| RUL | 75 (36.2) | 7 (29.2) | 19 (43.2) | 6 (27.3) | |||
| RLL | 48 (23.2) | 1 (4.2) | 2 (4.5) | 1 (4.5) | |||
| RML | 7 (3.4) | 0 (0) | 2 (4.5) | 0 (0) | |||
| Mediastinum | 3 (1.4) | 0 (0) | 1 (2.3) | 0 (0) | |||
| RUL + RLL | 2 (1.0) | 0 (0) | 1 (2.3) | 0 (0) | |||
| RLL + RML | 3 (1.4) | 1 (4.2) | 0 (0) | 1 (4.5) | |||
| RUL + RML | 0 | 1 (4.2) | 0 (0) | 1 (4.5) | |||
| Surgical type, n (%) | 0.02 | 0.57 | |||||
| Lobectomy | 111 (53.6) | 6 (25.0) | 14 (31.8) | 4 (18.2) | |||
| Segmentectomy | 30 (14.5) | 5 (20.8) | 9 (20.5) | 5 (22.7) | |||
| Wedge resection | 64 (30.9) | 12 (50.0) | 19 (43.2) | 12 (54.5) | |||
| Bilateral pneumonectomy | 1 (0.5) | 0 (0) | 1 (2.3) | 0 | |||
| Unilateral pneumonectomy | 1 (0.5) | 0 (0) | 1 (2.3) | 0 | |||
| Sleeve resection | 0 | 1 (4.2) | 0 | 1 (4.5) | |||
SV-VATS, spontaneous ventilation video-assisted thoracoscopic surgery; MV-VATS, mechanical ventilation video-assisted thoracoscopic surgery; PSM, propensity score matching; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe.
Comparison of intraoperative outcomes
Tables 2,3 summarises the intra- and postoperative outcomes. Among all included patients, there were no reported deaths or the need to convert to open surgery. During the procedures, the SV-VATS group did not need to convert to tracheal intubation.
Table 2
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| MV-VATS (n=207) | SV-VATS (n=24) | P value | MV-VATS (n=44) | SV-VATS (n=22) | P value | ||
| Surgical time (min) | 121 [56.0] | 100 [52.7] | 0.04 | 112 [41.4] | 103 [52.9] | 0.14 | |
| Anesthesia time (min) | 209 [63.5] | 186 [61.7] | 0.11 | 195 [48.5] | 191 [60.0] | 0.48 | |
| Ventilation time (min) | 183 [60.5] | 163 [61.8] | 0.14 | 172 [48.2] | 167 [60.3] | 0.36 | |
| Intraoperative blood loss (mL) | 59.8 [250] | 15.6 [10.8] | 0.03 | 21.1 [30.0] | 15.2 [11.0] | 0.56 | |
| Volume of chest drainage (mL) | 455 [320] | 309 [154] | 0.02 | 369 [187] | 305 [160] | 0.40 | |
| Volume of liquid infusion (mL) | 1280 [357] | 1210 [390] | 0.64 | 1230 [344] | 1220 [406] | 0.59 | |
| PACU stay (min) | 115 [49.8] | 88.1 [21.9] | 0.01 | 111.0 [38.8] | 88.8 [22.3] | 0.01 | |
| Resuscitation time (min) | 114 [49.9] | 87.5 [22.0] | 0.01 | 112 [40.4] | 88.8 [22.7] | 0.02 | |
| Chest tube duration (days) | 2.78 [2.08] | 2.08 [0.78] | 0.04 | 2.23 [0.743] | 1.95 [0.653] | 0.20 | |
| Postoperative hospital stay (days) | 4.45 [25.4] | 7.71 [5.94] | 0.57 | 4.91 [2.89] | 6.82 [6.10] | 0.57 | |
Data are presented as mean [SD]. SV-VATS, spontaneous ventilation video-assisted thoracoscopic surgery; MV-VATS, mechanical ventilation video-assisted thoracoscopic surgery; PSM, propensity score matching; PACU, post anesthesia care unit; SD, standard deviation.
Table 3
| Variables | MV-VATS (n=44) | SV-VATS (n=22) | P value |
|---|---|---|---|
| Values of PLR, mean [SD] | |||
| Preoperation | 152 [102.0] | 145 [81.1] | 0.81 |
| Postoperation | 248 [111] | 275 [136] | 0.48 |
| Values of SII, mean [SD] | |||
| Preoperation | 853 [1,170] | 648 [480] | >0.99 |
| Postoperation | 2,870 [2,460] | 2,410 [1,830] | 0.32 |
| Level of ACCI, n (%) | 0.09 | ||
| 4 | 27 (61.4) | 10 (45.5) | |
| 5 | 11 (25.0) | 8 (36.4) | |
| 6 | 1 (2.3) | 3 (13.6) | |
| ≥7 | 5 (11.4) | 1 (4.5) |
MV-VATS, mechanical ventilation video-assisted thoracoscopic surgery; SV-VATS, spontaneous ventilation video-assisted thoracoscopic surgery; PLR, platelet-to-lymphocyte ratio; SD, standard deviation; SII, systemic immune inflammation index; ACCI, age-adjusted Charlson comorbidity index.
Compared with the MV-VATS group after PSM, the SV-VATS group exhibited a significantly shorter duration of stay in the post-anesthesia care unit (PACU) (88.8±22.3 vs. 111±38.8 min, P=0.01) and shorter resuscitation time (88.8±22.7 vs. 112±40.4 min, P=0.02). No statistically differences were observed between the two groups in terms of surgical time (103±52.9 vs. 112±41.4 min, P=0.14), anaesthesia time (191±60.0 vs. 195±48.5 min, P=0.48), intraoperative ventilation time (167±60.3 vs. 172±48.2 min, P=0.36), the volume of intraoperative liquid infusion (1,220±406 vs. 1,230±344 mL, P=0.59), intraoperative blood loss (15.2±11.0 vs. 21.1±30.0 mL, P=0.56), chest tube duration (1.95±0.653 vs. 2.23±0.743 days, P=0.20), the total volume of chest drainage (305±160 vs. 369±187 mL, P=0.40), and postoperative hospital stay (6.82±6.10 vs. 4.91±2.89 days, P=0.57). The values of preoperative NLR, PLR, and SII exhibited no difference (3.64±2.62 vs. 4.48±6.50, 145±81.1 vs. 152±102.0, and 648±480 vs. 853±1,170, respectively, at P=0.22, P=0.81, and P>0.99, respectively). The values of postoperative NLR, PLR, and SII also exhibited no difference (15.4±11.4 vs. 15.2±11.5, 275±136 vs. 248±111, and 2,410±1,830 vs. 2,870±2,460, respectively, at P=0.87, P=0.48, and P=0.32, respectively). A high ACCI score was observed in the MV-VATS group (P=0.09).
Comparison of postoperative complications
Postoperative complications in the PACU, including chills, hypertension, and restlessness, were comparable between the two groups (P>0.05) (Table 4).
Table 4
| Variables | MV-VATS (n=44) | SV-VATS (n=22) | P value |
|---|---|---|---|
| Chill, n (%) | >0.99 | ||
| Yes | 2 (4.5) | 1 (4.5) | |
| No | 42 (95.5) | 21 (95.5) | |
| Hypertension, n (%) | 0.55 | ||
| Yes | 3 (6.8) | 0 (0) | |
| No | 41 (93.2) | 22 (100.0) | |
| Restlessness, n (%) | >0.99 | ||
| Yes | 3 (6.8) | 1 (4.5) | |
| No | 41 (93.2) | 21 (95.5) |
PACU, post-anesthesia care unit; MV-VATS, mechanical ventilation video-assisted thoracoscopic surgery; SV-VATS, spontaneous ventilation video-assisted thoracoscopic surgery.
Comparison of long-term survival
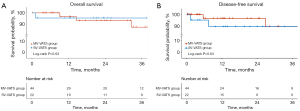
The median follow-up duration was 23.8 months. These results indicated that those who underwent SV-VATS had OS (32.0 vs. 25.5, P=0.64) and DFS (24.2 vs. 20.3, P=0.57) comparable to those who underwent MV-VATS (Figure 2).
Discussion
This study retrospectively analysed the short- and long-term outcomes of octogenarian patients with NSCLC who proceeded SV-VATS and MV-VATS. A significantly shorter duration of PACU stay and resuscitation was observed in the SV-VATS group. The ACCI scores were higher in the MV-VATS group, with more patients achieving scores ≥7 than in the SV-VATS group. Although intraoperative blood loss, chest tube duration and total volume of chest drainage, did not show statistical significance, the values were clinically better in the SV-VATS group. Of note, postoperative length of stay, though not statistically significant, was shorter in the MV-VATS group, possibly attributable to the extended postoperative monitoring initially required for SV-VATS in octogenarians to assess its efficacy and safety. Additionally, the SV-VATS group showed similar long-term results as the MV-VATS group, further supporting the safety and feasibility of performing SV-VATS in super-aged patients.
Although prior research has established SV-VATS as feasible and safe in a general patient cohort (9-11), these studies often did not include a sufficient number of octogenarian patients (>80 years), leaving a gap in evidence about this demographic. Furthermore, while studies focusing on the elderly (>65 years) exist (8), very few have specifically targeted the super-aged population, leading to insufficient data regarding the safety and practicality of SV-VATS in individuals aged over 80. This distinction is critical, as the physiological differences in octogenarian individuals may significantly impact surgical outcomes and feasibility. As the global population continues to age, the incidence of older adult patients with NSCLC requiring surgical intervention is increasing (13). Advanced age in these patients is correlated with an elevated incidence of perioperative complications arising from open-heart surgery and intubation under general anaesthesia (19), which have a potential negative impact on long-term survival outcomes (20). Consequently, it is imperative to comprehensively evaluate the impact of octogenarian on the outcomes of patients with NSCLC who undergo SV-VATS. The PACU time and resuscitation time of octogenarian patients in the SV-VATS group were significantly shortened, indicating that patients who underwent SV-VATS recovered faster immediately after surgery. This rapid recovery may be attributed to a significant reduction in the intraoperative opioid dosage. Because SV-VATS combined sedation and analgesia with nerve block to reduce the dosage of intravenous medication. Studies have suggested that opioid drug requirements can be reduced by approximately 50% with SV-VATS compared to MV-VATS (8).
Early reports based on single-centre and multicentre studies focusing on lung cancer surgery among octogenarians indicated a high postoperative mortality rate (21), poorer survival (22), and sleep apnoea (23). However, our results revealed that older adult patients in the SV-VATS group had comparable intraoperative variables, including the time of surgery and anaesthesia, blood loss, and liquid infusion. This indicated that changes in the anaesthesia method did not significantly affect surgical procedures and anaesthesia management. Furthermore, the duration of chest tube placement after surgery, volume of chest drainage, postoperative hospital stay, PLR and SII values showed similarity in the MV-VATS and SV-VATS groups, indicating that SV-VATS for super-aged patients achieved comparable rehabilitation outcomes during the ward stay as MV-VATS.
Octogenarian should not be considered an absolute contraindication for thoracic surgery. Recent studies have demonstrated favourable 5-year survival rates ranging from 36% to 49% in carefully selected patients aged over 80 years with NSCLC (24,25). Actually, surgery should not be waived just based on the chronological age, and the thoracic surgery remains the first-line regimen for early-stage NSCLC in octagenarian, as recommended in the current guidelines (1). With advancements in thoracoscopic techniques, the feasibility and safety of MV-VATS have been reported in octogenarian lung cancer patients (26).
Careful selection of patients of octogenarian continues to result in elevated rates of perioperative complications, ranging from 23% to 54% in those aged >80 years (27-29). Complications in the PACU had no dramatic difference between the MV-VATS- and SV-VATS groups. In the SV-VATS group, only two patients exhibited complications such as chills or restlessness in the PACU, and no other PACU-related complications were observed.
SV-VATS has demonstrated favourable short- and long-term survival outcomes, with OS and DFS rates similar to those of MV-VATS. Additionally, we considered cancer-specific survival (CSS) to mitigate potential bias from postoperative mortality unrelated to the surgical intervention. However, based on our observations, all recorded deaths in the study sample were cancer-related. As a result, the cancer-specific survival (CSS) outcomes are consistent with the overall survival (OS) outcomes. This result was inconsistent with our previous results (8), possibly due to the fact that all patients enrolled in this study were over 80 years old with limited postoperative follow-up duration, and the cause of postoperative mortality may be more complex rather than solely attributable to NSCLC. It is worth noting that the median follow-up duration in this study was nearly two years, which corresponds to the study’s design and intended timeframe and therefore should not be misconstrued as an insufficient period for assessing recurrence. In addition, this may be appropriate for elderly patients over 80 years old, since postoperative longer follow-up in extremely elderly patients is prone to death due to unrelated to surgery, which affecting postoperative outcome of this study. Benefiting from the results of this article, it is possible that SV-VATS might be a recommended surgical anesthesia for octogenarian patients, based on the consideration of the decrease in systemic multiorgan compensatory capacity caused by increasing age.
It is important to acknowledge certain limitations that influence the choice of the anaesthesia method. First, the transition from spontaneous breathing to intubation for general anaesthesia is considerably challenging, as evidenced by the high technical requirements and risks reported in previous studies (30). Second, chronic spontaneous breathing during SV-VATS can lead to hypercapnia and hypoxaemia, which are significant concerns for both patients and surgical teams (31). Accordingly, a comprehensive evaluation of each patient’s factors is imperative to enable the surgical team to make sensible decisions about the most appropriate anaesthesia method while respecting the patient’s autonomy and preferences. According to results of this study, the characteristics for SV-VATS in octogenarian NCLL patients could be raised that, the BMI ≤23 kg/m2, ASA level < III, more easier removing of tumors located in the upper lobe, wedge resection, and more safer with ACCI level <5. Pulmonary function is an independent factor of postoperative mortality in patients over 80 years old (26), since wedge resection can preserve more lung tissue than segmental resection in most cases. Therefore, wedge resection may be related with higher survival in octogenarian.
This study was an observation of thoracic surgery performed on patients with NSCLC aged over 80 years with a relatively large sample size (231 cases). The collected data involved important perioperative parameters, which was helpful for unravelling the significant novel insights in this study, However, it is essential to acknowledge several limitations in this study. First, despite conducting PSM to balance demographic characteristics, the retrospective design of a single institution may have introduced a selection bias in patient enrolment. In addition, including multiple covariates in PSM, especially when the sample size is limited, increases the risk of overfitting the model. This can lead to overly optimistic results and reduced generalizability. Second, as this was a single-centre study, external validation in other research settings is necessary to enhance the generalisability of the findings. Third, for a comprehensive evaluation and analysis, further follow-ups are required to assess the long-term survival outcomes of certain patients. Fourth, owing to the study’s retrospective nature, important outcome measures, such as patients’ quality of life scores, pain index scores, and medication usage, were not available. Therefore, prospective studies are warranted to investigate these aspects in octogenarian patients undergoing SV-VATS.
Conclusions
Our findings demonstrate the safety and feasibility of SV-VATS in octogenarian patients with NSCLC, as evidenced by shorter resuscitation time and PACU stays, as well as short- and long-term results comparable to those of MV-VATS. Careful patient selection and appropriate surgical strategies can yield favourable outcomes in this population. We believe that SV-VATS may serve as a viable alternative to MV-VATS in octogenarian patients.
Acknowledgments
The authors are pleased to acknowledge all resident doctors who participated in the collection and assembly of data. This study was accepted as a poster presentation at the 2023 World Conference on Lung Cancer (#WCLC23), September 9–12, 2023.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-725/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-725/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-725/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-725/coif). L.L. reports funding from the Guangzhou Municipal Science and Technology Bureau, The Project of Basic and Applied Basic Research Jointly Funded by the Municipality and University (Hospital) for the language modification and polishing (Fund No. 202201020584). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. ES-2024-018-01), and waived the requirement for informed consent owing to its retrospective characteristics.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert's opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann Oncol 2014;25:1270-83. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Miñambres E, Burón J, Ballesteros MA, et al. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg 2009;35:1056-62. [Crossref] [PubMed]
- Schneider T, Storz K, Dienemann H, et al. Management of iatrogenic tracheobronchial injuries: a retrospective analysis of 29 cases. Ann Thorac Surg 2007;83:1960-4. [Crossref] [PubMed]
- Frenkel M, Lien CA. Eliminating residual neuromuscular blockade: a literature review. Ann Transl Med 2024;12:65. [Crossref] [PubMed]
- Grewal HS, Dangayach NS, Ahmad U, et al. Treatment of Tracheobronchial Injuries: A Contemporary Review. Chest 2019;155:595-604. [Crossref] [PubMed]
- Grott M, Eichhorn M, Eichhorn F, et al. Thoracic surgery in the non-intubated spontaneously breathing patient. Respir Res 2022;23:379. [Crossref] [PubMed]
- Wang C, Wu D, Pang P, et al. Spontaneous Ventilation Video-Assisted Thoracoscopic Surgery for Geriatric Patients With Non-Small-Cell Lung Cancer. J Cardiothorac Vasc Anesth 2022;36:510-7. [Crossref] [PubMed]
- Liu J, Liang H, Cui F, et al. Spontaneous versus mechanical ventilation during video-assisted thoracoscopic surgery for spontaneous pneumothorax: A randomized trial. J Thorac Cardiovasc Surg 2022;163:1702-1714.e7. [Crossref] [PubMed]
- Liang H, Liu J, Wu S, et al. Nonintubated Spontaneous Ventilation Offers Better Short-term Outcome for Mediastinal Tumor Surgery. Ann Thorac Surg 2019;108:1045-51. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Wen Y, Liang H, Qiu G, et al. Non-intubated spontaneous ventilation in video-assisted thoracoscopic surgery: a meta-analysis. Eur J Cardiothorac Surg 2020;57:428-37. [Crossref] [PubMed]
- DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin 2019;69:452-67. [Crossref] [PubMed]
- Jaklitsch M, Billmeier S. Preoperative evaluation and risk assessment for elderly thoracic surgery patients. Thorac Surg Clin 2009;19:301-12. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Sublobar Resection for Non-Small Cell Lung Cancer in Octogenarians: A Prospective, Multicenter Study. Ann Thorac Surg 2023;116:543-51. [Crossref] [PubMed]
- Baldvinsson K, Oskarsdottir GN, Orrason AW, et al. Resection rate and operability of elderly patients with non-small cell lung cancer: Nationwide study from 1991 to 2014. Interact Cardiovasc Thorac Surg 2017;24:733-9. [Crossref] [PubMed]
- He J, Gonzalez-Rivas D, Liu H, et al. Chapter 37 - Tubeless Thoracic Procedures. In: Cohen E, editor. Cohen's Comprehensive Thoracic Anesthesia. Philadelphia: Elsevier; 2022. p. 533-43.
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Hino H, Karasaki T, Yoshida Y, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg 2018;53:980-6. [Crossref] [PubMed]
- Pagès PB, Mariet AS, Madelaine L, et al. Impact of video-assisted thoracic surgery approach on postoperative mortality after lobectomy in octogenarians. J Thorac Cardiovasc Surg 2019;157:1660-7. [Crossref] [PubMed]
- Bravo Iñiguez CE, Armstrong KW, Cooper Z, et al. Thirty-Day Mortality After Lobectomy in Elderly Patients Eligible for Lung Cancer Screening. Ann Thorac Surg 2016;101:541-6. [Crossref] [PubMed]
- Ehrsam JP, Aigner C. Surgery of old people-Thoracic surgery. Wien Klin Mag 2023;26:112-21. [Crossref] [PubMed]
- Sun F, Ma K, Yang X, et al. A nomogram to predict prognosis after surgery in early stage non-small cell lung cancer in elderly patients. Int J Surg 2017;42:11-6. [Crossref] [PubMed]
- Chan EY, Amirkhosravi F, Nguyen DT, et al. Lobectomy Provides the Best Survival for Stage I Lung Cancer Patients Despite Advanced Age. Ann Thorac Surg 2022;114:1824-32. [Crossref] [PubMed]
- Detillon DDEMA, Veen EJ. Postoperative Outcome After Pulmonary Surgery for Non-Small Cell Lung Cancer in Elderly Patients. Ann Thorac Surg 2018;105:287-93. [Crossref] [PubMed]
- Bongiolatti S, Gonfiotti A, Borgianni S, et al. Post-operative outcomes and quality of life assessment after thoracoscopic lobectomy for Non-small-cell lung cancer in octogenarians: Analysis from a national database. Surg Oncol 2021;37:101530. [Crossref] [PubMed]
- Sarkaria IS, Gorrepati ML, Mehendale S, et al. Lobectomy in octogenarians: real world outcomes for robotic-assisted, video-assisted thoracoscopic, and open approaches. J Thorac Dis 2019;11:2420-30. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Survival of Octogenarians with Early-Stage Non-small Cell Lung Cancer is Comparable Between Wedge Resection and Lobectomy/Segmentectomy: JACS1303. Ann Surg Oncol 2021;28:7219-27. [Crossref] [PubMed]
- He J, Liu J, Zhu C, et al. Expert consensus on spontaneous ventilation video-assisted thoracoscopic surgery in primary spontaneous pneumothorax (Guangzhou). Ann Transl Med 2019;7:518. [Crossref] [PubMed]
- Shi Y, Yu H, Huang L, et al. Postoperative pulmonary complications and hospital stay after lung resection surgery: A meta-analysis comparing nonintubated and intubated anesthesia. Medicine (Baltimore) 2018;97:e10596. [Crossref] [PubMed]


