
Validation of T stage classification strategy for >2 cm ground-glass opacity non-small cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• This study aimed to validate the T-staging classification strategy recommended by the International Association for the Study of Lung Cancer (IASLC) specifically for non-small cell lung cancer (NSCLC) patients with ground-glass opacity (GGO) lesions greater than 2 cm.
What is known and what is new?
• Although prior studies suggest that NSCLC patients with GGO lesions generally experience better prognoses than those with solid lesions, most research has focused on tumors with a diameter of 2 cm or less, leaving a gap in understanding the impact of larger GGO lesions.
• This study represents a large cohort analysis comparing the prognostic value of overall tumor size and solid component size for T-staging in NSCLC patients with GGO lesions greater than 2 cm. The results reinforce that T-staging based on solid component size, as per the IASLC recommendations, is more accurate in predicting survival outcomes.
What is the implication, and what should change now?
• For NSCLC patients with GGO lesions over 2 cm, using solid component size for T-staging offers a more precise survival prognosis and should guide treatment strategies. This approach supports individualized treatment planning, particularly in surgical and adjuvant therapy decisions for larger GGO tumors.
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality, responsible for approximately 1.8 million deaths annually (1). The National Lung Screening Trial in 2011 demonstrated that low-dose computed tomography (CT) screening significantly reduces lung cancer mortality (2). Subsequently, an increasing number of tumors characterized by ground-glass opacities (GGOs) have been detected. Numerous studies have indicated that NSCLC patients with GGOs generally have more favorable prognoses (3-7). Therefore, the presence of GGOs should influence both tumor staging and treatment decisions (8).
Tumor-node-metastasis (TNM) stage is one of the most important prognostic indicators in NSCLC. A previous study showed that using modified T descriptors, which measure the overall tumor size excluding the GGO component on CT, more accurately predicts the outcomes for patients with early-stage lung cancer (9). The International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Program also recommends prioritizing the measurement of the solid component over the overall size for T descriptors (10,11). Moreover, for larger lesions and advanced stages, clinical decisions should consider the solid-to-GGO ratio as it may impact treatment choices and outcomes (12). TNM stage guides the therapeutic approach. For lesions larger than 2 cm in diameter, lobectomy is a better option than sub-lobectomy (13-15). Additionally, postoperative adjuvant therapy in T2N0M0 (stage IB) patients improves prognosis (16-20). Thus, it is crucial that TNM staging effectively accounts for GGO components to optimize treatment and prognostication in NSCLC.
Although a large number of previous studies have confirmed the better prognosis of GGO lesions compared to equal-sized solid lesions, most recent studies of GGO lesions have been limited to small tumors ≤2.0 cm (7,13,14,21-23). The prognostic impact of the GGO component on tumors larger than 2 cm has not been adequately investigated (24). Only a few studies have examined tumors exceeding 3 cm in diameter, and these studies have been limited by small sample sizes, preventing conclusive results regarding the significance of GGO in larger tumors (24,25). Meanwhile, other articles concerning GGOs greater than 2 cm only mention the survival benefits brought about by the GGO component (26,27).
This study aimed to fill this gap by evaluating the effectiveness of the IASLC-recommended T-staging classification specifically for NSCLC patients with GGO-dominant tumors larger than 2 cm in diameter. By comparing the prognostic value of overall tumor size and solid component size as T-staging indicators, we sought to determine an optimal staging approach for this patient subgroup. Our findings could help to establish more precise and individualized treatment strategies for patients with larger GGO lesions, addressing an important clinical need in NSCLC management. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-664/rc).
Methods
Data source
The Western China Lung Cancer Database (WCLCD) was established in September 2005. Since then, all lung cancer patients who underwent surgery at the Department of Thoracic Surgery, West China Hospital, Sichuan University have been registered in the database. This was a retrospective cohort study with prospectively collected data, designed to evaluate the prognostic impact of GGO size in NSCLC patients with tumors larger than 2 cm. The exclusion criteria were as follows: patients with subsolid lesions having a maximum tumor diameter of 2 cm or less; those undergoing other surgical procedures such as segmental resection, wedge resection, sleeve resection, bilateral surgery, chest wall resection, or other extended resections; those diagnosed with other types of cancer, including small-cell lung cancer and pulmonary metastases; and patients with clinical N1–3 staging or those who were lost to follow-up.
Informed consent and ethical considerations
Informed consent was provided by all study participants prior to data collection, which ensured that their rights and privacy were protected throughout the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board (IRB) of West China Hospital (No. 2023-1694).
Surgical approach
All patients underwent lobectomy through a thoracic approach, with the entry route determined by tumor characteristics and patient health status. Standard protocols were followed, including intraoperative imaging (e.g., CT or ultrasound) to confirm tumor margins. Postoperatively, comprehensive lymph node dissection and sampling were mandatory to ensure accurate pathological staging and optimize subsequent treatment planning.
Radiological and clinicopathological evaluation
The preoperative CT scans of all patients were carefully reviewed by the authors (Y.L. and Z.Y.). Each tumor was further assessed using high-resolution CT to determine the consolidation-to-tumor ratio (CTR) for the GGO. The solid component was identified as areas of increased opacity that completely obscured the underlying vascular markers. In contrast, GGOs were defined as areas of slightly homogeneous increased density that did not obscure the underlying vascular markers. Following the Fleischner Society’s guidelines (28), the CTR was calculated as the ratio of the maximum diameter of the solid component to the tumor’s maximum diameter, based on high-resolution CT imaging. Postoperative pathology included assessments of tumor size, histologic subtype, visceral pleural invasion (VPI), and lymph node involvement according to IASLC and World Health Organization (WHO) guidelines (29).
Outcomes
Patients underwent regular follow-ups either via telephone visits or in the outpatient department. The long-term survival outcomes were overall survival (OS) and recurrence-free survival (RFS). OS was defined as the interval from surgery to death or the last follow-up, whereas RFS was defined as the time from surgery to the occurrence of recurrence, metastasis, death, or the last follow-up. Follow-up commenced on the day of surgery, with patient status evaluated at regular intervals using chest CT, brain magnetic resonance imaging (MRI) or CT, and upper abdominal CT. Follow-ups occurred every 3 to 6 months during the first 2 years, semiannually for the subsequent 3 years, and annually thereafter. To ensure the accuracy and reliability of the follow-up data, follow-up procedures were standardized, and data quality checks and updates were conducted periodically by independent data monitors. Additional diagnostics, such as bone scans, positron emission tomography (PET)-CT, or biopsies, were conducted as necessary. The latest follow-up was conducted in December 2023.
To assess which indicator is better for evaluating GGO patients, solid component size or overall size, we used propensity score matching (PSM) to compare the two groups (group matched by solid component size and group matched by overall size) of patients separately to minimize potential selection bias. Matching indicators included solid component size or overall size (categorical variables based on diameter size: 0–1, 1–2, 2–3 cm, and so on), gender (male, female), age at surgery, smoking history (smoker, non-smoker), Charlson Comorbidity Index (CCI), tumor location (right, left), lymph node dissection (yes, no), histologic type (adenocarcinoma, squamous, adenosquamous carcinoma, and other types of primary NSCLC), p-N status (N0, N1, N2, N3), and adjuvant postoperative therapy (yes, no). The patient inclusion process is shown in Figure 1.
Statistical analysis
All statistical analyses were performed using R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). Quantitative data were expressed as mean ± standard deviation. Independent samples t-tests were used to compare differences between groups. Categorical data were expressed as percentages, and differences between groups were compared using Fisher’s exact test or Chi-squared test. Preoperative variables such as solid component size or overall size, gender, age at surgery, smoking history, CCI score, side of surgery, lymph node dissection, histologic type, p-N status, and adjuvant postoperative therapy were considered covariates for PSM. The PSM process was performed using the nearest method, with the number of patients in each group being 1:1. Kaplan-Meier survival curves were used to describe the OS and RFS before and after PSM in both groups, and survival outcomes were compared using log-rank tests. Additionally, Cox regression models were used to determine independent prognostic factors for these patients, and multicollinearity was assessed using the variance inflation factor (VIF). Only the variables with VIF <10 were selected for modeling (30). All statistical tests were two-sided, with statistical significance set at P<0.05.
Results
Patient characteristics
A total of 8,041 NSCLC patients underwent lobectomy in the Department of Thoracic Surgery, West China Hospital, Sichuan University from December 2009 to December 2018. Of these patients, 3,569 were excluded based on the exclusion criteria, and the remaining 4,472 patients were included in the study (including 4,083 cases of solid lesions and 389 cases of subsolid lesions) (Figure 1). The subsequent PSM identified two groups: the Solid group (389 cases each, group matched by solid component size and group matched by solid component size) and the GGO group (389 cases) based on solid component size or overall size, gender, age at surgery, smoking history, CCI, side of surgery, lymph node dissection, histologic type, p-N status, and adjuvant postoperative therapy.
Unmatched population
Details of patient characteristics are shown in Table 1. The GGO group had more female patients but fewer smokers compared to the Solid group. The median follow-up time was 75.4 months. The mean Solid component size of the GGO group was smaller (1.73±1.34 vs. 2.89±1.65 cm, P<0.001). There were statistical differences among the two groups regarding histological type (P<0.001), pathological stage (P<0.001), p-N status (P<0.001), and postoperative adjuvant therapy (P<0.001).
Table 1
| Characteristics | GGO group (n=389) | Solid group (n=4,083) | P value |
|---|---|---|---|
| Age (years) | 59.28±9.53 | 58.26±10.27 | 0.06 |
| Sex | 0.001 | ||
| Male | 171 (44.0) | 2,170 (53.1) | |
| Female | 218 (56.0) | 1,913 (46.9) | |
| Tumor size (cm) | 3.04±0.94 | 2.89±1.65 | 0.08 |
| Solid component size (cm) | 1.73±1.34 | 2.89±1.65 | <0.001 |
| Smoking | <0.001 | ||
| Smoker | 105 (27.0) | 1,699 (41.6) | |
| Non-smoker | 284 (72.0) | 2,384 (58.4) | |
| Location | 0.008 | ||
| Right | 262 (67.4) | 2,465 (60.4) | |
| Left | 127 (32.6) | 1,618 (39.6) | |
| CCI score | 0.10 | ||
| 0 | 313 (80.5) | 3,211 (78.6) | |
| 1 | 66 (17.0) | 633 (15.5) | |
| 2 | 8 (2.1) | 205 (5.0) | |
| 3 | 2 (0.5) | 33 (0.8) | |
| 4 | 0 | 1 (0.0) | |
| Lymph node dissection | 0.05 | ||
| Yes | 383 (98.5) | 3,939 (96.5) | |
| No | 6 (1.5) | 144 (3.5) | |
| P-stage | <0.001 | ||
| 1 | 344 (88.4) | 2,797 (68.5) | |
| 2 | 23 (5.9) | 652 (16.0) | |
| 3 | 22 (5.7) | 634 (15.5) | |
| P-N status | <0.001 | ||
| 0 | 359 (92.3) | 3,100 (75.9) | |
| 1 | 10 (2.6) | 445 (10.9) | |
| 2 | 20 (5.1) | 535 (13.1) | |
| 3 | 0 | 3 (0.1) | |
| Histology | <0.001 | ||
| Adenocarcinoma | 328 (84.3) | 3,019 (73.9) | |
| Adenosquamous carcinoma | 9 (2.3) | 115 (2.8) | |
| Other | 37 (9.5) | 255 (6.2) | |
| Squamous carcinoma | 15 (3.9) | 694 (17.0) | |
| Post-therapy | <0.001 | ||
| Yes | 91 (23.4) | 1,577 (38.6) | |
| No | 298 (76.6) | 2,506 (61.4) |
Data are presented as mean ± SD or n (%). GGO, ground-glass opacity; CCI, Charlson Comorbidity Index; SD, standard deviation.
Of the 4,472 patients included in this study, the median follow-up duration was 75.4 months. Patients in the GGO group had significantly better OS and RFS than those in the solid group [the 5-year OS rates were 87.1% in the GGO group, 76.1% in the solid group, hazard ratio (HR) =0.55, 95% confidence interval (CI): 0.40–0.73, P<0.001; the 5-year RFS rates were 80.7% in the GGO group, 65.4% in the solid group, HR =0.53, 95% CI: 0.42–0.67, P<0.001] (Figures S1,S2).
The descriptions of recurrence sites or metastatic patterns are shown in Table 2. Pulmonary metastatic nodules and mediastinal lymph node metastasis were significantly more common in the solid group than in the GGO group (P=0.04 and P=0.02, respectively). For metastatic patterns, brain, lung, and liver metastases were also significantly higher in the solid group (P<0.001, P=0.02, and P=0.04, respectively).
Table 2
| Descriptions of recur sites or metastatic patterns | Solid group (n=4,144) | GGO group (n=389) | P value |
|---|---|---|---|
| Recur sites | 315 (7.6) | 13 (3.3) | 0.003 |
| Pulmonary metastatic nodules | 170 (4.1) | 7 (1.8) | 0.04 |
| Mediastinal lymph node metastasis | 67 (1.6) | 0 (0.0) | 0.02 |
| Pleural cavity implantation (including pleural nodules and malignant pleural effusion) | 60 (1.4) | 5 (1.3) | 0.97 |
| Residual recurrence | 53 (1.3) | 2 (0.5) | 0.28 |
| Other | 36 (0.9) | 1 (0.3) | 0.32 |
| Metastatic patterns | 1,103 (26.6) | 49 (12.6) | <0.001 |
| Brain | 449 (10.8) | 13 (3.3) | <0.001 |
| Bone | 402 (9.7) | 26 (6.7) | 0.06 |
| Lung | 266 (6.4) | 13 (3.3) | 0.02 |
| Liver | 136 (3.3) | 5 (1.3) | 0.04 |
| Adrenal gland | 44 (1.1) | 3 (0.8) | 0.78 |
| Cervical lymph nodes | 40 (1.0) | 3 (0.8) | 0.92 |
| Others | 209 (5.0) | 11 (2.8) | 0.07 |
Data are presented as n (%). Recur sites: refers to the anatomical or histological locations where NSCLC recurs after initial treatment; Others include the mediastinum, kidneys, pleura, lymph nodes, rectum, systemic metastases, etc. GGO, ground-glass opacity; NSCLC, non-small cell lung cancer.
Matched population
After PSM by solid component size or overall size, there were 389 patients in each group. The baseline clinical characteristics and surgical outcomes of PSM-matched patients are listed in Table 3. Matched patients were comparable in all data included in the PSM.
Table 3
| Characteristics | For overall size | For solid component size | |||||
|---|---|---|---|---|---|---|---|
| GGO group (n=389) | Group matched by overall size (n=389) | P value | GGO group (n=389) | Group matched by solid component size (n=389) | P value | ||
| Age (years) | 59.28±9.53 | 59.58±10.02 | 0.68 | 59.28±9.53 | 60.14±9.49 | 0.21 | |
| Sex | 0.83 | 0.72 | |||||
| Male | 171 (44.0) | 167 (42.9) | 171 (44.0) | 165 (42.4) | |||
| Female | 218 (56.0) | 222 (57.1) | 218 (56.0) | 224 (57.6) | |||
| Smoking | 0.94 | 0.81 | |||||
| Smoker | 105 (27.0) | 103 (26.5) | 105 (27.0) | 109 (28.0) | |||
| Non-smoker | 284 (73.0) | 286 (73.5) | 284 (73.0) | 280 (72.0) | |||
| Location | 0.64 | 1 | |||||
| Right | 262 (67.4) | 269 (69.2) | 262 (67.4) | 261 (67.1) | |||
| Left | 127 (32.6) | 120 (30.8) | 127 (32.6) | 128 (32.9) | |||
| CCI score | 0.78 | 0.26 | |||||
| 0 | 313 (80.5) | 318 (81.7) | 313 (80.5) | 324 (83.3) | |||
| 1 | 66 (17.0) | 66 (17.0) | 66 (17.0) | 50 (12.9) | |||
| 2 | 8 (2.1) | 5 (1.3) | 8 (2.1) | 14 (3.6) | |||
| 3 | 2 (0.5) | 0 (0.0) | 2 (0.5) | 1 (0.3) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Overall size (cm) | 3.04±0.94 | 3.21±1.35 | 0.05 | 3.04±0.94 | 1.88±1.16 | <0.001 | |
| Solid component size (cm) | 1.73±1.34 | 3.21±1.35 | <0.001 | 1.73±1.34 | 1.88±1.16 | 0.09 | |
| Lymph node dissection | 0.50 | 0.50 | |||||
| Yes | 383 (98.5) | 386 (99.2) | 383 (98.5) | 379 (97.4) | |||
| No | 6 (1.5) | 3 (0.8) | 6 (1.5) | 10 (2.6) | |||
| P-stage | 0.33 | 0.61 | |||||
| 0 | 359 (92.3) | 355 (91.3) | 359 (92.3) | 348 (89.5) | |||
| 1 | 10 (2.6) | 12 (3.1) | 10 (2.6) | 16 (4.1) | |||
| 2 | 20 (5.1) | 22 (5.7) | 20 (5.1) | 25 (6.4) | |||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Histology | 0.86 | 0.42 | |||||
| Adenocarcinoma | 328 (84.3) | 332 (85.3) | 328 (84.3) | 332 (85.3) | |||
| Adenosquamous carcinoma | 9 (2.3) | 11 (2.8) | 9 (2.3) | 12 (3.1) | |||
| Other | 37 (9.5) | 31 (8.0) | 37 (9.5) | 26 (6.7) | |||
| Squamous carcinoma | 15 (3.9) | 15 (3.9) | 15 (3.9) | 19 (4.9) | |||
| Post-therapy | 0.73 | 0.44 | |||||
| Yes | 91 (23.4) | 86 (22.1) | 91 (23.4) | 81 (20.8) | |||
| No | 298 (76.6) | 303 (77.9) | 298 (76.6) | 308 (79.2) | |||
Data are presented as mean ± SD or n (%). PSM, propensity score matching; GGO, ground-glass opacity; CCI, Charlson Comorbidity Index; SD, standard deviation.
Compared to the group matched by overall size, the GGO group had better OS and RFS (the 5-year OS rates were 87.1% in the GGO group, 78.9% in the group matched by overall size, HR =0.60, 95% CI: 0.43–0.85, P=0.004; the 5-year RFS rates were 80.7% in the GGO group, 68.5% in group matched by overall size HR =0.59, 95% CI: 0.44–0.79, P<0.001) (Figure 2). However, there was no significant difference between the GGO group and the group matched by solid component size on OS and RFS (the 5-year OS rates were 87.1% in the GGO group, 85.6% in the group matched by solid component size, HR =0.89, 95% CI: 0.61–1.28, P=0.52; the 5-year RFS rates were 80.7% in the GGO group, 78.3% in the group matched by solid component size, HR =0.92, 95% CI: 0.67–1.26, P=0.61) (Figure 2).

Following multivariable Cox regression analysis of the GGO group and the two matched groups, we identified that the size of the solid component (HR =1.70, 95% CI: 1.27–2.26, P<0.001), age (HR =1.02, 95% CI: 1.01–1.04, P=0.005), pathological T stage (HR =2.53, 95% CI: 1.53–4.17, P<0.001), and pathological N stage (HR =2.74, 95% CI: 2.06–4.16, P<0.001) were independent prognostic factors for OS (Table 4). Overall tumor size did not independently predict OS (HR =0.77, 95% CI: 0.57–1.04, P=0.23). Additionally, there was no evidence of multicollinearity, with the VIF ranging from 1.04 to 8.63.
Table 4
| Predictors of survival | HR | Lower 95% | Upper 95% | P value |
|---|---|---|---|---|
| Age at surgery | 1.02 | 1.01 | 1.04 | 0.005 |
| Gender (ref = male) | 1.15 | 0.78 | 1.68 | 0.29 |
| Tumor size | 0.77 | 0.57 | 1.04 | 0.23 |
| Solid component size | 1.70 | 1.27 | 2.26 | <0.001 |
| Smoking history (ref = non-smoker) | 1.32 | 0.88 | 1.98 | 0.24 |
| CCI (ref >1) | 0.79 | 0.56 | 1.12 | 0.18 |
| Location (ref = right side) | 0.83 | 0.63 | 1.09 | 0.15 |
| Lymph node dissection (ref = no) | 0.53 | 0.24 | 1.15 | 0.17 |
| pT stage (ref = T1) | 2.53 | 1.53 | 4.17 | <0.001 |
| pN stage (ref = N0) | 2.74 | 2.06 | 4.16 | <0.001 |
| Histology (ref = adenocarcinoma) | 1.03 | 0.73 | 1.46 | 0.87 |
| Post-therapy (ref = no) | 0.79 | 0.57 | 1.10 | 0.16 |
Lower 95%, lower bound of the 95% CI; upper 95%, upper bound of the 95% CI. OS, overall survival; HR, hazard ratio; CCI, Charlson Comorbidity Index; CI, confidence interval.
Subgroup analysis
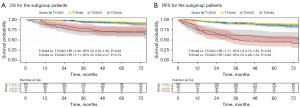
We subsequently reassessed clinical T-staging (9th edition TNM stage) for 389 patients in the GGO group according to the solid component size and matched these patients in the solid group. The results showed no difference in OS and RFS between the restaged patients (T1 and T2, since there were only five T3 patients, we did not match for T3) and the corresponding patients in the Solid group (for OS, HR =1.06, 95% CI: 0.61–1.83, P=0.83; HR =1.11, 95% CI: 0.60–2.07, P=0.73, respectively and RFS, HR =1.17, 95% CI: 0.75–1.82, P=0.49; HR =0.80, 95% CI: 0.48–1.34, P=0.39, respectively) (Figure 3).
Discussion
Based on our findings, staging recommendations of IASLC are valid for GGO tumors larger than 2 cm, and solid component size should be used instead of overall size as an indicator for TNM staging. New clinical TNM staging that takes into account the GGO component may provide more accurate survival prediction and treatment guidance for patients with lung cancer tumors over 2 cm in diameter. With numerous studies confirming that patients with GGO have better survival rates, the IASLC recommended a significant change in the definition of clinical T descriptors, using only the size of the solid component as a descriptor (3,4,10). Our study confirmed that in patients with GGO >2 cm, the solid component size is a better predictor of outcomes compared to the overall size. Our findings validate the previous IASLC staging recommendations for GGO tumors larger than 2 cm and suggest that the solid component size should be used instead of the overall size in TNM staging.
In our study, significant differences in OS and RFS were observed between the group matched by overall size and the GGO group, whereas no differences in OS and RFS were noted between the group matched by solid component size and the GGO group. This observation is consistent with previous studies showing that even a small percentage of GGO components is associated with a better prognosis compared to solid lesions (24,25). These findings support the hypothesis of a linear multistep progression in which a precursor lesion evolves into adenocarcinoma in situ and subsequently into invasive adenocarcinoma, particularly in cases of lung adenocarcinomas (LUADs) with a radiographic GGO component (24,31). A recent study indicated that adenocarcinoma in situ/minimally invasive adenocarcinoma and GGO-like LUAD exhibit a lower frequency of oncogene mutations and reduced activity in cell proliferation-related pathways compared to solid LUAD lesions (32). Notably, in our unmatched baseline, the GGO group had fewer lymph node metastases than the solid group (7.7% vs. 24.1%), a lower proportion of VPI (11.8% vs. 27.0%), and a higher proportion of LUAD (84.3% vs. 73.9%). These results are consistent with previous studies and may affect prognosis (24,33-36). However, using PSM to balance variables such as histologic type, pathological N status, and adjuvant postoperative therapy resulted in more balanced matched groups. Consequently, our findings offer greater applicability to postoperative tumor staging and subsequent treatment options. There was still no difference in the T1 and T2 stage subgroups using the same matching method. We hypothesize that the indolent nature of the GGO component underlies this phenomenon, suggesting that the GGO component does not significantly impact tumor progression.
This study validates the IASLC recommendation that for patients with GGO >2 cm, the solid component size should be used instead of the overall size. Our findings provide updated data to support this recommendation and improve the choice of surgical approach and postoperative treatment for GGO >2 cm. In multivariate Cox regression analyses, we verified that the solid component size differentiated patient survival more significantly than the overall size. Other significant factors included age and postoperative T stage and N stage. Notably, in our study cohort, adjuvant therapy did not result in better survival outcomes for the GGO group. In subgroup analysis, adjuvant therapy was also not significant for patients in the T2 GGO group after modified staging (Figure S3). The use of adjuvant therapy in patients with GGO >2 cm needs further consideration.
The limitations of this study should also be acknowledged. We acknowledge that there is a significant disproportion in the number of patients between the two groups, which potentially could have influenced the generalizability of our findings. To address this, we applied PSM to balance key variables such as gender distribution, smoking history, and tumor size, thus controlling for these factors in our analysis. However, although PSM effectively minimizes bias for observed variables, it cannot completely eliminate residual bias, especially from unmeasured confounding factors that may also impact survival outcomes. Additionally, the retrospective nature of the study, though based on prospectively collected data, may have introduced inherent observational bias. Another limitation lies in the relatively long study period [2009–2018], during which treatment practices and staging criteria may have evolved, potentially influencing the interpretation of outcomes. Future studies with more balanced cohorts and adjustments for potential unobserved confounders are recommended to validate our findings and further refine T-staging criteria for NSCLC patients with larger GGO lesions.
Conclusions
Based on our findings, the IASLC staging recommendations are valid for GGO tumors larger than 2 cm in diameter, and the solid component size should be used instead of the overall size as an indicator for TNM staging. New clinical TNM staging that considers the GGO component may provide more accurate survival prediction and treatment guidance for patients with lung cancer over 2 cm.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-664/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-664/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-664/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-664/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from all study participants prior to data collection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The use of the data for this study was approved by the Institutional Review Board (IRB) of West China Hospital (No. 2023-1694).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-2110.e1. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. [Crossref] [PubMed]
- Park S, Lee SM, Choe J, et al. Recurrence Patterns and Patient Outcomes in Resected Lung Adenocarcinoma Differ according to Ground-Glass Opacity at CT. Radiology 2023;307:e222422. [Crossref] [PubMed]
- Liu C, Yang Z, Li Y, et al. Intentional wedge resection versus segmentectomy for ≤2 cm ground-glass-opacity-dominant non-small cell lung cancer: a real-world study using inverse probability of treatment weighting. Int J Surg 2024;110:4231-9. [Crossref] [PubMed]
- Fu F, Zhang Y, Wen Z, et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J Thorac Oncol 2019;14:2133-42. [Crossref] [PubMed]
- Henschke CI, Yip R, Shaham D, et al. A 20-year Follow-up of the International Early Lung Cancer Action Program (I-ELCAP). Radiology 2023;309:e231988. [Crossref] [PubMed]
- Nakamura S, Fukui T, Taniguchi T, et al. Prognostic impact of tumor size eliminating the ground glass opacity component: modified clinical T descriptors of the tumor, node, metastasis classification of lung cancer. J Thorac Oncol 2013;8:1551-7. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Wen Z, Fu F, Zhao Y, et al. Residual tumor descriptors proposed by the International Association for the Study of Lung Cancer may not be applicable to stage I and ground-glass opacity-featured non-small cell lung cancer. Transl Lung Cancer Res 2023;12:2157-68. [Crossref] [PubMed]
- Sun F, Huang Y, Yang X, et al. Solid component ratio influences prognosis of GGO-featured IA stage invasive lung adenocarcinoma. Cancer Imaging 2020;20:87. [Crossref] [PubMed]
- Hattori A, Suzuki K, Takamochi K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer with radiologically pure-solid appearance in Japan (JCOG0802/WJOG4607L): a post-hoc supplemental analysis of a multicentre, open-label, phase 3 trial. Lancet Respir Med 2024;12:105-16. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Hammad AY, Fernando HC. Commentary: is segmentectomy curative in patients with early-stage ground glass opacity predominant non-small cell lung cancer? AME Clin Trials Rev 2024;2:8.
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Casiraghi M, Petrella F, Bardoni C, et al. Surgically Treated pT2aN0M0 (Stage IB) Non-Small Cell Lung Cancer: A 20-Year Single-Center Retrospective Study. J Clin Med 2023;12:2081. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. Erratum in: Lancet Oncol 2006;7:797.
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Miyoshi T, Ito H, Wakabayashi M, et al. Risk factors for loss of pulmonary function after wedge resection for peripheral ground-glass opacity dominant lung cancer. Eur J Cardiothorac Surg 2023;64:ezad365. [Crossref] [PubMed]
- Aokage K, Suzuki K, Saji H, et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med 2023;11:540-9. [Crossref] [PubMed]
- Koike T, Hasebe T, Nakamura M, et al. Towards better outcomes: segmentectomy for ground-glass opacity-dominant non-small cell lung cancer 3 cm or less—insights from JCOG1211. AME Clin Trials Rev 2023;1:5.
- Fan F, Zhang Y, Fu F, et al. Subsolid Lesions Exceeding 3 Centimeters: The Ground-Glass Opacity Component Still Matters. Ann Thorac Surg 2022;113:984-92. [Crossref] [PubMed]
- Berry MF, Gao R, Kunder CA, et al. Presence of Even a Small Ground-Glass Component in Lung Adenocarcinoma Predicts Better Survival. Clin Lung Cancer 2018;19:e47-51. [Crossref] [PubMed]
- Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer 2015;88:174-80. [Crossref] [PubMed]
- Sun K, Xie H, Zhao J, et al. A clinicopathological study of lung adenocarcinomas with pure ground-glass opacity > 3 cm on high-resolution computed tomography. Eur Radiol 2022;32:174-83. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90.
- K M N MN. K R S, Paul Sreeram M. Muscling mussels: Understanding the invasive potential of the South American bivalve Mytella strigata (Hanley, 1843) in the Northern Indian Ocean. Sci Total Environ 2024;916:170243. [Crossref] [PubMed]
- Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer 2011;74:7-11. [Crossref] [PubMed]
- Shang J, Jiang H, Zhao Y, et al. Differences of molecular events driving pathological and radiological progression of lung adenocarcinoma. EBioMedicine 2023;94:104728. [Crossref] [PubMed]
- Wang C, Shao J, Song L, et al. Persistent increase and improved survival of stage I lung cancer based on a large-scale real-world sample of 26,226 cases. Chin Med J (Engl) 2023;136:1937-48. [Crossref] [PubMed]
- Zhang Y, Fu F, Wen Z, et al. Segment Location and Ground Glass Opacity Ratio Reliably Predict Node-Negative Status in Lung Cancer. Ann Thorac Surg 2020;109:1061-8. [Crossref] [PubMed]
- Li W, Zhou F, Wan Z, et al. Clinicopathologic features and lymph node metastatic characteristics in patients with adenocarcinoma manifesting as part-solid nodule exceeding 3 cm in diameter. Lung Cancer 2019;136:37-44. [Crossref] [PubMed]
- Suzuki S, Sakurai H, Yotsukura M, et al. Clinical Features of Ground Glass Opacity-Dominant Lung Cancer Exceeding 3.0 cm in the Whole Tumor Size. Ann Thorac Surg 2018;105:1499-506. [Crossref] [PubMed]



