
Association between immunotherapy timing and efficacy in non-small cell lung cancer: a comprehensive analysis at a high-volume specialist centre
Highlight box
Key findings
• The timing of the first immune checkpoint inhibitor (ICI) dose did not significantly impact progression-free survival (PFS) or overall survival (OS) in advanced NSCLC patients.
• Patients receiving more than 50% of their doses in the afternoon showed significantly poorer OS.
What is known and what is new?
• Previous studies suggested a potential chronotherapy effect on ICI efficacy, with morning administrations associated with better outcomes.
• This study showed that the timing of the first dose did not impact ICI efficacy in a large cohort.
What is the implication, and what should change now?
• Current clinical practices regarding the timing of ICI administration may not need immediate alteration based on the first dose timing.
• Prospective trials are needed to provide robust evidence before making any recommendations that would impact the scheduling in chemotherapy units.
Introduction
Circadian rhythms are endogenous oscillations with a period of approximately 24 hours that are entrained by external factors such as light and persist under constant conditions (1). Circadian rhythmicity governs the majority of human physiological functions, regulating organs and tissues via molecular clocks (2). As drug metabolism is among the functions affected by circadian rhythms, the concept of optimizing the timing of drug administration (chronotherapy) has long been explored as a strategy for improving drug efficacy and minimising side effects (3). Chrono-chemotherapy entails timing the cytotoxic administration considering circadian-regulated processes such as drug metabolism and target expression (4). It is a decades-old concept that has been tested in several solid tumours, mainly in colorectal carcinoma (5). Most trials showed a decrease in toxicity, but a clear and consistent benefit in overall survival (OS) has not been demonstrated and therefore this approach has not been widely implemented (6).
The immune system is also under circadian control, and periodic oscillations in both the innate and adaptive response have been demonstrated (7). Based on preclinical findings, a cluster-randomised trial compared antibody response to influenza vaccination depending on vaccine timing (8). It showed a significant increase in antibody levels in the group that was vaccinated in the morning (9–11 am) compared to those vaccinated in the afternoon (3–5 pm) (8). Although the mechanism of action of immune checkpoint inhibitors (ICIs) is fundamentally different, some clinical observations led to retrospective studies investigating a time-of-the-day effect in their efficacy. A seminal study evaluating patients with stage IV melanoma showed that ICI infusions administered in the evening (after 16:30) led to significantly shorter progression-free survival (PFS) and OS (9) compared to those administered before 16:30. This led to subsequent retrospective studies being conducted in a variety of solid tumours treated with ICIs. Regarding metastatic non-small cell lung cancer (NSCLC), a large retrospective study showed that patients who received over 20% of their ICI doses in the evening had a worse survival (10). Another study found that among patients who received at least four doses, those who received between two and four doses in the morning had significantly longer OS [hazard ratio (HR) =0.28, P=0.003] (11). Recently, a meta-analysis of 13 studies in several solid tumour types showed that morning infusions led to a significantly longer PFS [HR =0.51, 95% confidence interval (CI): 0.42–0.61; P<0.00001] and OS (HR 0.50, 95% CI: 0.42–0.58; P<0.00001) (12). Notably, the cutoff and percentage of “afternoon” doses defining the morning and afternoon groups varied widely among the included studies (12).
Despite several hypotheses attempting to explain this phenomenon, questions remain about the validity of these findings and whether the proportion of doses or the timing of the first dose plays a more critical role. The primary aim of our study was to evaluate the potential influence of the timing of the first dose on survival outcomes in patients with advanced NSCLC. The secondary objective was to assess the association between the percentage of doses administered after 14:30 and survival outcomes. As an exploratory aim, we compared the seasonal variations in the differential response between morning and afternoon doses. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-571/rc).
Methods
Study design
We conducted a retrospective, single centre, non-interventional cohort study in a tertiary cancer centre (The Christie NHS Foundation Trust). This study was registered and approved as a clinical audit by the Quality Improvement and Clinical Audit Committee at The Christie NHS Foundation Trust (registration No. 3673). Clinical data was pseudonymised for analysis. The audit procedures were compliant with the precepts of Good Clinical Practice guidelines with regards to the collection, storage, processing and disclosure of personal information, and the principles outlined in the Declaration of Helsinki (as revised in 2013) for all human investigations. Patient data was collected through an electronic case report form (eCRF) through medical chart review. Collected data included patient demographics, clinical characteristics at the time of initiation of first-line therapy, and clinical outcomes. Individual consent for this retrospective analysis was waived.
Patients
Eligible patients were ≥18 years old at time of diagnosis and met the following criteria: histologically or cytologically confirmed NSCLC; programmed cell death ligand 1 (PD-L1) expression ≥50%; had received first-line palliative treatment with single agent atezolizumab or pembrolizumab initiated between June 2017 and August 2023. For variables with truly missing data (e.g., demographic information), cases were excluded using listwise deletion. For specific variables where “unknown” was a relevant and observed category (e.g., certain laboratory measurements), these cases were retained and analysed within the “unknown” group to preserve clinical relevance.
Patients were divided into two groups, the morning group (first dose before 14:30) and the afternoon group (first dose after 14:30). This cut-off was selected pragmatically, guided by published literature and aligned with the typical scheduling of immunotherapy infusions at our centre. In further, exploratory analysis, we stratified patients who received at least 4 doses into two groups depending on the percentage of doses received after 14:30, those who received at least 20% or those who received at least 50% of the doses after that timepoint. Finally, to explore the possible seasonal influence, we stratified the analysis into patients who received the first dose between the autumn and spring equinox and those who received it between the spring equinox and the autumn equinox.
Outcomes
Objective response according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 was assessed by the responsible clinician using computed tomography (CT) scans. A centralized independent blinded review was not performed. The radiological response types, including partial response (PR), complete response (CR), stable disease (SD), and progressive disease (PD), were recorded as reported in the eCRF, along with the date of imaging that confirmed the patient’s best therapeutic response. The objective response rate (ORR) was determined by the percentage of patients achieving either CR or PR. PFS was defined as the time from treatment initiation to either radiological disease progression or death from any cause, whichever came first. Patients who were still alive without evidence of disease progression at their last follow-up were censored. OS was measured as the time from the start of treatment to the date of death, with patients alive at the last follow-up being censored.
Statistical analysis
Continuous variables were reported as medians with corresponding ranges, while categorical variables were summarized as frequencies and percentages. To compare two variables, the Chi-squared test or Fisher’s exact test was applied for categorical data, and the Wilcoxon rank-sum test or Pearson’s correlation analysis was used for continuous data. A P value below 0.05 was considered statistically significant. The variables examined included gender, age, Eastern Cooperative Oncology Group performance status (ECOG PS), and the presence of liver or brain metastases at baseline. Survival outcomes were assessed using the Kaplan-Meier method. All statistical analyses were performed using SPSS Version 24.0 (SPSS Inc., Portsmouth, Hampshire, UK).
Results
Patients
A total of 349 patients were included in the study with a median age of 70 years (range, 45–87 years) and 181 (51.9%) were males. The majority (n=288, 82.5%) of patients received pembrolizumab as first-line therapy. In the overall cohort, patients received a median of 8 cycles (range, 1–35 cycles). One hundred eighty-eight patients (53.9%) received their first dose before 14:30. Comprehensive clinical and demographic characteristics stratified by the first dose timing are shown in Table 1.
Table 1
| Characteristics | Total (n=349) | First dose <14:30 (n=188) | First dose ≥14:30 (n=161) | P value |
|---|---|---|---|---|
| Sex | 0.33 | |||
| Female | 168 (48.1) | 86 (45.7) | 82 (50.9) | |
| Male | 181 (51.9) | 102 (54.3) | 79 (49.1) | |
| Age (years) | 70 [45–87] | 70 [45–87] | 70 [45–87] | 0.41 |
| ECOG PS | 0.45 | |||
| 0 | 47 (13.5) | 29 (15.4) | 18 (11.2) | |
| 1 | 269 (77.1) | 143 (76.1) | 126 (78.3) | |
| 2 | 33 (9.5) | 16 (8.5) | 17 (10.6) | |
| Smoking status | 0.84 | |||
| Never smoker | 15 (4.3) | 7 (3.7) | 8 (5.0) | |
| Former smoker | 239 (68.5) | 129 (68.6) | 110 (68.3) | |
| Current smoker | 95 (27.2) | 52 (27.7) | 43 (26.7) | |
| Histology | 0.80 | |||
| Squamous | 102 (29.2) | 56 (29.8) | 115 (71.4) | |
| Non-squamous | 247 (70.8) | 132 (70.2) | 46 (28.6) | |
| PD-L1 | 0.39 | |||
| <90% | 193 (55.3) | 100 (53.2) | 93 (57.8) | |
| ≥90% | 156 (44.7) | 88 (46.8) | 68 (42.2) | |
| LIPI score | 0.11 | |||
| Good | 85 (24.4) | 45 (23.9) | 40 (24.8) | |
| Intermediate | 158 (45.3) | 84 (44.7) | 74 (46.0) | |
| Poor | 86 (24.6) | 43 (22.9) | 43 (26.7) | |
| Unknown | 20 (5.7) | 16 (8.5) | 4 (2.5) | |
| Liver metastases | 0.81 | |||
| No | 303 (86.8) | 164 (87.2) | 139 (86.3) | |
| Yes | 46 (13.2) | 24 (12.8) | 22 (13.7) | |
| Brain metastases | 0.23 | |||
| No | 304 (87.1) | 160 (85.1) | 144 (89.4) | |
| Yes | 45 (12.9) | 28 (14.9) | 17 (10.6) | |
| Treatment regimen | 0.75 | |||
| Pembrolizumab | 288 (82.5) | 154 (81.9) | 134 (83.2) | |
| Atezolizumab | 61 (17.5) | 34 (18.1) | 27 (16.8) | |
| IMD | 0.30 | |||
| Below D5 | 241 (69.1) | 125 (66.5) | 116 (72.0) | |
| Above D5 | 108 (30.9) | 63 (33.5) | 45 (28.0) |
Data are presented as n (%) or median [range]. ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; LIPI, Lung Immune Prognostic Index; IMD, Index of Multiple Deprivation; D5, decile 5; n, number.
After excluding patients (29.1%) who received less than 4 cycles, the cohort consisted of 243 patients. Of these, 145 (59.7%) patients received at least 20% of the doses after 14:30 and 33 (13.6%) patients received at least 50% of the doses after 14:30.
Clinical outcomes
The majority (n=314, 90.0%) of patients were evaluable for response. Among these, ORR was 41.3%, with 17 (4.9%) and 153 (43.8%) patients achieving CR and PR, respectively. Sixty-four (18.3%) patients experienced PD as best response. At the time of data cut-off (February 2024), 274 (78.5%) patients had experienced either disease progression or death. Minimum and median follow-up in censored patients were 4.0 and 27.3 months (IQR, 13.1–46.4 months), respectively. The median PFS in the entire cohort was 8.8 (95% CI: 7.4–10.2) months, with a median OS of 18.6 (95% CI: 15.6–21.5) months.
First dose timing analysis
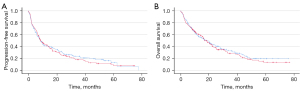
The outcomes of patients who received the first dose before 14:30 was not significantly different compared to those who received the first dose after 14:30. Median PFS was 8.9 (95% CI: 7.1–10.7) and 8.8 (95% CI: 6.5–11.0) months in the early and late groups (P=0.61), respectively (Figure 1A). OS was 19.0 (95% CI: 14.2–23.9) and 18.6 (95% CI: 15.6–21.5) months, without a significant difference (P=0.59) (Figure 1B). When we explored different administration time cut-offs (12:30, 13:30, 15:30, 16:30) the differences remained not significant.
Percentage of doses after 14:30
When we analysed patients who received at least 4 doses (n=243), those who received less than 20% of the doses after 14:30 (n=99) had a numerically, but not statistically, longer PFS: 20.3 (95% CI: 15.2–25.4) vs. 15.1 (95% CI: 11.2–19.1) months (P=0.20). OS was also numerically higher: 36.8 (95% CI: 23.6–50.1) vs. 24.5 (95% CI: 19.1–29.8) months (P=0.08).
When we increased the threshold to 50% of the doses given after 14:30 (n=33), the difference in OS was statistically significant: 32.7 (95% CI: 25.1–40.3) vs. 16.8 (95% CI: 11.6–22.1) months for the morning and afternoon groups, respectively (P<0.001). A statistically significant difference was also seen with regards to PFS: 19.1 (95% CI: 15.2–23.0) vs. 8.2 (95% CI: 5.7–10.7) months (P<0.001). The clinical and demographic characteristics of patients who received less than or greater than 50% of their doses after 14:30 are presented in Table 2.
Table 2
| Characteristics | Total (n=243) | 50% doses before 14:30 (n=210) | 50% doses after 14:30 (n=33) | P value |
|---|---|---|---|---|
| Sex | 0.21 | |||
| Female | 113 (46.5) | 101 (48.1) | 12 (36.4) | |
| Male | 130 (53.5) | 109 (51.9) | 21 (63.6) | |
| Age (years) | 69.4 [45–87] | 69.6 [45–87] | 68.1 [53–86] | 0.37 |
| ECOG PS | 0.93 | |||
| 0 | 41 (16.9) | 35 (16.7) | 6 (18.2) | |
| 1 | 183 (75.3) | 159 (75.7) | 24 (72.7) | |
| 2 | 19 (7.8) | 16 (7.6) | 3 (9.1) | |
| Smoking status | 0.87 | |||
| Never smoker | 11 (4.5) | 10 (4.8) | 1 (3.0) | |
| Former smoker | 162 (66.7) | 139 (66.2) | 23 (69.7) | |
| Current smoker | 70 (28.8) | 61 (29.0) | 9 (27.3) | |
| Histology | 0.66 | |||
| Squamous | 73 (30.0) | 62 (29.5) | 11 (33.3) | |
| Non-squamous | 170 (70.0) | 148 (70.5) | 22 (66.7) | |
| PD-L1 | 0.18 | |||
| <90% | 128 (52.7) | 107 (51.0) | 21 (63.6) | |
| ≥90% | 115 (47.3) | 103 (49.0) | 12 (36.4) | |
| LIPI score | 0.70 | |||
| Good | 63 (25.9) | 55 (26.2) | 8 (24.2) | |
| Intermediate | 116 (47.7) | 99 (47.1) | 17 (51.5) | |
| Poor | 50 (20.6) | 45 (21.4) | 5 (15.2) | |
| Unknown | 14 (5.8) | 11 (5.2) | 3 (9.1) | |
| Liver metastases | 0.91 | |||
| No | 215 (88.5) | 186 (88.6) | 29 (87.9) | |
| Yes | 28 (11.5) | 24 (11.4) | 4 (12.1) | |
| Brain metastases | 0.041 | |||
| No | 219 (90.1) | 186 (88.6) | 33 (100.0) | |
| Yes | 24 (9.9) | 24 (11.4) | 0 (0.0) | |
| IMD | 0.94 | |||
| Below D5 | 168 (69.1) | 145 (69.0) | 23 (69.7) | |
| Above D5 | 75 (30.9) | 65 (31.0) | 10 (30.3) |
Data are presented as n (%) or median [range]. ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; LIPI, Lung Immune Prognostic Index; IMD, Index of Multiple Deprivation; D5, decile 5; n, number.
Seasonal analysis
Given the significant seasonal variation in daylight in Manchester, we stratified patients into two groups: those who received the first dose within three months before or after the winter solstice (“Winter group”) and those who received it within three months before or after the summer solstice (“Summer group”). We analysed differences in survival outcomes among patients who received the first dose before or after 14:30 in each group. In the winter group, patients who received the first dose after 14:30 had similar PFS (8.7 vs. 9.4 months, P=0.93) and OS (16.8 vs. 14.5 months, P=0.99) compared to those who received the first dose before 14:30. In the summer group, patients who received the first dose after 14:30 did not show a significantly shorter PFS (8.8 vs. 8.6 months, P=0.47) or OS (20.3 vs. 19.1 months, P=0.45) compared to those who received the dose earlier.
Immune-related toxicities
Safety was consistent with previous reports. Overall, 179 (51.3%) patients experienced any immune-related adverse event (irAE), and 47 (13.5%) patients had a grade 3 or higher adverse event. Treatment was discontinued due to side effects in 49 (14.0%) patients and five treatment-related deaths were recorded (two pneumonitis, one pericarditis, one colitis, one disseminated intravascular coagulation). There were no significant differences in the incidence of irAEs between the morning and afternoon groups (5.19% vs. 44.7%, P=0.18).
Discussion
Our study found that the timing of the first ICI dose did not impact on survival outcomes in patients diagnosed with advanced NSCLC and treated with immunotherapy monotherapy. Differently from most of the published evidence, we did not find any significant difference in those patients who received more than 20% of the doses in the afternoon (after 14:30). We explored the potential impact of the time of year but did not find significant differences in survival outcomes related to this factor. Nonetheless, solar timing should be considered in future prospective studies to fully assess its potential influence. Although we did find a significant OS difference when we increased the threshold to 50%, the afternoon group was small (n=33) and therefore prone to confounding factors and bias. Furthermore, we did not find a difference in the incidence of irAEs depending on the timing.
Although there is strong evidence of a circadian regulation of the immune system, ICIs half-life and receptor occupancy are longer than 4 weeks (13). Therefore, the biological rationale behind a possible differential efficacy when ICIs are administered in the afternoon is not well understood. A retrospective study published by Rousseau et al. found that patients with NSCLC who received at least 20% of the doses after 16:30 had a worse OS, but that difference was not significant when the total number of ICI infusions was considered (10). Another multicentre, retrospective study of NSCLC patients treated with ICIs found numerically longer PFS and OS in the morning group, but that difference vanished after including the number of cycles in the propensity score matched analysis (14). Most published studies did not perform an analysis considering the number of cycles, which could at least partly account for the differences found.
We acknowledge that our study has several limitations. Firstly, due to its retrospective nature, patients were not randomly allocated to the morning or afternoon administration. Although the clinical characteristics were well balanced and administration time is usually offered based on treatment slot availability, we cannot rule out subtle socioeconomic or clinical bias between the two groups. This could explain the difference in OS found in the group that received more than 50% of the doses after 14:30, as patients who live in a rural environment or have a worse performance status may attend later than younger patients from an urban setting. Furthermore, being a single-center study impacts its generalizability to other populations and healthcare settings. Multicentric, prospective studies could address the aforementioned issues. Secondly, disease evaluation was performed according to local practice, so the PFS could be longer than that reported in clinical trials. However, this should affect both groups equally. Thirdly, it is a single centre analysis performed in a population living in a north latitude with strong daylight variations throughout the year. Some preclinical evidence suggests that ICI efficacy is higher when administered 2 hours after first light exposure in mice (15). Finally, treatment duration could influence the percentage of doses received in the afternoon. Patients who receive fewer doses are more susceptible to random variation, which may result in a higher proportion of doses being administered after 14:30. To mitigate this, we excluded patients who received fewer than four doses from the analysis. However, the potential influence of this factor cannot be entirely ruled out.
Despite the findings from previous studies and our own work, the true impact of immunotherapy timing remains unclear. If prospective studies confirm that timing plays a significant role, immunotherapy infusion schedules should be adjusted accordingly. However, this shift could introduce disparities, as patients from rural areas or lower socioeconomic backgrounds may face challenges in attending morning appointments. It will be crucial to consider these factors carefully if future research confirms the importance of timing and leads to changes in clinical practice.
Conclusions
Our study did not show a significantly worse PFS or OS in patients who received either the first ICI dose or more than 20% of the doses after 14:30. Although we found a difference in patients who received more than 50% of the doses after 14:30, the limited sample size warrants caution. Further prospective research is needed to better understand the influence of dosing time on ICI efficacy before changing treatment schedule.
Acknowledgments
We would like to thank the patients, their families, and all investigators involved in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-571/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-571/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-571/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-571/coif). I.G.R. has received consulting fees from Janssen. M.P. has received consulting fees from Janssen. R.C. reports grants paid to institution from Roche, AstraZeneca, Pfizer, Clovis, Lilly Oncology, MSD, BMS, Abbvie, Takeda, Janssen, Novartis, and has received consulting fees from AstraZeneca, Boeringher Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, BMS, Takeda, Janssen, Bayer, Novartis, and holds stock or stock options of The Christie Private Care. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved as a clinical audit by the Quality Improvement and Clinical Audit Committee at The Christie NHS Foundation Trust (registration No. 3673). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Quante M, Mariani S, Weng J, et al. Zeitgebers and their association with rest-activity patterns. Chronobiol Int 2019;36:203-13. [Crossref] [PubMed]
- Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 2020;21:67-84. [Crossref] [PubMed]
- Kisamore CO, Elliott BD, DeVries AC, et al. Chronotherapeutics for Solid Tumors. Pharmaceutics 2023;15:2023. [Crossref] [PubMed]
- Ohdo S, Koyanagi S, Matsunaga N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol Ther 2019;202:72-90. [Crossref] [PubMed]
- Amiama-Roig A, Verdugo-Sivianes EM, Carnero A, et al. Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers (Basel) 2022;14:5071. [Crossref] [PubMed]
- Printezi MI, Kilgallen AB, Bond MJG, et al. Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol 2022;23:e129-43. [Crossref] [PubMed]
- Scheiermann C, Gibbs J, Ince L, et al. Clocking in to immunity. Nat Rev Immunol 2018;18:423-37. [Crossref] [PubMed]
- Long JE, Drayson MT, Taylor AE, et al. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 2016;34:2679-85. Erratum in: Vaccine 2016;34:4842. [Crossref] [PubMed]
- Qian DC, Kleber T, Brammer B, et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol 2021;22:1777-86. [Crossref] [PubMed]
- Rousseau A, Tagliamento M, Auclin E, et al. Clinical outcomes by infusion timing of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Eur J Cancer 2023;182:107-14. [Crossref] [PubMed]
- Karaboué A, Collon T, Innominato P, et al. Improved survival on morning pembrolizumab with or without chemotherapy during initial treatment for stage IV non-small cell lung cancer. J Clin Oncol 2023;41:e21055. [Crossref]
- Landré T, Karaboué A, Buchwald ZS, et al. Effect of immunotherapy-infusion time of day on survival of patients with advanced cancers: a study-level meta-analysis. ESMO Open 2024;9:102220. [Crossref] [PubMed]
- Centanni M, Moes DJAR, Trocóniz IF, et al. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet 2019;58:835-57. [Crossref] [PubMed]
- Cortellini A, Barrichello A, Alessi JV, et al. 512 Time-of-day of pembrolizumab infusion and clinical outcomes of patients with NSCLC: too soon to promote morning infusions. J Immunother Cancer 2022; [Crossref]
- Lichterman JN, Srinivasan T, Li W, et al. Abstract 2658: The circadian clock modulates cancer immune checkpoint therapy through immune cell trafficking. Cancer Res 2024;84:2658. [Crossref]


