
Methodological aspects of studies on survival after immunotherapy in stage IV non-small cell lung cancer
We would like to thank Dr. Tsuruda and colleagues (1) for taking the time to read and comment on our manuscript, “Personal and clinical characteristics associated with immunotherapy effectiveness in stage IV non-small cell lung cancer” (2). The point they have raised in regards to the possibility of immortal time bias is a worthwhile consideration, and we’re grateful to have the opportunity to respond.
Tsuruda et al. suggest that measuring survival time from date of first systemic treatment is the most appropriate approach. We have now conducted such analysis, and found that the results are consistent with those previously published, namely that those patients treated with chemoimmunotherapy had better survival compared to those patients treated with chemotherapy alone (+5.5 months, Wilcoxon P<0.0001; Figure 1A).
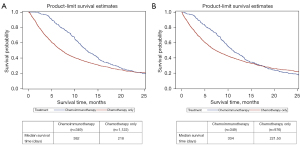
To their next point, patients treated with chemoimmunotherapy had to survive long enough to receive both chemotherapy and immunotherapy. To account for this, we performed a landmark analysis by generating stratified Kaplan-Meier curves beginning at the first chemoimmunotherapy death (48 days). Individuals who only received chemotherapy and died prior to 48 days of survival were excluded from this analysis. Survival time was calculated from first date of treatment to death or end to follow-up, although shifting by the 48-day landmark. Results, while attenuated, still showed better survival with combination systemic treatment (+3.8 months, Wilcoxon P<0.0001; Figure 1B).
While we are appreciative of Dr. Tsuruda et al., for highlighting this important consideration, ultimately, we are showing here that immortal time bias has not caused a substantial overestimation of survival. Instead, the observed better survival of non-small cell lung cancer (NSCLC) patients with the addition of immunotherapy treatment is robust to various methods of statistical correction. Lastly, we feel it is worth noting that while a potential source of time bias, the period between chemotherapy and immunotherapy treatments is none-the-less clinically and biologically important, and to remove it partly or completely from consideration is itself an avenue of bias introduction. We acknowledge that this was an analysis of observational data, and as such there were inherent limitations, including bias in regards to survival time. Unfortunately, no perfect method exists to balance these conflicting considerations; yet we feel strongly that our multiple methodologies to address the issue of immortal time bias, having yielded similar results, strengthen the conclusions drawn in our original paper.
We are grateful to Dr. Tsuruda et al. and editors for the opportunity to respond.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1119/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsuruda KM, Hektoen HH, Costa DR, et al. Immortal time bias in survival outcomes when comparing treatment with chemotherapy versus immunochemotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2025; [Epub ahead of print]. [Crossref]
- Patel KH, Alpert N, Tuminello S, et al. Personal and clinical characteristics associated with immunotherapy effectiveness in stage IV non-small cell lung cancer. Transl Lung Cancer Res 2023;12:1210-20. [Crossref] [PubMed]


