
Clinical features and outcomes of unresectable locally advanced lung adenocarcinoma with uncommon EGFR mutations: a retrospective multi-center Chinese study
Highlight box
Key findings
• Concurrent chemoradiotherapy (CRT) combined with sensitivity epidermal growth factor receptor (EGFR) gene-tyrosine kinase inhibitors (TKIs) depending on mutation genotype may be a promising treatment choice for unresectable locally advanced lung adenocarcinoma with uncommon EGFR mutation.
What is known and what is new?
• Low frequency and wide variety have limited the development of clinical trials of uncommon EGFR mutations.
• Radiotherapy (RT) combined with first generation EGFR-TKIs did not yield discernible survival advantages relative to CRT.
What is the implication, and what should change now?
• Future studies are needed to refine treatment strategies of combining RT with EGFR-TKIs or CRT for unresectable locally advanced lung adenocarcinoma with uncommon EGFR mutation.
Introduction
The phase 3 PACIFIC trial established a new standard of care for unresectable stage III non-small cell lung cancer (NSCLC) patients, particularly those receiving durvalumab consolidation therapy following definitive chemoradiotherapy (CRT) (1). However, the identification of epidermal growth factor receptor (EGFR) mutations, which have shown significant responsiveness to tyrosine kinase inhibitors (TKIs), presents a challenge in therapeutic decision-making for locally advanced NSCLC (2-5). In patients with EGFR mutations, the combination of EGFR-TKIs with radiotherapy (RT) has demonstrated superior survival outcomes compared to CRT with durvalumab or CRT alone (6-9).
Approximately 40–50% of Chinese patients with advanced non-squamous lung cancer harbor EGFR mutations (10-11). The most common mutations, exon 19 deletion(19DEL) and exon 21-L858R mutation, exhibit a superior response and survival rate compared to traditionally chemotherapy treatment (2-5). However, most clinical trials have excluded uncommon EGFR mutations, a highly heterogeneous group of mutations within exons 18–21, including T790M, G719X, and L861Q. Studies have demonstrated varying sensitivities to first, second, and third-generation EGFR-TKIs among patients with these uncommon mutations, with first-generation EGFR-TKIs being notably less effective (12-14).
Previously, our REFRACT program revealed that the frequency of EGFR mutations was significantly lower than that reported in advanced-stage Asian patients (6). Additional evidence indicates that the prevalence of EGFR-mutant stage III lung adenocarcinoma is lower (17–31%) compared to advanced-stage disease (40–50%), with limited reporting on uncommon mutations (15-22). Furthermore, uncommon mutations were not included in the phase 3 LAURA trial, which has reshaped treatment standards for locally advanced NSCLC with common mutations (9). The low incidence and wide variety of these mutations have constrained the development of clinical trials targeting uncommon EGFR mutations. To address this gap, we conducted a multi-center study focusing on patients with locally advanced lung adenocarcinoma harboring uncommon EGFR mutations to elucidate their clinical demographics and survival outcomes under different treatment modalities. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-751/rc).
Methods
Data sources and treatment cohorts
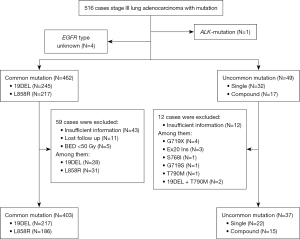
This study included data from the multi-center REFRACT program (trial number: NCT04304638). We extracted information from this database, including mutation type, treatment details, and survival endpoints. Patients with unknown EGFR mutations or mutations in other genes were excluded from enrollment. The data analysis flow is presented in Figure 1. Patients were stratified into three cohorts based on their initial treatment strategy involving RT or EGFR-TKIs therapy. Those who received concurrent or sequential CRT were included in the study. EGFR mutations were assessed using either polymerase chain reaction (PCR) amplification or next-generation sequencing. The follow-up strategy and RT plans have been previously described in detail (6).
Definition of endpoints
Overall survival (OS) was defined as the time from histological diagnosis to death or the last follow-up. Progression-free survival (PFS) was defined as the time from histological diagnosis to the first event of tumor progression or death. Locoregional recurrence was defined as disease recurrence in the ipsilateral lung and regional lymph nodes, while new lesions outside of the loco-regional areas were considered distant recurrences.
Statistical analysis
Data comparisons were conducted using Chi-squared tests, t-tests, and non-parametric Mann-Whitney U tests. Survival data were evaluated using the Kaplan-Meier method, and differences were examined using the log-rank test. Hazard ratios were estimated using the Cox proportional hazards model. Variables with a univariable significance of P<0.10 were entered into the multivariable Cox model, and a two-sided P value <0.05 was considered statistically significant. Statistical analyses were performed using STATA 16.0 or SPSS 26.0.
Ethical statement
The study was conducted in accordance with the standards set forth in Good Clinical Practice guidelines and the principles of outlined in the Declaration of Helsinki (as revised in 2013). This study protocol was approved by the Medical Research Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (2020042915382802), and the other participant units were also agreed the study. Written informed consent was waived since this is a retrospective analysis.
Results
Clinical characteristics
A total of 511 patients were identified in the current cohorts, including 245 patients (47.9%) with an exon 19 deletion, 217 patients (42.5%) with the L858R exon mutation, and 49 patients (9.6%) with an uncommon mutation. Among these, 162 patients (31.7%) were smokers, 349 (68.3%) were non-smokers, and 306 (59.9%) were female. The median age was 60 years, with a range of 30–89 years. Of the total cohort, 168 patients (32.9%) were diagnosed with stage IIIA and 343 patients (67.1%) with stage IIIB. Additionally, 482 patients (94.3%) were in good physical condition.
The median age at diagnosis was 57 years (range, 37–80 years) for patients with common EGFR mutations and 61 years (range, 30–89 years) for those with uncommon mutations. The baseline characteristics, including sex, performance status, smoking status, and stage, were similar between the two groups. Detailed baseline information was presented in Table 1.
Table 1
| Characteristic | Total (N=511) | Uncommon (N=49) | Common (N=462) | P |
|---|---|---|---|---|
| Age, years | 0.14 | |||
| Median [range] | 60 [30–89] | 57 [37–80] | 61 [30–89] | |
| ≥60, n (%) | 249 (48.7) | 29 (59.2) | 220 (47.6) | |
| <60, n (%) | 262 (51.3) | 20 (40.8) | 242 (52.4) | |
| Sex, n (%) | 0.76 | |||
| Female | 306 (59.9) | 28 (57.1) | 278 (60.2) | |
| Male | 205 (40.1) | 21 (42.9) | 184 (39.8) | |
| Smoking, n (%) | >0.99 | |||
| Yes | 162 (31.7) | 15 (30.6) | 147 (31.8) | |
| No | 349 (68.3) | 34 (69.4) | 315 (68.2) | |
| ECOG PS, n (%) | >0.99 | |||
| <2 | 482 (94.3) | 47 (95.9) | 435 (94.2) | |
| ≥2 | 29 (5.7) | 2 (4.1) | 27 (5.8) | |
| Stage, n (%) | 0.87 | |||
| IIIA | 168 (32.9) | 15 (30.6) | 153 (33.1) | |
| IIIB | 343 (67.1) | 34 (69.4) | 309 (66.9) | |
| PET-CT, n (%) | 0.08 | |||
| Yes | 131 (25.6) | 18 (36.7) | 113 (24.5) | |
| No | 380 (74.4) | 31 (63.3) | 349 (75.5) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PET-CT, positron emission tomography-computed tomography.
Frequencies
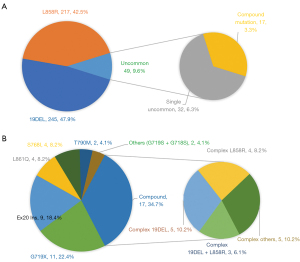
Among patients with uncommon EGFR mutations, 9 (18.4%) had a single exon 20 insertion (EX20 Ins), while 11 (22.4%) had the G719X point mutation occurring in exon 18. The L861Q and S768I mutations were each found in 4 patients (eight patients in total), and the T790M mutation was identified in 2 patients. Complex mutations were observed in 17 patients (34.7%), including 19DEL + L858R in 3 patients and 19DEL or L858R + T790M in 6 patients. Other infrequently reported uncommon mutations included G719S (n=1), G718S (n=1), G719X + S768I (n=1), G719X + L861Q (n=1), L861Q + S768I (n=1), S768I + G719S (n=1), 19DEL + L861Q (n=1), L858R + G719S (n=1), L858R + S768I (n=1), and G719X + T790M (n=1). The frequencies of the uncommon and common subtypes were presented in Figure 2.
Treatment
At the time of analysis, 440 of the 511 patients with EGFR mutations had received systemic anticancer treatment and had adequate follow-up (baseline clinical characteristics are shown in Table S1). In the uncommon mutation group, 9 patients (23.4%) received a combination of EGFR-TKIs and RT (EGFR-TKIs + RT), while 16 patients (43.2%) were treated with EGFR-TKIs. In the CRT subgroup (N=12), with 9 patients (24.3%) receiving concurrent CRT and 3 patients (8.1%) receiving sequential CRT. The RT dose ranged from 54.0 to 61.6 Gy, with the most common concurrent chemotherapy regimen being pemetrexed and cisplatin (n=8, 88.9%). In EGFR-TKIs group, 14 patients (87.5%) received first-generation TKIs (10 gefitinib, 3 icotinib, and 1 erlotinib), 1 patient received a second-generation TKI (afatinib), and 1 patient received a third-generation EGFR-TKIs (osimertinib). In the EGFR-TKIs + RT subgroup, 8 patients (88.9%) received first-generation TKIs (5 gefitinib, 2 icotinib, and 1 erlotinib), and 1 patient received experimental EGFR-TKI. The RT dose in this subgroup ranged from 50.0 to 60.0 Gy. One patient received concurrent EGFR-TKIs without chemotherapy, 8 patients received pemetrexed and cisplatin before RT, 2 patients received sequential EGFR-TKIs before RT, and 6 patients received EGFR-TKIs after CRT. In the common mutation group, 93 patients (23.4%) received EGFR-TKIs + RT, 214 patients (53.8%) were treated with EGFR-TKIs alone, and 91 patients (22.9%) received CRT.
Survival
The median follow-up period was 35.9 months [95% confidence interval (CI), 32.5–39.3]. During this time, 320 patients (72.7%) experienced tumor recurrence, and 164 patients (37.2%) died. A significant difference in PFS for uncommon and common mutation group [median 11.9 (95% CI: 8.1–15.7) vs. 17.5 (95% CI: 15.7–19.4) months; P=0.005, Figure 3A]. Multivariable Cox regression analysis confirmed this difference [P=0.006, hazard ratio (HR): 1.67, 95% CI: 1.16–2.40]. However, there was no significant difference in OS for uncommon and common mutation group [median 42.6 (95% CI: 29.3–55.9) vs. 54.2 (95% CI: 46.6–61.7) months; P=0.14, Figure 3B].
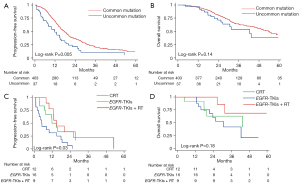
For the uncommon mutation group, the median PFS for the CRT, EGFR-TKIs, and EGFR-TKIs + RT subgroups were 11.9 months (95% CI, 6.5–17.3), 5.0 months (95% CI, 3.3–6.6), and 14.8 months (95% CI, 9.8–19.8), respectively (P=0.03; Figure 3C). The median OS for the three subgroups was 43.6 months (95% CI, 9.0–78.2), 30.9 months (95% CI, 19.8–42.0), and not reached (NR), respectively (P=0.18; Figure 3D). Patients who received CRT or EGFR-TKIs showed significantly superior PFS compared to those who received TKIs alone (P=0.02, 0.04, respectively). The EGFR-TKIs + RT regimen did not result in increased PFS compared to CRT alone (P=0.90). Similarly, the CRT (P=0.49) and EGFR-TKIs + RT (P=0.07) groups showed a trend toward superior OS compared to the EGFR-TKIs group, though the difference was not statistically significant. There was no difference in OS between EGFR-TKIs + RT and CRT alone (P=0.21).
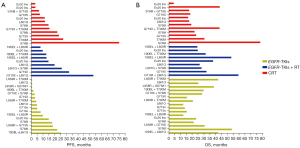
The median PFS was significantly shorter in patients with uncommon EGFR mutations compared to those with common mutations who were treated with EGFR-TKIs [median 5.0 (95% CI: 3.3–6.6) vs. 16.9 (95% CI: 14.0–19.8) months, P<0.001]. However, PFS was similar in patients treated with CRT (P=0.39) and EGFR-TKIs + RT (P=0.09). OS was shorter in patients with uncommon EGFR mutations compared to those with common mutations treated with TKIs [median 30.9 (95% CI: 19.8–49.3) vs. 49.3 (95% CI: 36.8–61.7), P=0.09, Figure 3]. However, OS was similar in the CRT (P=0.48) and EGFR-TKIs + RT (P=0.73) groups. The survival outcomes for patients with uncommon EGFR mutations were shown in Figure 4.
Recurrence patterns
Among the 37 patients with uncommon mutations, tumor recurrence was identified in 34 patients (91.9%), compared to 286 patients (71.0%) with common mutations (P=0.006). Distant metastases or loco-regional failure as the first site of recurrence were similar between the uncommon and common mutation groups (P>0.99, P=0.27, respectively). Brain metastases were also comparable between the two groups (24.3% vs. 24.1%, P>0.99, Table 2).
Table 2
| First site of progression | Total, n (%) | Common, n (%) | Uncommon, n (%) | P |
|---|---|---|---|---|
| All | 320 (72.7) | 286 (71.0) | 34 (91.9) | 0.006 |
| Distant only | 125 (28.4) | 115 (28.5) | 10 (27.0) | >0.99 |
| Loco-regional only | 138 (31.4) | 123 (30.5) | 15 (40.5) | 0.27 |
| Distant + loco-regional | 57 (13.0) | 48 (11.9) | 9 (24.3) | 0.04 |
| Brain | 106 (24.1) | 97 (24.1) | 9 (24.3) | >0.99 |
EGFR, epidermal growth factor receptor.
Compound mutations
Of the 17 patients with compound mutations, 9 were male, and all were current or former smokers, with an age range of 37–79 years (median, 58 years). Six patients were diagnosed with stage IIIA, and 11 with stage IIIB. Patients with compound mutations had similar OS [median 42.6 (95% CI: 29.8–55.4) vs. 26.7 (95% CI: 21.8–31.7), P=0.32] and PFS [median 10.0 (95% CI: 2.3–17.7) vs. 11.9 (95% CI: 6.2–17.6) months, P=0.30] compared to patients with common mutations. The baseline characteristics of patients between the two groups were balanced with respect to sex, performance status, smoking status, and stage (Tables S2,S3). Recurrence rates were similar between the two groups (P=0.26, Table S4).
Discussion
In this study, we analyzed the frequency and characteristics of EGFR mutations in patients with unresectable locally advanced adenocarcinoma. We found that uncommon mutations accounted for 9.6% of all EGFR mutations, with G719X (2.2%, 11/511) and EX20 Ins (1.7%, 9/511) being the most common single uncommon mutation sites. Additionally, we observed that 3.3% (17/511) of the patients had EGFR compound mutations, including combinations of G719X, L858R, and S768I. These findings were consistent with prevalence rates reported in other studies (11,23-26). The demographic and clinical characteristics of the two groups in our study those with uncommon and common mutations were similar regarding sex, age, smoking status, and disease stage.
The uncommon EGFR mutations generally exhibited lower responses rates to TKI than observed in common mutations, especially for first-generation EGFR-TKIs. The first-line chemotherapy remains relatively effective for NSCLC, especially with uncommon EGFR mutations (13,27). Our results indicated that patients receiving standard CRT as first-line treatment had significantly longer PFS compared to those who received EGFR-TKIs. Furthermore, there was no significant difference in survival between patients with uncommon and common mutations within the CRT group (median PFS 11.9 vs. 12.6 months, P=0.39; median OS 43.6 vs. 53.9 months, P=0.48). These findings aligned with those from a small retrospective study on CRT for patients with uncommon mutations (18). These results suggest that CRT remains the standard of care for this patient subgroup.
Previous real-world data and clinical trials have suggested that patients with uncommon EGFR mutations generally have a poorer treatment response to EGFR-TKIs compared to those with common mutations, particularly with first-generation EGFR-TKIs (12-14,23). Our data corroborated these findings, showing that PFS and OS were significantly shorter in patients with uncommon mutations treated with EGFR-TKIs (median PFS 5.0 vs. 16.9 months, P<0.001; median OS 30.9 vs. 49.3 months, P=0.14) compared to those with common mutations. However, afatinib has demonstrated a favorable response in patients with certain uncommon mutations, including G719X, L861Q, and S768I, according to the LUX-Lung 2, 3, and 6 studies (12). Similarly, a phase II clinical trial of osimertinib reported favorable outcomes and tolerable toxicity in patients with G719X (n=19; 53%), L861Q (n=9; 25%), and S768I (n=8; 22%) mutations. Notably, EX20 Ins mutations have shown poor responses to both first- and second-generation EGFR-TKIs (28). Few patients with EX20 Ins have responded to EGFR-TKIs, and these patients were excluded from trials involving third-generation EGFR-TKIs (osimertinib) (14,29). Based on our findings, EGFR-TKIs should not be recommended as the first choice for locally advanced NSCLC with uncommon mutations. The LAURA trial established that osimertinib combined with CRT significantly prolonged PFS compared to placebo in unresectable stage III EGFR-common mutation NSCLCs (9). A systematic review supported the use of second-generation TKI afatinib for G719X, S768I, E709X, and L747X mutations and for compound uncommon mutations. For other uncommon mutations such as L861Q, exhibited 75% (95% CI: 56.6–88.5%) response rates to osimertinib (27). Therefore, the optimal treatment strategy for patients with uncommon mutations remains a challenge, and a combination of RT and sensitivity EGFR-TKIs may be a promising option depending on the specific mutation genotype.
Few studies have specifically examined the prevalence and survival outcomes of compound mutations. In our cohort, the frequency of EGFR compound mutations was 3.3% (n=17/511), slightly lower than reported in a previous study (30). In subgroup analyses, patients with EGFR compound mutations had similar PFS compared to those with individual uncommon mutations (median PFS 10.0 vs. 11.9 months, P=0.30). Previous research has indicated that the PFS for patients with EGFR compound mutations was significantly higher than for those with individual uncommon mutations (median PFS 11.9 vs. 6.5 months, P=0.01) among patients receiving EGFR-TKIs alone (31). Another study reported different results for PFS (median 9.3 vs. 7.0 months, P=0.45) but found a significant difference in OS (median 31.4 vs. 12.4 months, P<0.001) (32). Due to the limited sample size, these results require validation through further studies.
Several limitations of this study should be acknowledged. The selection bias could not be excluded owing to retrospectively design. The most significant limitation was the small size of the uncommon mutation cohort. In particular, 88.0% of patients with uncommon mutation was treated with the first generation of EGFR-TKIs, which are known to be less effective. This highlighted the need for further data on the next generation of EGFR-TKIs. Moreover, it is necessary to conduct prospective data validation to facilitate a comparison of the results obtained from the different treatment. Additionally, the detection of uncommon mutations was constrained by sequencing methods and sample availability. PCR-based or direct sequencing methods may have underestimated the prevalence of EGFR uncommon mutations compared to next-generation sequencing. Plasma genetic testing was feasible but less accurate and sensitive than tissue biopsy. Moreover, clinical decision-making for locally advanced NSCLC with EGFR mutations varies among institutions and physicians. Finally, concurrent RT and EGFR-TKIs pose concerns regarding pulmonary toxicity. A systematic literature review indicated that the rate of severe lung pneumonitis was 4.9% (95% CI, 2.4–8.1%) for concurrent EGFR-TKIs and RT, significantly higher than the 0.4% (95% CI, 0.0–3.1%) rate for sequential therapy (33).
Conclusions
In conclusion, this study provided a comprehensive overview of the prevalence and outcomes of uncommon EGFR mutations in unresectable locally advanced lung adenocarcinoma patients. Our findings suggested that uncommon mutations are associated with worse survival outcomes when treated with EGFR-TKIs. However, CRT remained the primary treatment, with RT combined with EGFR-TKIs emerging as a promising approach. Future studies are needed to validate these results and refine treatment strategies for patients with uncommon EGFR mutations.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-751/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-751/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-751/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-751/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the standards set forth in Good Clinical Practice guidelines and the principles of outlined in the Declaration of Helsinki (as revised in 2013), and the participant hospitals were also informed and agreed the study. This study was registered with Clinical Trials.gov. (trial number: NCT04304638). This study protocol was approved by the Medical Research Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (2020042915382802). Written informed consent was waived since this is a retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Ochiai S, Nomoto Y, Watanabe Y, et al. The impact of epidermal growth factor receptor mutations on patterns of disease recurrence after chemoradiotherapy for locally advanced non-small cell lung cancer: a literature review and pooled analysis. J Radiat Res 2016;57:449-59. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Bi N, Xu K, Ge H, et al. Real-world treatment patterns and clinical outcomes in EGFR-mutant locally advanced lung adenocarcinoma: A multi-center cohort study. J Natl Cancer Cent 2023;3:65-71. [Crossref] [PubMed]
- Nassar AH, Kim SY, Aredo JV, et al. Consolidation Osimertinib Versus Durvalumab Versus Observation After Concurrent Chemoradiation in Unresectable EGFR-Mutant NSCLC: A Multicenter Retrospective Cohort Study. J Thorac Oncol 2024;19:928-40. [Crossref] [PubMed]
- Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for Stage III EGFR-Mutated NSCLC After Definitive Chemoradiotherapy. J Thorac Oncol 2021;16:1030-41. [Crossref] [PubMed]
- Lu S, Kato T, Dong X, et al. Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N Engl J Med 2024;391:585-97. [Crossref] [PubMed]
- Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol 2015;10:438-45. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014;9:189-94. [Crossref] [PubMed]
- Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. [Crossref] [PubMed]
- Qin Q, Peng B, Li B. The impact of epidermal growth factor receptor mutations on the efficacy of definitive chemoradiotherapy in patients with locally advanced unresectable stage III non-small cell lung cancer: a systematic review and meta-analysis. Expert Rev Anticancer Ther 2019;19:533-9. [Crossref] [PubMed]
- Tanaka K, Hida T, Oya Y, et al. EGFR Mutation Impact on Definitive Concurrent Chemoradiation Therapy for Inoperable Stage III Adenocarcinoma. J Thorac Oncol 2015;10:1720-5. [Crossref] [PubMed]
- Lim YJ, Chang JH, Kim HJ, et al. Superior Treatment Response and In-field Tumor Control in Epidermal Growth Factor Receptor-mutant Genotype of Stage III Nonsquamous Non-Small cell Lung Cancer Undergoing Definitive Concurrent Chemoradiotherapy. Clin Lung Cancer 2017;18:e169-78. [Crossref] [PubMed]
- Park SE, Noh JM, Kim YJ, et al. EGFR Mutation Is Associated with Short Progression-Free Survival in Patients with Stage III Non-squamous Cell Lung Cancer Treated with Concurrent Chemoradiotherapy. Cancer Res Treat 2019;51:493-501. [Crossref] [PubMed]
- Nakamura M, Kageyama SI, Niho S, et al. Impact of EGFR Mutation and ALK Translocation on Recurrence Pattern After Definitive Chemoradiotherapy for Inoperable Stage III Non-squamous Non-small-cell Lung Cancer. Clin Lung Cancer 2019;20:e256-64. [Crossref] [PubMed]
- Yagishita S, Horinouchi H, Katsui Taniyama T, et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015;91:140-8. [Crossref] [PubMed]
- Akamatsu H, Kaira K, Murakami H, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol 2014;37:144-7. [Crossref] [PubMed]
- Hayashi H, Okamoto I, Kimura H, et al. Clinical outcomes of thoracic radiotherapy for locally advanced NSCLC with EGFR mutations or EML4-ALK rearrangement. Anticancer Res 2012;32:4533-7. [PubMed]
- Pilotto S, Rossi A, Vavalà T, et al. Outcomes of First-Generation EGFR-TKIs Against Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Post Hoc Analysis of the BE-POSITIVE Study. Clin Lung Cancer 2018;19:93-104. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016;107:1179-86. [Crossref] [PubMed]
- Lohinai Z, Hoda MA, Fabian K, et al. Distinct Epidemiology and Clinical Consequence of Classic Versus Rare EGFR Mutations in Lung Adenocarcinoma. J Thorac Oncol 2015;10:738-46. [Crossref] [PubMed]
- Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer 2017;114:96-102. [Crossref] [PubMed]
- Borgeaud M, Parikh K, Banna GL, et al. Unveiling the Landscape of Uncommon EGFR Mutations in NSCLC-A Systematic Review. J Thorac Oncol 2024;19:973-83. [Crossref] [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016;9:4181-6. [Crossref] [PubMed]
- Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol 2014;19:594-600. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Li H, Wang C, Wang Z, et al. Efficacy and long-term survival of advanced lung adenocarcinoma patients with uncommon EGFR mutations treated with 1st generation EGFR-TKIs compared with chemotherapy as first-line therapy. Lung Cancer 2019;130:42-9. [Crossref] [PubMed]
- Meng Y, Sun H, Wang S, et al. Treatment-Related Pneumonitis of EGFR Tyrosine Kinase Inhibitors Plus Thoracic Radiation Therapy in Patients With Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Int J Radiat Oncol Biol Phys 2024;118:415-26. [Crossref] [PubMed]


