
Lorlatinib in frontline treatment of advanced ALK-positive non-small cell lung cancer: a highly efficient new standard of care but still challenging to manage
Since 2014, the frontline treatment of patients with metastatic anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC) has been based on ALK targeting tyrosine kinase inhibitors (TKIs), crizotinib (defining the 1st generation of ALK TKIs) being the first to demonstrate superiority over platinum-based chemotherapy doublets (1). The second generation of ALK TKIs with alectinib, brigatinib and ensartinib characterized by higher potency on native ALK kinase and activity on certain crizotinib resistance mutations, as well as better central nervous system (CNS) penetration, proved superior to crizotinib in terms of progression-free survival (PFS) (2-4) and overall survival (OS) (5). Lorlatinib is a 3rd-generation inhibitor based on a macrocyclic chemical structure ensuring excellent brain penetration, active on virtually all single mutations resistant to 1st- and 2nd-generation ALK TKIs. The initial results of the phase III CROWN trial, comparing lorlatinib to crizotinib in first-line demonstrated an increase in PFS by blinded independent central review (BICR) [hazard ratio (HR) for PFS 0.28, 95% confidence interval (CI): 0.19–0.41] and an impressive intracranial efficacy [HR for intracranial PFS 0.07 (95% CI: 0.03–0.17)] consistent with the CNS penetration (6). Based on these promising efficacy data, lorlatinib joined the 2nd-generation TKIs as standard of care from the first-line [European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines] without defining a hierarchy (7).
Recently, updated results from the CROWN study with a median follow-up of 60.2 months showed that the median PFS was not reached yet in the lorlatinib arm (8). Do these unprecedented results appear convincing enough to definitively change the standard of treatment for ALK+ advanced NSCLC and formally establish lorlatinib as 1st-line therapy?
In the absence of comparative trials, and since cross-study comparisons do not provide sufficient level of evidence, it is crucial to consider all factors that support the prescription of a new frontline targeted therapy. These factors include systemic and CNS efficacy, tolerance, resistance mechanisms, and quality of life (Figure 1), to help guide physicians in making first-line treatment decisions. The multicenter CROWN phase III trial included 296 patients randomized 1:1 between lorlatinib given at the dose of 100 mg once daily and crizotinib (250 mg twice daily) with PFS as the primary objective, centrally assessed for the first three years, and by investigator evaluation beyond three years. Patients were stratified based on the presence of brain metastases at diagnosis and ethnicity (Asian or non-Asian). Cross-over was not permitted in the study. The main efficacy results are reported in Table 1. The updated 5-year results demonstrated a very impressive sustained systemic efficacy, with a 5-year PFS rate of 60% [HR (95% CI) for PFS 0.19 (0.13–0.27)]. Intracranial efficacy was also impressive, with 92% (95% CI: 85–96%) of patients not developing intracranial progression over five years and with 83% of those with brain metastases at baseline remaining progression-free at 5 years. The intracranial objective response rate (IC-ORR) was 60%, with 49% achieving complete responses. Among patients without baseline brain metastases (n=114), only four patients developed brain metastases (by investigator assessment).
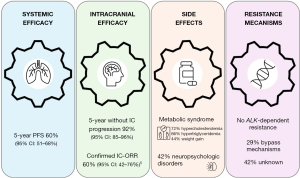
Table 1
| Outcomes | Lorlatinib (CROWN) | Alectinib (ALEX) | Brigatinib (ALTA-1L) |
|---|---|---|---|
| Median PFS, months (95% CI) (investigator-assessed) | NR (64.3–NR) | 34.8 (17.7–NR) | 30.8 (21.3–40.6) |
| PFS rates, % (95% CI) | 60 (51–68) at 5 years | 43.7 at 4 years | 36.0 (26–46) at 4 years |
| HR for PFS versus crizotinib (95% CI) (BIRC-assessed) | 0.19 (0.13–0.27) | 0.50 (0.36–0.70) | 0.48 (0.35–0.66) |
| Median intracranial PFS, months (95% CI) (ITT population)† | NR | NA | 44.1 (32.2–NR) |
| HR for intracranial progression versus crizotinib (95% CI) | 0.06 (0.03–0.12) | 0.37 (0.23–0.58) | 0.25 (0.14–0.46) |
†, intracranial PFS events are not defined in an identical manner in the three trials. ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; CI, confidence interval; HR, hazard ratio; BIRC, blinded independent review committee; ITT, intent-to-treat; NR, not reached; NA, not available.
Interestingly, the lorlatinib PFS curve shows a “plateau” which was not previously observed with other TKIs, raising questions about the emergence of “long-responders” as described with treatment with anti-PD(L)-1. This could be explained by the absence of an ALK-dependent resistance mechanism and a major protective effect against brain metastases. These data are remarkable in the context of advanced ALK-positive lung cancer, which has a strong propensity for CNS progression, often marking a decisive turning point in the natural history of the disease and its management.
The impact of fusion variants and co-mutations was also analyzed in 89% of patients. However, the fusion was not detected in more than 50% of patients. In the lorlatinib group, among the 15% of patients harboring an EML4 v1 variant and 13% with an EML4 v3a/b variant, no difference in PFS was observed. This could be linked with the absence of emergence of G1202R mutations with lorlatinib, which is more frequently associated with v3 variants in patients receiving 2nd-generation ALK TKIs. However, due to the small sample size, these results should be interpreted with caution. Outcomes were also evaluated with regard to TP53 mutations and as expected, the presence of TP53 mutations was associated with shorter PFS. This probably reflects higher tumor heterogeneity, the involvement of alternative cell survival pathways and its immunomodulatory role in the tumor microenvironment.
Mechanisms of resistance to frontline lorlatinib are largely unknown. Analysis of circulating tumor DNA samples from patients who progressed on first-line lorlatinib (n=31) in CROWN did not reveal any ALK-dependent resistance mechanisms, a finding never observed with other ALK TKIs. These resistance mechanisms appear to differ from those reported with lorlatinib administered sequentially after prior treatment with 1st- or 2nd-generation ALK TKIs (9,10). This could be explained by the activity of lorlatinib on most of single ALK-mutations and to prevent their emergence. Bypass mechanisms (MAPK, PI3K/MTOR/PTEN, RTK, cell cycle pathways aberration) were the most common identified routes of escape (n=9, 29%), mechanisms of resistance remaining unknown for the majority of patients in the lorlatinib arm (42%). Data on histological transformation, which would also be important to consider, are not yet available. This raises the question of how to manage ALK-independent relapses in the future. In the absence of ALK-resistance mutations, the use after frontline lorlatinib of 4th-generation inhibitors active on compound ALK-mutations such as NVL-655 would not be appropriate (11) and chemotherapy will probably remain the preferred option. Further research on ALK-independent resistance pathways is warranted to guide physicians’ choice for subsequent treatment lines, also opening the door to potential novel therapeutic approaches.
The tolerance profile is crucial for ALK TKIs as patients will remain under treatment for years, which is especially true for lorlatinib. In the CROWN trial, the proportion of grade ≥3 treatment-related adverse event (TRAE) was 66% for lorlatinib with a median exposure of 57 months (range, 13.9–63.3 months). Lorlatinib has a very specific safety profile, as compared with other ALK inhibitors, making its management more challenging in clinical practice.
Nearly all patients on lorlatinib will develop metabolic syndrome, involving hyperlipidemia and weight gain. Hypercholesterolemia was reported in 72% of patients, including 32/149 (21.5%) with grade ≥3. Hypertriglyceridemia was reported in 66% of patients, with 17% and 8% experiencing grade 3 or 4, respectively. Hypolipidemic treatment is recommended starting at grade 2, affecting 47% of patients. The median time to onset of hyperlipidemia was 15 days and a median duration of 37 months. Most patients (71%) with hyperlipidemia were managed with oral medication without lorlatinib interruption or dose reduction. Nevertheless, 55% of hyperlipidemia cases were unresolved or only partially resolved. Weight gain affected 44% of patients, negatively impacting both physical and psychological quality of life, half of them were grade 3 (meaning a gain of ≥20% of weight at baseline). Initial onset occurred at 4–5 months and may persist over the long-term, with no increase in prevalence over time. Twenty-one percent of patients gaining weight had no associated subcutaneous edema, suggesting distinct pathophysiological mechanisms, notably hyperphagia.
The key question is whether lorlatinib-induced hyperlipidemia may translate into an increased risk of arterial ischemic events, which must be distinguished from venous thrombo-embolic (VTE) events. Curiously, the analysis of the “Standardized MedDRA Queries (SMQ) ischemic heart disease” group included isolated elevation of blood creatine phosphokinase (CPK), which skews the interpretation, representing 19/21 SMQ events. Indeed, elevated CPK is commonly seen with ALK TKIs and often asymptomatic, with generally no clinical consequences. When focusing on relevant ischemic cardiac toxicity, there appear to be slightly more events in the lorlatinib group than in the crizotinib group. Myocardial ischemia/infarction was reported in three patients, heart failure in seven patients, stroke in four, hemorrhagic syndrome in four, and arterial sclerosis in two. The mechanisms behind lorlatinib-induced hyperlipidemia remain largely unknown and the follow-up should be longer (than the 30 days post-treatment planned in the study) to conclude about potential long-term cardiovascular toxicity. Moreover, comparison to crizotinib does not seem to be relevant as there is a clear imbalance between median follow-up of the two groups. In contrast, no significant difference in VTE events were reported in the lorlatinib arm (n=13) compared to crizotinib (n=16). Furthermore, no occurrence of pulmonary arterial hypertension was noticed on lorlatinib as previously described in the literature (12).
CNS adverse events (CNS-AEs) is probably the most challenging side effects of lorlatinib for clinicians, both underestimated and difficult to handle. The authors report 42% of patients experiencing CNS-AEs of any grade, classified into four categories: cognitive, mood, speech, and psychotic effects. Although usually only AEs of grades ≥3 are considered as serious, grade 2 CNS-AEs are already likely to interfere significantly with quality of life, since they are defined as moderate cognitive disability, interfering with work/life performance, nightmares, mood alteration, short term memory loss, impairment in attention, disorientation, limiting instrumental activities of daily living. Additionally, grading this type of effects is probably difficult and not easily reproducible by investigators. In the lorlatinib group, 29/149 patients (19.5%) had grade ≥2 CNS toxicity. Only three patients discontinued treatment due to neuropsychological side effects, a relatively low number compared to the incidence and potential impact of these events on quality of life. This suggests the possible reluctance of clinicians to discontinue such an active treatment in the context of a life-threatening metastatic disease. Furthermore, patient-reported outcomes (PROs) were evaluated as a secondary endpoint using the EORTC QLQ-C30 questionnaire, administered at baseline and on day 1 of each cycle until the end of treatment. While overall, all the safety parameters studied were improving, cognitive functioning was worse with lorlatinib compared to crizotinib, with an estimated difference of −3.18 (95% CI: −6.47, 0.12). Notably, patients in the lorlatinib arm without brain metastases showed a greater decline in cognitive functioning scores from baseline (13).
In the second interim analysis of CROWN, the authors detailed the management of CNS-AEs in 52 lorlatinib-treated patients (14). Overall, 38% of CNS-AEs remained unresolved at the time of analysis, among which three quarters did not receive medical intervention (concomitant medication or dose reduction/interruption). A possible association between CNS-AEs and a history of brain radiotherapy has been suggested. Out of the nine patients who had previously undergone brain radiotherapy [modality not specified-stereotactic body radiation therapy (SBRT), whole brain radiation therapy (WBRT)], six developed CNS-AEs, while in the remaining population (n=140), 41% of patients experienced CNS-AEs. Brain radiotherapy seems more likely to be a confounding factor rather than an explanatory one. The possible role of radiotherapy (among other baseline factors) has also been suggested as associated with the development of CNS-AEs in the prospective Massachusetts General Hospital study. In this cohort, 60% of patients experienced neurocognitive AEs on lorlatinib (15). It would therefore appear essential to develop new tools to identify and treat these neuropsychological disorders.
The high incidence of CNS-AEs which are mainly managed by dose-reductions raises the question of whether the lorlatinib dose of 100 mg might be too high in some patients. There is a need for studies collecting pharmacokinetic data, especially in patients experiencing CNS side effects, to search for correlations and guide dose reduction if necessary. Further research on dose optimization could be pivotal to improve the efficacy-toxicity ratio of lorlatinib in context of long-term clinical use.
Regarding post-hoc efficacy data in cases of lorlatinib dose reduction, caution is needed in interpreting these results. In fact, the study of PFS and time to intracranial progression suggests that patients with a dose reduction in the first 16 weeks had no impact on their outcome compared with patients who maintain the 100 mg dose over this period. However, it might be more relevant to take into account the dose-intensity of treatment over the entire treatment period to really analyze the impact of dose reductions.
Updated results from the CROWN trial show for lorlatinib a level of activity never achieved before in any oncogenic addiction, with 60% of patients still alive and free of tumor progression after five years of treatment. The intracerebral efficacy and the ability to protect from the emergence of brain metastases are also quite remarkable. These results make undeniably lorlatinib the new standard of first-line treatment for patients with ALK+ metastatic NSCLC. Nevertheless, the highly specific toxicity profile of lorlatinib, especially CNS side effects, weight gain and uncertainty about the long-term consequences of hyperlipidemia, remains for oncologists a major challenge in terms of detection and management. If it seems reasonable not to offer lorlatinib to patients with a severe cardiovascular or neuropsychiatric history, these circumstances are rare in ALK+ patients. They are mostly non-smokers and younger than the average lung cancer patient, still active in their professional and family lives. Managing side effects is crucial, as they can significantly affect treatment adherence and daily quality of life. Given the expected long duration of lorlatinib treatment, it is essential to regularly address and discuss these challenges with patients, emphasizing the importance of balancing effectiveness with side effects and quality of life. Furthermore, the absence of extensive long-term safety data, especially regarding cardiovascular events, underscores the necessity of continued pharmacovigilance and real-world studies to better understand lorlatinib enduring effects. For patients with severe toxicity, particularly neurological toxicity, switching to a 2nd-generation treatment may be considered. Nevertheless, as alectinib and brigatinib have a different safety profile than lorlatinib notably without CNS side-effects and may produce prolonged responses in some patients, identifying the molecular profile of these long-term responders could be valuable. This approach may aid in selecting the most appropriate upfront ALK-targeted therapy in a more personalized manner.
Another challenge is that of subsequent treatment during progression on lorlatinib, which appears to be mediated mainly by bypasses, relies mainly on chemotherapy. OS that remains still immature (as 70% of survival events are required to perform the second interim analysis as per protocol) is the only way to take the whole treatment sequence into account (6). In fact, until now in the field of oncogenic addictions, using the most active treatment from the outset has almost always proved to be the most effective strategy. Thus, preventing the emergence of resistance mutations, which accounts for the long duration of efficacy, combined with its intracerebral activity and protective capacity, is a major argument in favor of using lorlatinib in frontline treatment.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-903/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-903/coif). M.P. reports consulting fee from Roche, Ipsen, Esai, GlaxoSmithKline, Eli Lilly, and Novocure; honoraria from Bristol-Myers Squibb, Merck Sharp and Dohme, AstraZeneca, AnHeart Therapeutics, Sanofi, Pfizer, Takeda, Janssen, Amgen, and Daiichi Sankyo; payment for expert testimony from Bristol-Myers Squibb, AstraZeneca, Roche, and Janssen; support for attending meetings and/or travel from Bristol-Myers Squibb, Merck Sharp and Dohme, AstraZeneca, Roche, Pfizer, and Takeda; and participation on a Data Safety Monitoring Board or Advisory Board of Roche and Pharmamar. A.S. reports honoraria from Merck Sharp and Dohme, AstraZeneca, Roche, Daiichi Sankyo, Janssen, Pfizer, Takeda, Sanofi, and Amgen; and support for attending meetings and/or travel from Roche, Janssen, Pfizer, and Takeda. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol 2021;16:2091-108. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin Cancer Res 2018;24:2771-9. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. [Crossref] [PubMed]
- Solomon BJ, Liu G, Felip E, et al. Lorlatinib Versus Crizotinib in Patients With Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J Clin Oncol 2024;42:3400-9. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Drilon AE, Lin JJ, Johnson ML, et al. 1253O Phase I/II ALKOVE-1 study of NVL-655 in ALK-positive (ALK+) solid tumours. Ann Oncol 2024;35:S802-3. [Crossref]
- Hlavaty A, Roustit M, Montani D, et al. Identifying new drugs associated with pulmonary arterial hypertension: A WHO pharmacovigilance database disproportionality analysis. Br J Clin Pharmacol 2022;88:5227-37. [Crossref] [PubMed]
- Mazieres J, Iadeluca L, Shaw AT, et al. Patient-reported outcomes from the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive non-small cell lung cancer. Lung Cancer 2022;174:146-56. [Crossref] [PubMed]
- Solomon BJ, Bauer TM, Ignatius Ou SH, et al. Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study. J Clin Oncol 2022;40:3593-602. [Crossref] [PubMed]
- Dagogo-Jack I, Abbattista A, Murphy JF, et al. Factors Associated With Developing Neurocognitive Adverse Events in Patients Receiving Lorlatinib After Progression on Other Targeted Therapies. J Thorac Oncol 2023;18:67-78. [Crossref] [PubMed]


