
Patient-reported outcomes in the early postoperative period following resection of unilateral multiple pulmonary nodules: implications for surgical decision-making
Highlight box
Key findings
• For patients with unilateral multiple pulmonary nodules, multiple-lobe surgery had a greater patient-reported symptom and functional burden in the early postoperative period than single-lobe surgery. In addition, the operation time and recovery time of symptoms and functional impairment were also longer in the multiple-lobe surgery group.
What is known and what is new?
• Previous studies mainly focused on the diagnosis, treatment and prognosis of multiple pulmonary nodules, and there were few comparative studies on postoperative symptoms, functional recovery and complications.
• Currently, more and more patients are beginning to focus on quality of life and rapid recovery after surgery. The results of this study provide new insights into the patient-reported outcomes in the early postoperative period following nodule resection, emphasizing patient-centered metrics.
What is the implication, and what should change now?
• Multiple-lobe surgery is associated with more severe symptoms and functional impairment than single-lobe surgery. Our results advocate for a conservative surgical approach, favoring long-term monitoring over extensive resection for patients with unilateral multiple nodules without clear signs of malignancy or progression.
Introduction
Lung cancer remains a significant global health challenge, leading both in incidence and as the primary cause of cancer-related mortality worldwide. The International Agency for Research on Cancer (IARC) reported that in 2022, there were approximately 2.5 million new lung cancer cases and 1.8 million deaths attributable to the disease globally (1). Advances in early lung cancer screening have notably increased the detection rates of early-stage lung cancer and multiple pulmonary nodules (2). Surgery, particularly radical resection, is one of the effective treatments for multiple pulmonary nodules (3). This is especially true for specific patients, such as individuals where nodules are highly suspicious for malignancy based on imaging characteristics or growth patterns, and where comprehensive preoperative assessment supports the likelihood of a surgical cure. The lack of standardized treatment strategies often leads to diverse therapeutic approaches, particularly in deciding whether to monitor, biopsy, or resect, with significant variations possible between different doctors or treatment centers. According to a survey among members of the International Association for the Study of Lung Cancer (IASLC), 63% recommended surgical removal, with the number increasing to 81% among surgeons. The survey also highlighted divergent opinions on surgical extent, with 75 respondents advocating for the removal of only the primary lesion, while 63 supported excising both primary and additional lesions (4). The controversy surrounding the prognostic reliance on the primary lesion continues to complicate surgical decision-making regarding the extent of resection required (5,6).
Traditionally, research has focused on the diagnosis and prognosis of multiple pulmonary nodules, with less emphasis on comparing postoperative outcomes such as symptoms, functional recovery, and complications. Modern surgical advancements have shifted the focus from merely excising the lesion to prioritizing management of postoperative symptoms and functional recovery. Evidence suggests that patient-reported symptoms and complications provide a reliable and complementary assessment to those conducted by medical personnel (7). Recognizing this, the U.S. Food and Drug Administration (FDA) in 2006 endorsed patient-reported outcomes (PROs) as a vital clinical endpoint, now extensively employed in clinical trials, drug efficacy evaluations, and healthcare quality assessments (8-11). PROs cover a broad spectrum, from symptoms and functional status to quality of life, offering insights that are often overlooked in conventional clinical evaluations. PROs, such as those measuring pain, shortness of breath, and fatigue, provide insights into the day-to-day challenges patients face after surgery, which are not always apparent through clinical assessments alone. For example, while a patient may have a clinically uneventful recovery, they may still report significant levels of fatigue or disturbed sleep, which can substantially impact their quality of life. The real-time data captured by PROs offer an unparalleled reflection of the patient’s current condition. Substantial research supports the utility of PROs in enhancing treatment planning, prognosis, collaborative decision-making, and communication between healthcare providers and patients (7,12-14).
The physical and emotional toll of lung cancer surgery often results in prolonged postoperative discomfort, including pain and shortness of breath, significantly affecting patients’ quality of life (15). A multicenter study has demonstrated that managing symptoms based on PROs can significantly mitigate the burden of symptoms and reduce complications compared to traditional care (16). Moreover, recent studies highlight the critical role of PRO-based evaluations in guiding surgical strategies, underscoring their importance in clinical decision-making (17-19). Given the potential for both overtreatment and undertreatment in managing multiple pulmonary nodules, understanding the impact of different treatment strategies from the patients’ viewpoint is crucial. This approach is particularly pertinent as the management strategies for pulmonary nodules evolve and become more patient-centered.
This study leverages the Perioperative Symptom Assessment for Patients Undergoing Lung Surgery (PSA-Lung) to develop a comprehensive database of early postoperative symptoms and functional statuses based on PROs. It compares the postoperative conditions between patients undergoing single-lobe versus multiple-lobe surgeries for unilateral multiple pulmonary nodules. By adopting a patient-centered perspective, this research aims to delineate the relative benefits and limitations of varying surgical extents in treating unilateral multiple pulmonary nodules. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-702/rc).
Methods
Study design and participants
This longitudinal, observational study included 550 patients undergoing unilateral multiple pulmonary nodule resection via single-port video-assisted thoracoscopic surgery (VATS) from December 2021 to December 2023. The cohort was divided into two groups based on the surgical extent: single-lobe and multiple-lobe resections. Based on the findings of Dai et al., thoracoscopic lobectomy and sublobar resection produce similar symptom burden and functional impairment in the early postoperative period (18). Therefore, only patients undergoing lobectomy and sublobar resection were included in this study, whereas sleeve lobectomy, combined lobectomy and total pneumonectomy were excluded. Comprehensive preoperative evaluations assessed each patient’s general health and tumor status, adhering to the IASLC lung cancer tumor node metastasis (TNM) staging system, 8th edition (20). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 22/301-3503), with informed consent obtained from all participants.
Inclusion criteria: adults ≥18 years old; medically cleared for surgery in preoperative assessments; multiple nodules confined to unilateral lobes; understanding of the study procedures. Exclusion criteria: history of thoracic surgeries or malignancies at other sites; radiographic evidence suggestive of metastatic disease; prior preoperative treatments; conversion to open thoracotomy or unplanned secondary surgeries; types of cancer other than adenocarcinoma; non-compliance with data collection protocols.
Surgical procedure and management
A consistent surgical technique was ensured by the same leading surgeon performing all procedures. The incision was made between the anterior and mid-axillary lines at the third to fifth intercostal spaces, standardized at 4 cm. A disposable endoscopic cutter was used for severing vessels, bronchi, and lung tissues. Postoperatively, a silicone drainage tube was inserted, with size adjusted based on intraoperative findings. Patients followed a standardized perioperative protocol, which excluded the use of intercostal nerve blocks. Pain management was primarily through intravenous analgesics, supplemented by oral medications as needed. Patients resumed a liquid diet and initiated ambulation (800–1,000 m) on the first postoperative day. Drainage tubes were removed under strict criteria: effective lung re-expansion, no sign of air leaks, and drainage below 200 mL in 24 hours. Follow-ups were scheduled at 4 weeks post-discharge.
Outcomes and measurements
The study primarily compared early postoperative symptoms and functional statuses between surgical approaches using the PSA-Lung scale (21-23), which includes seven symptom items and two functionality items, each rated from 0 (none) to 10 (most severe). Yu et al. have validated the PSA-lung scale for understandability, reliability, sensitivity, and surgical specificity (24). The PSA-Lung scale was specifically chosen for its relevance to pulmonary surgical outcomes, providing focused insights into respiratory-specific symptoms and post-surgical recovery that scales like 36-Item Short Form Survey (SF-36) or EuroQol 5-Dimensions (EQ-5D) might not fully capture. The PSA-Lung scale allows for a more detailed assessment of the surgical impact on lung function and patient comfort, which is crucial for the population under study. Symptom items include pain, coughing, shortness of breath, disturbed sleep, fatigue, drowsy, and distress; function items include walking and activity. Measurements were taken preoperatively, daily for the first four postoperative days, and weekly for four weeks thereafter, totaling nine assessments. The secondary objective of this study was to evaluate the short-term clinical outcomes of single-lobe versus multiple-lobe surgeries. Specific outcomes assessed included the duration of surgery, postoperative drainage time, hospital stay length, discharge activity levels, and the rate of early postoperative complications. These complications were evaluated according to the classification provided by the Japanese Society of Clinical Oncology (25). Additionally, we conducted comparative analyses of demographic and clinical data between the two groups. These analyses covered a range of variables: gender, age, body mass index (BMI), educational background, smoking and alcohol use, family cancer history, the time from disease onset to hospitalization, American Society of Anesthesiologists (ASA) classification (26), age-adjusted Charlson Comorbidity Index (aCCI) (27), forced expiratory volume in one second (FEV1), FEV1%, maximum voluntary ventilation (MVV), MVV%, diffusing capacity of the lung for carbon monoxide single breath (DLCO SB), DLCO SB%, and characteristics of the pulmonary nodules (diameter, nature, location). Additionally, we assessed the approaches used for lymph node management and the histopathological types of the nodules. Nodal characteristics refer to the dominant nodule. The “dominant nodule” was defined as the nodule with the largest diameter in the presence of multiple pulmonary nodules. Nodule diameter refers to the overall size of the nodule observed on preoperative imaging.
Statistical analysis
Descriptive statistics summarized the demographic and clinical data. Continuous variables were presented as means ± standard deviation or medians and interquartile ranges based on their distribution. Categorical data were analyzed using frequencies and percentages. The t-test and Mann-Whitney U test were applied to normally and non-normally distributed continuous variables, respectively, while the chi-squared test was used for categorical variables. Mixed-effects models analyzed the differences in PRO scores over time, with two-piecewise linear growth models evaluating the early postoperative symptom and functional statuses. Kaplan-Meier curves and log-rank tests determined differences in recovery times, defined as the days required for symptom and functionality scores to decrease from ≥4 to ≤3 (19). All statistical tests were two-tailed with significance set at P<0.05, conducted using SPSS version 22.0 and R software, version 4.3.1.
Results
Patient characteristics
Figure 1 depicts the enrollment process of the 550 patients in the study, with 416 undergoing single-lobe and 134 undergoing multiple-lobe uniport VATS surgeries. In the single-lobe group, 159 patients underwent lobectomy and 257 patients underwent sublobar resection. In the multiple-lobe group, 40 patients underwent lobectomy plus sublobar resection and 94 patients underwent sublobar combined sublobar resection. Table 1 details the demographic and clinical characteristics of these patients. Both groups had a median age of 57 years, with females comprising over 60% of each group; the proportion was significantly higher in the multiple-lobe group (P=0.001). No significant differences were observed between groups in terms of BMI, education level, smoking status, alcohol consumption, family cancer history, time from disease onset to hospitalization, ASA score, adjusted CCI score, or lung function indices including FEV1, FEV1%, MVV, DLCO SB, and DLCO SB%.
Table 1
| Variables | Single-lobe surgery (N=416) | Multiple-lobe surgery (N=134) | P value |
|---|---|---|---|
| Sex | 0.001 | ||
| Male | 149 (35.8) | 28 (20.9) | |
| Female | 267 (64.2) | 106 (79.1) | |
| Age (years) | 57.00 (50.00–64.00) | 57.00 (49.75–66.00) | 0.66 |
| BMI (kg/m2) | 23.88 (22.03–25.55) | 23.74 (21.64–25.52) | 0.60 |
| Education level | 0.93 | ||
| ≤ high school | 275 (66.1) | 88 (65.7) | |
| > high school | 141 (33.9) | 46 (34.3) | |
| Smoking status | 0.12 | ||
| Never | 314 (75.5) | 112 (83.6) | |
| Current | 33 (7.9) | 9 (6.7) | |
| Former | 69 (16.6) | 13 (9.7) | |
| Alcohol consumption | 0.15 | ||
| Never | 335 (80.5) | 117 (87.3) | |
| Current | 65 (15.6) | 12 (9.0) | |
| Former | 16 (3.8) | 5 (3.7) | |
| Family history of cancer | 0.19 | ||
| No | 280 (67.3) | 82 (61.2) | |
| Yes | 136 (32.7) | 52 (38.8) | |
| Time between onset and treatment (months) | 0.46 | ||
| ≤3 | 190 (45.7) | 52 (38.8) | |
| >3 to 6 | 69 (16.6) | 26 (19.4) | |
| >6 to 12 | 42 (10.1) | 18 (13.4) | |
| >12 | 115 (27.6) | 38 (28.4) | |
| ASA classification | 0.90 | ||
| 1 | 257 (61.8) | 82 (61.2) | |
| >1 | 159 (38.2) | 52 (38.8) | |
| aCCI score | 0.53 | ||
| ≤2 | 196 (47.1) | 59 (44.0) | |
| >2 | 220 (52.9) | 75 (56.0) | |
| FEV1 (L) | 2.52 (2.13–3.03) | 2.52 (2.19–2.78) | 0.56 |
| FEV1% | 99.70 (87.50–109.18) | 100.80 (89.25–112.32) | 0.23 |
| MVV (L/min) | 83.13 (64.08–103.44) | 87.81 (69.22–103.44) | 0.30 |
| MVV% | 82.69±23.58 | 88.32±21.03 | 0.01 |
| DLCO SB (mmol/min/kPa) | 7.30 (6.33–8.72) | 7.10 (6.27–8.48) | 0.21 |
| DLCO SB% | 90.10 (79.20–102.48) | 89.45 (81.53–100.55) | >0.99 |
Data are presented as median (interquartile range), n (%) or mean ± standard deviation. BMI, body mass index; ASA, American Society of Anesthesiologists; aCCI, age adjusted Charlson comorbidity index; FEV1, forced expiratory volume in 1 second; MVV, maximal voluntary ventilation; DLCO SB, diffusion capacity of carbon monoxide single breath.
As shown in Table 2, the median nodule diameter was slightly smaller in the multiple-lobe group (1.2 cm) compared to the single-lobe group (1.3 cm). Nodule type was predominantly sub-solid nodes between the two groups, with the P value being marginal (P=0.050). It appeared that the right lung was more likely to have multiple nodules, with more than half of the patients with multiple nodules in the right lung in both groups, and the proportion was significantly higher in the multiple-lobe group than in the single-lobe group (P<0.001). Systematic lymph node dissection was more commonly performed in the multiple-lobe group (P=0.03). There were no significant differences in histological types or drainage tube diameter between the groups.
Table 2
| Variables | Single-lobe surgery (N=416) | Multiple-lobe surgery (N=134) | P value |
|---|---|---|---|
| Nodule diameter (cm) | 1.30 (0.93–2.00) | 1.20 (0.90–2.00) | 0.51 |
| Nodule type | 0.050 | ||
| Pure GGO | 131 (31.5) | 49 (36.6) | |
| Subsolid | 192 (46.2) | 68 (50.7) | |
| Pure solid | 93 (22.4) | 17 (12.7) | |
| Nodule location | <0.001 | ||
| Left lung | 197 (47.4) | 39 (29.1) | |
| Right lung | 219 (52.6) | 95 (70.9) | |
| Surgical procedures | 0.12 | ||
| Lobectomy | 159 (38.2) | 40 (29.9) | |
| Segmentectomy | 187 (45.0) | 63 (47.0) | |
| Wedge resection | 70 (16.8) | 31 (23.1) | |
| Type of lymphadenectomy | 0.03 | ||
| Lymph node sampling | 139 (33.4) | 31 (23.1) | |
| Systematic lymph node dissection | 277 (66.6) | 103 (76.9) | |
| Histological type | 0.16 | ||
| Adenocarcinoma in situ | 77 (18.5) | 25 (18.7) | |
| Minimally invasive adenocarcinoma | 112 (26.9) | 47 (35.1) | |
| Invasive adenocarcinoma | 227 (54.6) | 62 (46.3) | |
| TNM stage | 0.92 | ||
| 0 | 77 (18.5) | 25 (18.7) | |
| IA | 315 (75.7) | 100 (74.6) | |
| IB | 24 (5.8) | 9 (6.7) | |
| Drainage tube diameter | 0.74 | ||
| Thin (size: 15 Fr, 5 mm) | 115 (27.6) | 39 (29.1) | |
| Thick (size: 22 Fr, 7.3 mm) | 301 (72.4) | 95 (70.9) |
Data are presented as median (interquartile range) or n (%). GGO, ground-glass opacity; TNM, tumor node metastasis.
Patient-reported symptoms and functional status
Using mixed-effects models for the initial univariate analysis, key clinical characteristics affecting symptoms and functionality were identified (Table S1). These characteristics were included in the final model, which demonstrated that within one-month post-surgery, the multiple-lobe group reported significantly worse outcomes in terms of symptoms and functionality (Figure 2). Specifically, this group experienced higher levels of pain (P=0.04), shortness of breath (P<0.001), disturbed sleep (P=0.007), and fatigue (P=0.01) (Figure 2A,2C-2E). Functional impairment such as walking (P=0.002) and performing daily activities (P=0.002) were also markedly worse in the multiple-lobe group (Figure 2H,2I). Additionally, though not statistically significant, scores for cough, drowsiness, and distress were higher in this group (Figure 2B,2F,2G).
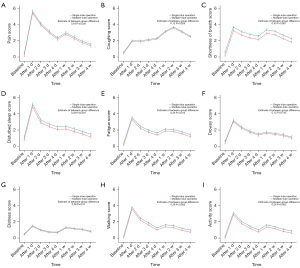
We then analyzed the proportion of patients with moderate-to-severe symptoms and functional impairment in the early postoperative period in both groups (Figure 3). Further analysis showed that a larger proportion of patients in the multiple-lobe group suffered from moderate-to-severe shortness of breath (P<0.001) and disturbed sleep (P<0.001) (Figure 3C,3D). Functional impairments, specifically difficulty in walking (P=0.001) and daily activities (P<0.001) (Figure 3H,3I), were also significantly more prevalent in the multiple-lobe group. Distribution of moderate-to-severe symptoms and functional impairment was shown in Table S2.

Recovery times
Kaplan-Meier analysis compared the time to recovery from symptoms and functional interference between the two groups (Table 3). Only 69.1% of the patients were able to return their cough symptoms to no/minimal levels within 1 month after surgery. For other symptoms and functional interference, more than 80% of patients could recover to the level of none/mild. The mean recovery time for pain (P=0.02) and drowsiness (P=0.005) was significantly longer in the multiple-lobe group compared to the single-lobe group. The time to alleviate disturbed sleep was also longer in multiple-lobe group, bordering on statistical significance (P=0.050).
Table 3
| Items | Single lobe operation (n=416) | Multiple lobe operation (n=134) | Total number (%) |
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean days to recovery (95% CI) | Number (%) |
Censored | Mean days to recovery (95% CI) | Number (%) |
Censored | ||||
| Pain | 2.1 (1.9–2.3) | 357 (94.9) | 40 | 2.8 (2.1–3.5) | 118 (96.7) | 12 | 475 (95.4) | 0.02 | |
| Coughing | 9.0 (8.1–9.9) | 201 (66.8) | 115 | 8.9 (7.6–10.3) | 85 (75.2) | 21 | 286 (69.1) | 0.92 | |
| Shortness of breath | 4.9 (4.3–5.6) | 233 (81.5) | 130 | 5.7 (4.4–7.0) | 91 (80.5) | 21 | 324 (81.2) | 0.29 | |
| Disturbed sleep | 3.1 (2.7–3.6) | 308 (88.0) | 66 | 4.2 (3.2–5.3) | 108 (89.3) | 13 | 416 (88.3) | 0.050 | |
| Fatigue | 2.8 (2.4–3.3) | 230 (88.8) | 157 | 3.5 (2.5–4.6) | 83 (90.2) | 42 | 313 (89.2) | 0.23 | |
| Drowsy | 2.1 (1.8–2.4) | 201 (94.8) | 204 | 3.4 (2.5–4.3) | 76 (96.2) | 55 | 277 (95.2) | 0.005 | |
| Distress | 4.6 (3.6–5.7) | 94 (78.3) | 296 | 4.1 (2.5–5.7) | 39 (92.9) | 92 | 133 (82.1) | 0.58 | |
| Walking | 2.2 (1.8–2.5) | 248 (96.5) | 159 | 2.3 (1.7–2.9) | 85 (92.4) | 42 | 333 (95.4) | 0.73 | |
| Activity | 2.1 (1.7–2.4) | 158 (96.3) | 252 | 2.2 (1.7–2.7) | 64 (95.5) | 67 | 222 (96.1) | 0.64 | |
CI, confidence interval.
Short-term clinical outcomes
Table 4 summarizes the short-term clinical outcomes. Median surgical time was notably longer for the multiple-lobe group (P<0.001). No significant differences were observed in median postoperative hospital stays, drainage times, discharge score, or complication rates between the groups.
Table 4
| Variables | Single lobe surgery (N=416) | Multiple lobe surgery (N=134) | P value |
|---|---|---|---|
| Operative time (min) | 92.00 (68.00–113.00) | 106.50 (83.00–140.25) | <0.001 |
| Postoperative hospital stay (days) | 4.00 (3.00–5.00) | 4.00 (3.00–6.00) | 0.11 |
| Drainage time (days) | 3.00 (3.00–4.00) | 3.00 (3.00–5.00) | 0.10 |
| Discharge score | 70.00 (65.00–80.00) | 75.00 (65.00–80.00) | 0.22 |
| Complications | 63 (15.1) | 23 (17.2) | 0.58 |
Data are presented as median (interquartile range) or n (%).
Discussion
This study elucidates the differences in symptom burden, functional status, short-term clinical outcomes, and recovery times between single-lobe and multiple-lobe surgeries for unilateral multiple pulmonary nodules. Patients undergoing multiple-lobe surgeries reported more severe symptoms and functional impairments, a higher incidence of moderate-to-severe symptoms, and longer surgical and recovery times compared to those undergoing single-lobe surgeries. This investigation is pioneering in its use of PROs to determine the optimal surgical extent for treating multiple pulmonary nodules, a dimension not previously explored.
Traditionally, surgical evaluations for multiple pulmonary nodules have relied on clinical indices such as operation time and hospital stay (28,29). These metrics, while useful, often overlook the subjective experiences of patients, which can provide critical insights into the postoperative quality of life and recovery. With the adoption of enhanced recovery protocols in thoracic surgery, the limitations of traditional clinical indices have become more apparent, necessitating a shift towards more holistic and patient-centered care models. Our study leverages PRO-based symptom reporting to provide a nuanced understanding of postoperative recovery, aligning with recent shifts in healthcare towards patient-centered outcomes (30).
Our findings indicate that more extensive surgery, associated with multiple-lobe resections, exacerbates early postoperative symptoms such as pain, shortness of breath, and disturbed sleep. These symptoms are likely exacerbated by the larger volume of lung tissue resected, which can significantly disrupt normal physiological functions and delay recovery. Additionally, functional impairments, including difficulties in walking and daily activities, were more pronounced in the multiple-lobe group, reflecting the greater physical toll of these surgeries. Considering that the differences in recovery times and symptom scores between the single-lobe and multiple-lobe groups were usually small, caution is still warranted in interpreting the significant differences in the proportions of patients with moderate-to-severe symptoms and functional impairment between the two groups.
Interestingly, the recovery from cough, a common postoperative symptom, was prolonged in both surgical groups, with the lowest proportion of patients achieving resolution within the first month post-surgery. This symptom persistence underscores the need for targeted interventions to alleviate cough and improve patient comfort during recovery (31).
Demographically, a significant majority of our study participants were female, mirroring trends seen in other studies where women and non-smokers are more likely to develop multiple pulmonary nodules (32,33). The timing of treatment post-detection showed a bimodal distribution, with a considerable number of patients either opting for immediate surgery within 3 months of diagnosis or delaying treatment for over a year. The predominance of nodules in the right lung is consistent with anatomical expectations and the observed high incidence of invasive adenocarcinoma necessitated frequent systematic lymph node dissections. However, approximately half of the patients in both groups underwent surgery for adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA), which are often low-risk, early-stage lesions. While these tumors are associated with an excellent prognosis and may not impact survival in the long term, surgical resection was considered based on the patient preferences, tumor characteristics, and the potential risk of progression. In clinical practice, these decisions are individualized and surgery may be recommended even for low-risk lesions. However, we acknowledge that the long-term benefits of surgery in these patients warrant further exploration, and future studies should aim to better define the optimal management approach for AIS and MIA. Furthermore, while lesser resections might lead to faster recoveries and less immediate postoperative distress as suggested by our findings, it is essential to verify that these benefits do not compromise long-term oncological safety. Therefore, we suggest future research efforts should focus on longitudinal studies that track both PROs and survival outcomes to ensure that the surgical strategy not only optimizes immediate recovery but also maximizes the long-term health and survival of patients.
While our study provides novel insights into the impact of surgical extent on patient outcomes using PROs, it is not without limitations. The single-surgeon design of this study, with all procedures performed by the same leading surgeon, may limit the generalizability of the findings. The need for multicenter studies to validate and extend these results is emphasized. Although statistically significant differences were observed between the single-lobe and multiple-lobe surgery groups for several postoperative symptoms and functional impairments, the effect sizes were small. This highlights the need for caution when interpreting the clinical relevance of statistically significant findings. While PROs offer valuable insights into the patient experience, it is important to acknowledge their limitations. PROs inherently reflect subjective patient perceptions, which can vary widely between individuals due to factors such as psychological state, cultural background, and personal coping mechanisms. This variability can introduce a degree of inconsistency in the data, which may impact the interpretation of results. Additionally, the reliability of PROs is heavily dependent on the use of validated measurement tools. In our study, we employed the PSA-Lung scale, which has been demonstrated to be both reliable and sensitive in capturing postoperative symptoms and functional impairments. However, even validated scales are not immune to biases, and their ability to capture the full range of a patient’s experience may be limited by their design. Future studies should continue to refine and validate PRO scales to ensure they capture the most relevant symptoms and functional domains while minimizing subjectivity. The decision to measure PROs weekly post-discharge may have led to missed opportunities to capture more granular changes in patient status, suggesting that future research might explore different measurement frequencies. Lastly, the restriction of PRO measurements to the first postoperative month may omit longer-term changes in patient health and recovery, advocating for extended follow-up periods in future studies.
Conclusions
This study demonstrates that extensive surgical resection involving multiple lobes for unilateral multiple pulmonary nodules significantly increases the postoperative symptom burden and functional impairments. Patients undergoing multiple-lobe surgeries experienced longer operation times and extended periods of recovery for symptoms and functionality compared to those undergoing single-lobe surgeries. These findings suggest that an aggressive resection approach is not necessarily advisable for all cases of unilateral multiple pulmonary nodules. For nodules across different lobes that do not exhibit clear malignant tendencies or progressive behavior, a strategy of long-term monitoring may be more appropriate than extensive surgical intervention. This approach aligns with patient-centered care principles, potentially leading to better overall patient outcomes by minimizing unnecessary surgical risks and enhancing quality of life postoperatively.
Acknowledgments
Special thanks to Qiuling Shi from School of Public Health, Chongqing Medical University, Chongqing, China, for her permission to use, reproduce and/or distribute copies of the PSA-Lung scale.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-702/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-702/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-702/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-702/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 22/301-3503), with informed consent obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Chen K, Nie Y, Park S, et al. Development and Validation of Machine Learning-based Model for the Prediction of Malignancy in Multiple Pulmonary Nodules: Analysis from Multicentric Cohorts. Clin Cancer Res 2021;27:2255-65. [Crossref] [PubMed]
- Cheng M, Ding R, Wang S. Diagnosis and treatment of high-risk bilateral lung ground-glass opacity nodules. Asian J Surg 2024;47:2969-74. [Crossref] [PubMed]
- Leventakos K, Peikert T, Midthun DE, et al. Management of Multifocal Lung Cancer: Results of a Survey. J Thorac Oncol 2017;12:1398-402. [Crossref] [PubMed]
- Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer 2015;88:174-80. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Radiological classification of multiple lung cancers and the prognostic impact based on the presence of a ground glass opacity component on thin-section computed tomography. Lung Cancer 2017;113:7-13. [Crossref] [PubMed]
- Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624-32. [Crossref] [PubMed]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [Crossref]
- Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA 2018;319:483-94. [Crossref] [PubMed]
- Basch E. Patient-Reported Outcomes - Harnessing Patients' Voices to Improve Clinical Care. N Engl J Med 2017;376:105-8. [Crossref] [PubMed]
- Yi H, Ou-Yang X, Hong Q, et al. Patient-reported outcomes in lung cancer surgery: A narrative review. Asian J Surg. 2024; Epub ahead of print. [Crossref] [PubMed]
- Araujo LH, Baldotto CS, Monteiro MR, et al. Patient-centered outcomes in non-small-cell lung cancer: a real-world perspective. Future Oncol 2021;17:1721-33. [Crossref] [PubMed]
- Nipp RD, El-Jawahri A, Ruddy M, et al. Pilot randomized trial of an electronic symptom monitoring intervention for hospitalized patients with cancer. Ann Oncol 2019;30:274-80. [Crossref] [PubMed]
- Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017;318:197-8. [Crossref] [PubMed]
- Heiden BT, Subramanian MP, Liu J, et al. Long-term patient-reported outcomes after non-small cell lung cancer resection. J Thorac Cardiovasc Surg 2022;164:615-626.e3. [Crossref] [PubMed]
- Dai W, Feng W, Zhang Y, et al. Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial. J Clin Oncol 2022;40:988-96. [Crossref] [PubMed]
- Dai W, Dai Z, Wei X, et al. Early Patient-Reported Outcomes After Uniportal vs Multiportal Thoracoscopic Lobectomy. Ann Thorac Surg 2022;114:1229-37. [Crossref] [PubMed]
- Dai W, Chang S, Pompili C, et al. Early Postoperative Patient-Reported Outcomes After Thoracoscopic Segmentectomy Versus Lobectomy for Small-Sized Peripheral Non-small-cell Lung Cancer. Ann Surg Oncol 2022;29:547-56. [Crossref] [PubMed]
- Wei X, Yu H, Dai W, et al. Patient-Reported Outcomes of Video-Assisted Thoracoscopic Surgery Versus Thoracotomy for Locally Advanced Lung Cancer: A Longitudinal Cohort Study. Ann Surg Oncol 2021;28:8358-71. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- 28th Annual Conference of the International Society for Quality of Life Research. Qual Life Res 2021;30:1-177. [Crossref] [PubMed]
- Gao X, Dai W, Zhang Q, et al. Longitudinal patient-reported outcomes 1 year after thoracoscopic segmentectomy versus lobectomy for early-stage lung cancer: a multicentre, prospective cohort study protocol. BMJ Open 2023;13:e067841. [Crossref] [PubMed]
- Su X, Huang Y, Gong R, et al. Undergoing Lung Surgery (PSA-Lung) was appropriate for symptom assessment after discharge. Qual Life Res 2024;33:1807-18. [Crossref] [PubMed]
- Yu H, Lei C, Wei X, et al. Electronic symptom monitoring after lung cancer surgery: establishing a core set of patient-reported outcomes for surgical oncology care in a longitudinal cohort study. Int J Surg 2024;110:6591-600. [Crossref] [PubMed]
- Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 2016;46:668-85. [Crossref] [PubMed]
- Sankar A, Johnson SR, Beattie WS, et al. Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. Br J Anaesth 2014;113:424-32. [Crossref] [PubMed]
- Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008;112:2384-92. [Crossref] [PubMed]
- Chang LK, Su PK, Chan PS, et al. Single-Stage Image-Guided Percutaneous Ablation with Thoracoscopic Resection for Multiple Pulmonary Lesions in a Hybrid Operating Room: A Retrospective Study. Cancers (Basel) 2024;16:3512. [Crossref] [PubMed]
- Gao RW, Berry MF, Kunder CA, et al. Survival and risk factors for progression after resection of the dominant tumor in multifocal, lepidic-type pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:2092-2099.e2. [Crossref] [PubMed]
- Dai W, Wang Y, Liao J, et al. Electronic Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: Long-Term Results of a Multicenter, Randomized, Controlled Trial. J Clin Oncol 2024;42:2126-31. [Crossref] [PubMed]
- Yang D, Hong Q, Zhao C, et al. Postoperative Patient-Reported Outcomes after Uniportal Video-Assisted Thoracoscopic Surgery Using the Perioperative Symptom Assessment for Lung Surgery Scale. Curr Oncol 2022;29:7645-54. [Crossref] [PubMed]
- Shintani Y, Okami J, Ito H, et al. Clinical features and outcomes of patients with stage I multiple primary lung cancers. Cancer Sci 2021;112:1924-35. [Crossref] [PubMed]
- Kim TJ, Goo JM, Lee KW, et al. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer 2009;64:171-8. [Crossref] [PubMed]



