
Efficacy of adjuvant tegafur-uracil (UFT) in early-stage non-small cell lung cancer with poor prognostic factors
Highlight box
Key findings
• Efficacy of tegafur-uracil (UFT): adjuvant UFT significantly improves recurrence-free survival and overall survival in early-stage non-small cell lung cancer (NSCLC) patients with poor prognostic factors.
What is known and what is new?
• Adjuvant therapies have been used to address postoperative recurrence in early-stage NSCLC, but their impact on high-risk pathological features has not been well-defined.
• This study reports that UFT effectively enhances survival outcomes in patients with high-risk features, such as vascular or pleural invasion.
What is the implication, and what should change now?
• The findings suggest that UFT should be considered as a treatment option for early-stage NSCLC patients with poor prognostic factors. Clinicians are encouraged to incorporate UFT into their treatment plans to improve patient outcomes. Further research is needed to refine treatment protocols and explore the effectiveness of UFT in other patient groups.
Introduction
Surgery is the most effective curative treatment for stage I to II non-small cell lung cancer (NSCLC) (1). However, even in early-stage lung cancers, postoperative recurrence is a common issue, with recurrence rates of approximately 35% for stage IB and 50% for stage IIA (2).
Adjuvant therapy after surgery plays a crucial role in the prevention of recurrence. In Japan, tegafur-uracil (UFT) is recommended for such patients (3-5). However, the clinical trial was conducted according to the older version of the tumor-node-metastasis (TNM) classification (6,7), which does not align with the current staging system (3). Furthermore, various pathological factors associated with a poor prognosis exist in lung cancer, and even in early-stage lung cancer, the presence or absence of these factors significantly affects recurrence rates and survival outcomes (8-15). Despite this, reports examining the efficacy of UFT in relation to these pathological factors are scarce, leaving a clinical question regarding the appropriateness of administering UFT.
Given this background, this retrospective study was designed to evaluate the association between poor prognostic factors and the efficacy of UFT. This manuscript was written in accordance with the STROBE checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-820/rc).
Methods
Patients
This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This retrospective study was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (approval No. 24-02). The requirement for informed consent from each patient was waived because of the retrospective nature of the study.
The subjects were patients who underwent lung resection for primary lung cancer and were diagnosed with pathological stage IB or IIA disease according to the TNM classification 8th edition (16) in our department between May 2000 and December 2019. Clinical data were collected from electronic medical records. The exclusion criteria were (I) patients with insufficient clinical data and (II) patients who received postoperative adjuvant therapy other than UFT.
Prognostic factors
In this study, poor prognostic factors were defined as (I) vascular invasion; (II) lymphatic invasion; (III) pleural invasion; and (IV) pathological grade ≥3 (8-15). The presence of any one of these was defined as the presence of poor prognostic factors.
Adjuvant therapy with UFT
The decision to administer UFT as adjuvant therapy was based on the judgment of the attending physician and the patient’s will. The treatment duration was set at 2 years, and patients who completed the full 2-year course were defined as having achieved completion. Patients who did not take the medication due to adverse events or personal intention within 1 month were classified as non-adherent, whereas those who took the medication for more than 1 month but discontinued it within 2 years were classified as having discontinued treatment.
Statistical analyses
Patients were divided into two cohorts, with and without prognostic factors, and were analyzed. The clinical endpoints in both cohorts were recurrence-free survival (RFS) and overall survival (OS) in patients with or without UFT as adjuvant therapy. RFS and OS were calculated using the Kaplan-Meier method, and differences were assessed using the log-rank test. Cox proportional hazard models were used to estimate univariate and multivariate hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs). RFS and OS were censored on the date on which survival was last confirmed for patients without documented disease progression. Statistical significance was set at P <0.05. All statistical analyses were performed using RStudio (version 4.3.2; RStudio Team, Boston, MA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (17).
Results
Patient characteristics
The consort diagram of the study is presented in Figure 1, and patient characteristics are summarized in Table 1. In total, 330 patients were included in the analysis. Among them, 283 patients (85.8%) had poor prognostic factors, while 47 (14.2%) had no poor prognostic factors. The median observation period was 61 months. The median age was 69 years, and 253 patients (76.7%) were male. Patients with poor prognostic factors were significantly younger (P=0.01) and had a higher proportion of males (P<0.001) than those without poor prognostic factors. The most common poor prognostic factors were vascular (64.5%) and pleural (67.9%) invasion. Epidermal growth factor receptor (EGFR) mutation testing was performed in 76.7% of patients, of whom 24.5% tested positive for EGFR mutations. UFT was administered to 163 patients (49.4%), with a completion rate of 64.4%. No significant differences were observed between the two cohorts regarding these factors. Postoperative recurrence was confirmed in 95 patients (28.8%), and was significantly higher in patients with poor prognostic factors (32.2% vs. 8.5%; P<0.001). Among the 115 patients (34.8%) who died, those with poor prognostic factors had a higher rate of lung cancer-specific death (55.7% vs. 22.2%), whereas those without poor prognostic factors had a higher rate of death from other causes (44.3% vs. 77.8%), with a significant difference between the two cohorts (P=0.01).
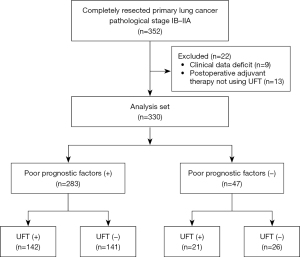
Table 1
| Characteristics | All (n=330) | Patients with poor prognostic factors (n=283) | Patients without poor prognostic factors (n=47) |
P value |
|---|---|---|---|---|
| Observation period (months) | 61 [0–230] | 61 [0–230] | 61 [0–197] | 0.85 |
| Age (years) | 69 [39–90] | 69 [39–90] | 75 [51–90] | 0.01 |
| Sex | <0.001 | |||
| Male | 253 (76.7) | 229 (80.9) | 24 (51.1) | |
| Female | 77 (23.3) | 54 (19.1) | 23 (48.9) | |
| Smoking index | 720 [0–4,400] | 760 [0–4,400] | 350 [0–2,500] | <0.001 |
| Procedures | 0.19 | |||
| Wedge resection | 26 (7.9) | 24 (8.5) | 2 (4.3) | |
| Segmentectomy | 91 (27.6) | 73 (25.8) | 18 (38.3) | |
| Lobectomy | 213 (64.5) | 186 (65.7) | 27 (57.4) | |
| Pathological subtype | 0.06 | |||
| Adenocarcinoma | 224 (67.9) | 185 (65.4) | 39 (83.0) | |
| Squamous cell carcinoma | 94 (28.5) | 87 (30.7) | 7 (14.9) | |
| Others | 12 (3.6) | 11 (3.9) | 1 (2.1) | |
| Vascular invasion | <0.001 | |||
| V (−) | 117 (35.5) | 70 (24.7) | 47 (100.0) | |
| V (+) | 213 (64.5) | 213 (75.3) | 0 | |
| Lymphatic permeation | <0.001 | |||
| Ly (−) | 229 (69.4) | 182 (64.3) | 47 (100.0) | |
| Ly (+) | 101 (30.6) | 101 (35.7) | 0 | |
| Pleural invasion | <0.001 | |||
| PL (−) | 106 (32.1) | 59 (20.8) | 47 (100.0) | |
| PL (+) | 224 (67.9) | 224 (79.2) | 0 | |
| Pathological grade | <0.001 | |||
| G1 | 75 (22.7) | 49 (17.3) | 26 (55.3) | |
| G2 | 159 (48.2) | 138 (48.8) | 21 (44.7) | |
| G3–4 | 96 (29.1) | 96 (33.9) | 0 | |
| Poor prognostic factors | NA | |||
| Yes | 283 (85.8) | 283 (100.0) | 0 | |
| No | 47 (14.2) | 0 | 47 (100.0) | |
| Postoperative complications | >0.99 | |||
| Yes | 68 (20.6) | 59 (20.8) | 9 (19.1) | |
| No | 262 (79.4) | 224 (79.2) | 38 (80.9) | |
| EGFR mutation | 0.75 | |||
| Positive | 62 (18.8) | 52 (18.4) | 10 (21.3) | |
| Negative | 191 (57.9) | 163 (57.6) | 28 (59.6) | |
| Unknown | 77 (23.3) | 68 (24.0) | 9 (19.1) | |
| Postoperative adjuvant therapy with UFT | 0.53 | |||
| Yes | 163 (49.4) | 142 (50.2) | 21 (44.7) | |
| No | 167 (50.6) | 141 (49.8) | 26 (55.3) | |
| Medication progress of UFT | n=163 | n=142 | n=21 | 0.82 |
| Completion | 105 (64.4) | 91 (64.1) | 14 (66.7) | |
| Discontinuance | 58 (35.6) | 51 (35.9) | 7 (33.3) | |
| Recurrence | <0.001 | |||
| Yes | 95 (28.8) | 91 (32.2) | 4 (8.5) | |
| No | 235 (71.2) | 192 (67.8) | 43 (91.5) | |
| Outcome | 0.62 | |||
| Survival | 215 (65.2) | 186 (65.7) | 29 (61.7) | |
| Death | 115 (34.8) | 97 (34.3) | 18 (38.3) | |
| Cause of death | n=115 | n=97 | n=18 | 0.01 |
| Lung cancer | 58 (50.4) | 54 (55.7) | 4 (22.2) | |
| Others | 57 (49.6) | 43 (44.3) | 14 (77.8) | |
Data are presented as median [range] or n (%). (+), with; (−), without. EGFR, epidermal growth factor receptor; UFT, tegafur-uracil; NA, not available.
Outcomes
In patients with poor prognostic factors, the 5-year RFS rate in the UFT group [74.3% (95% CI: 66.1–80.7%)] was significantly greater than that in the non-UFT group [62.6% (95% CI: 53.0–70.8%); P=0.048; Figure 2A]. Similarly, the 5-year OS of the UFT group [85.6% (95% CI: 78.5–90.4%)] was significantly greater than that in the non-UFT group [62.4% (95% CI: 53.0–70.4%); P<0.001; Figure 2B].
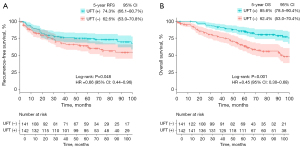
In patients without poor prognostic factors, the 5-year RFS rate was 90.5% (95% CI: 67.0–97.5%) in the UFT group and 95.2% (95% CI: 70.7–99.3%) in the non-UFT group, which did not amount to a significant difference (P=0.40; Figure 3A). However, the 5-year OS rate in the UFT group [90.2% (95% CI: 66.2–97.5%)] was significantly greater than that in the non-UFT group [57.2% (95% CI: 34.8–74.4%); P=0.03; Figure 3B].

Factor analysis associated with outcomes
In the multivariate analysis of patients with poor prognostic factors, age was identified as a significant prognostic factor for RFS (HR =1.61; 95% CI: 1.05–2.46; P=0.03) and OS (HR =1.80; 95% CI: 1.15–2.84; P=0.01). Additionally, the administration of UFT was also a significant prognostic factor for OS (HR =0.57; 95% CI: 0.36–0.89; P=0.02) (Tables 2,3).
Table 2
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥75 vs. <75 years) | 1.73 (1.14–2.61) | 0.01 | 1.61 (1.05–2.46) | 0.03 | |
| Male (vs. female) | 1.25 (0.74–2.12) | 0.41 | – | – | |
| Squamous cell carcinoma (vs. adenocarcinoma) | 1.36 (0.87–2.14) | 0.17 | – | – | |
| Postoperative complications (yes vs. no) | 1.34 (0.82–2.18) | 0.24 | – | – | |
| EGFR mutation (yes vs. no) | 1.13 (0.68–1.88) | 0.64 | – | – | |
| UFT for adjuvant therapy (yes vs. no) | 0.66 (0.44–0.96) | 0.048 | 0.74 (0.49–1.14) | 0.17 | |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; UFT, tegafur-uracil.
Table 3
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥75 vs. <75 years) | 2.44 (1.62–3.68) | <0.001 | 1.80 (1.15–2.84) | 0.01 | |
| Male (vs. female) | 2.22 (1.18–4.16) | 0.01 | 1.66 (0.87–3.17) | 0.13 | |
| Squamous cell carcinoma (vs. adenocarcinoma) | 2.16 (1.43–3.27) | <0.001 | 1.43 (0.91–2.23) | 0.12 | |
| Postoperative complications (yes vs. no) | 1.64 (1.04–2.58) | 0.03 | 1.41 (0.87–2.28) | 0.16 | |
| EGFR mutation (yes vs. no) | 0.80 (0.46–1.39) | 0.43 | – | – | |
| UFT for adjuvant therapy (yes vs. no) | 0.45 (0.30–0.68) | <0.001 | 0.57 (0.36–0.89) | 0.02 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; UFT, tegafur-uracil.
Among patients without poor prognostic factors, squamous cell carcinoma was a significant prognostic factor for RFS (HR =18.3; 95% CI: 1.65–204.3; P=0.02) and OS (HR =7.23, 95% CI: 2.41–21.7; P<0.001). The administration of UFT was also a significant prognostic factor for OS (HR =0.35; 95% CI: 0.12–0.99; P=0.047) (Tables 4,5).
Table 4
| Factors | Univariate | |
|---|---|---|
| HR (95% CI) | P value | |
| Age (≥75 vs. <75 years) | 0.48 (0.05–4.90) | 0.54 |
| Male (vs. female) | 1.03 (0.14–7.28) | 0.98 |
| Squamous cell carcinoma (vs. adenocarcinoma) | 18.3 (1.65–204.3) | 0.02 |
| Postoperative complications (yes vs. no) | NA | – |
| EGFR mutation (yes vs. no) | NA | – |
| UFT for adjuvant therapy (yes vs. no) | 2.58 (0.26–25.2) | 0.40 |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; NA, not applicable; EGFR, epidermal growth factor receptor; UFT, tegafur-uracil.
Table 5
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥75 vs. <75 years) | 2.01 (0.78–5.23) | 0.15 | – | – | |
| Male (vs. female) | 2.21 (0.79–6.21) | 0.13 | – | – | |
| Squamous cell carcinoma (vs. adenocarcinoma) | 7.35 (2.50–21.6) | <0.001 | 7.23 (2.41–21.7) | <0.001 | |
| Postoperative complications (yes vs. no) | 2.26 (0.80–6.41) | 0.13 | – | – | |
| EGFR mutation (yes vs. no) | 0.36 (0.08–1.67) | 0.19 | – | – | |
| UFT for adjuvant therapy (yes vs. no) | 0.29 (0.10–0.82) | 0.03 | 0.35 (0.12–0.99) | 0.047 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; UFT, tegafur-uracil.
Discussion
In patients with stage IB and IIA NSCLC with poor prognostic factors, UFT significantly prolonged RFS and OS. However, in the absence of poor prognostic factors, no benefit of UFT for RFS was observed. This lack of effect may be influenced by the small number of patients and events, suggesting that omitting UFT could be considered for patients without poor prognostic factors. Although there was a significant difference in OS, it should be noted that 77.8% of the deaths were due to other causes. This likely reflects the impact of underlying conditions and overall health status that prevented some patients from undergoing UFT, leading to poor survival outcomes. Nonetheless, one of the key findings of this study is that adjuvant UFT therapy may not be necessary for stage IB and IIA patients without poor prognostic factors.
A major issue with using UFT for postoperative adjuvant chemotherapy is that it is based on the old TNM classification, which does not differentiate between invasive and noninvasive tumor components. Reports have analyzed the effects of UFT based on the 8th edition of the TNM classification, but the results have been inconsistent and controversial (18,19). In contrast, reports using real-world data from patients excluded from the JCOG0707 trial have confirmed that UFT prolongs OS in tumors of >3 cm in diameter without ground-glass opacity (GGO) (20,21). This is consistent with our findings, suggesting that pure solid tumors without GGO are invasive lung cancers with many poor prognostic factors. Furthermore, although platinum-based chemotherapy is sometimes used in clinical practice for stage IB patients with poor prognostic factors, a retrospective study comparing UFT and platinum-based chemotherapy found no significant difference between the two treatments (22). Combined with the results of this study, there is no need to expand the use of platinum-based chemotherapy, even in patients with poor prognostic factors, and UFT is sufficient.
Currently, there is an expanding range of adjuvant therapies for stage ≥ II cancer, including options such as IMpower010 (23), ADAURA (24,25), and ALINA (26). Additionally, the ADAURA2 trial is underway for early-stage EGFR-positive lung cancer (IA2–IA3), with results expected in 2027 (27). Therefore, it is necessary to reconsider the position of UFT in postoperative adjuvant therapy.
Limitations
The present study was associated with several limitations. First, this was a retrospective study conducted at a single institution. Second, 23% of the patients had an unknown EGFR mutation status. Recently, several studies have reported that the efficacy of UFT is extremely limited in EGFR-positive lung cancer (28-31), which could have influenced our results. Additionally, measurement of programmed death ligand 1 (PD-L1) tumor progression score (TPS) has rarely been performed. PD-L1 TPS is particularly important for decision making between using atezolizumab or UFT in stage IIA; however, the results of this study should not be used to guide such decisions.
Future perspectives
Future research should focus on stratifying patients based on genetic and molecular markers, such as EGFR mutations or PD-L1 expression, to optimize treatment selection. Additionally, prospective trials comparing UFT with newer therapies in specific subgroups, including those with distinct pathological or molecular characteristics, are warranted. Real-world evidence studies that incorporate long-term outcomes and quality-of-life measures will also be essential to validate UFT’s efficacy in contemporary clinical settings. In fact, a large-scale real-world database study of EGFR-positive lung cancer has demonstrated that the efficacy of conventional adjuvant therapies is limited (32). Similarly, an appropriate adjuvant therapy for PD-L1-expressing lung cancer with driver mutations has yet to be established, highlighting the need for further research (33).
Furthermore, research into circulating tumor DNA (ctDNA) is gaining attention in the context of adjuvant therapy decision-making. Recent studies have reported that postoperative ctDNA is a risk factor for recurrence and a monitoring tool for the effectiveness of adjuvant therapy (34-36). ctDNA has also been identified as an important risk factor for recurrence in early-stage lung cancer (35,36), which is likely to further accelerate the era of personalized adjuvant therapy.
Ultimately, integrating UFT into a broader framework of tailored adjuvant strategies could provide a cost-effective option for high-risk patients, especially in regions where access to advanced therapies is limited. This approach underscores the importance of a nuanced application of evidence-based therapies to achieve the best possible outcomes for diverse patient populations.
Conclusions
In patients with completely resected stage IB and IIA NSCLC with poor prognostic factors (such as vascular invasion, lymphatic invasion, pleural invasion, and poor differentiation), postoperative adjuvant therapy with UFT is expected to extend RFS and OS and should be actively implemented.
Acknowledgments
We thank the patients, their families, and all the staff of the Respiratory Disease Center. We also extend our gratitude to Rina Watanabe (design SATO) for her assistance in designing the figures.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-820/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-820/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-820/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-820/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This retrospective study was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (approval No. 24-02). The requirement for informed consent from each patient was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riely GJ, Wood DE, Ettinger DS, et al. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024;22:249-74. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 2005;23:4999-5006. [Crossref] [PubMed]
- Hamada C, Tsuboi M, Ohta M, et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 2009;4:1511-6. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Mountain CF. A new international staging system for lung cancer. Chest 1986;89:225S-33S. [Crossref] [PubMed]
- Okiror L, Harling L, Toufektzian L, et al. Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;156:785-93. [Crossref] [PubMed]
- Hsu CP, Hsia JY, Chang GC, et al. Surgical-pathologic factors affect long-term outcomes in stage IB (pT2 N0 M0) non-small cell lung cancer: a heterogeneous disease. J Thorac Cardiovasc Surg 2009;138:426-33. [Crossref] [PubMed]
- Shimada Y, Saji H, Yoshida K, et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 2012;7:1263-70. [Crossref] [PubMed]
- Moon Y, Choi SY, Park JK, et al. Prognostic factors in stage IB non-small cell lung cancer according to the 8(th) edition of the TNM staging system after curative resection. J Thorac Dis 2019;11:5352-61. [Crossref] [PubMed]
- Yasukawa M, Sawabata N, Kawaguchi T, et al. Histological Grade: Analysis of Prognosis of Non-small Cell Lung Cancer After Complete Resection. In Vivo 2018;32:1505-12. [Crossref] [PubMed]
- Yasukawa M, Ohbayashi C, Kawaguchi T, et al. Analysis of Histological Grade in Resected Lung-invasive Adenocarcinoma. Anticancer Res 2019;39:1491-500. [Crossref] [PubMed]
- Wang J, Chen J, Chen X, et al. Blood vessel invasion as a strong independent prognostic indicator in non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2011;6:e28844. [Crossref] [PubMed]
- Ichinose Y, Yano T, Asoh H, et al. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg 1995;110:601-5. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Adachi H, Nishii T, Yamamoto T, et al. Retrospective study of efficacy of adjuvant chemotherapy using tegafur-uracil in patients with non-small cell lung cancer with primary tumor size of 4.1-5.0 cm. J Thorac Dis 2019;11:3103-11. [Crossref] [PubMed]
- Tsuboi M, Hamada C, Kato H, et al. The Effect of Tegafur-Uracil on Survival in T Categories as Defined in the Eighth Edition of the TNM Classification: An Exploratory Analysis of Postoperative Adjuvant Tegafur-Uracil on Survival in Patients with Adenocarcinoma of the Lung. Chemotherapy 2017;62:357-60.
- Shukuya T, Takamochi K, Sakurai H, et al. Efficacy of Adjuvant Chemotherapy With Tegafur-Uracil in Patients With Completely Resected, Node-Negative NSCLC-Real-World Data in the Era of Molecularly Targeted Agents and Immunotherapy. JTO Clin Res Rep 2022;3:100320. [Crossref] [PubMed]
- Kunitoh H, Tsuboi M, Wakabayashi M, et al. A phase III study of adjuvant chemotherapy in patients with completely resected, node-negative non-small cell lung cancer (JCOG 0707). JTCVS Open 2020;4:90-102. [Crossref] [PubMed]
- Liang SK, Wu CW, Chang CI, et al. Oral uracil-tegafur compared with intravenous chemotherapy as adjuvant therapy for resected early-stage non-small cell lung cancer patients. Cancer Med 2023;12:17993-8004. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Wu YL, Dziadziuszko R, Ahn JS, et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:1265-76. [Crossref] [PubMed]
- Tsutani Y, Goldman JW, Dacic S, et al. Adjuvant Osimertinib vs. Placebo in Completely Resected Stage IA2-IA3 EGFR-Mutated NSCLC: ADAURA2. Clin Lung Cancer 2023;24:376-80. [Crossref] [PubMed]
- Tsutani Y, Ito M, Shimada Y, et al. The impact of epidermal growth factor receptor mutation status on adjuvant chemotherapy for patients with high-risk stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2022;164:1306-1315.e4. [Crossref] [PubMed]
- Aoki M, Miyata R, Kamimura G, et al. Effect of Tegafur-Uracil in Resected Stage IB Lung Adenocarcinoma According to Presence or Absence of Epidermal Growth Factor Receptor Gene Mutation: A Retrospective Cohort Study. Ann Thorac Cardiovasc Surg 2024;30:23-00134. [Crossref] [PubMed]
- Miyoshi T, Aokage K, Watanabe SI, et al. The effect of epidermal growth factor receptor mutation on adjuvant chemotherapy with tegafur/uracil for patients with completely resected, non-lymph node metastatic non-small cell lung cancer (> 2 cm): a multicenter, retrospective, observational study as exploratory analysis of the CSPOR-LC03 study. Jpn J Clin Oncol 2024;54:1185-93. [Crossref] [PubMed]
- Nomura K, Aokage K, Kaminuma Y, et al. EGFR mutation impacts recurrence in high-risk early-stage lung adenocarcinoma in the IASLC grading system. Int J Clin Oncol 2024;29:248-57. [Crossref] [PubMed]
- Katsumata S, Shimokawa M, Hamada A, et al. Impact of central nervous system metastasis after complete resection of lung adenocarcinomas harboring common EGFR mutation - A real-world database study in Japan: The CReGYT-01 EGFR study. Eur J Cancer 2024;201:113951. [Crossref] [PubMed]
- Nomura K, Takada K, Kinoshita F, et al. Prognostic impact of PD-L1 expression in surgically resected EGFR-mutant lung adenocarcinoma: A real-world database study in Japan (CReGYT-01 EGFR study). Int J Cancer 2024; Epub ahead of print. [Crossref] [PubMed]
- Oh Y, Yoon SM, Lee J, et al. Personalized, tumor-informed, circulating tumor DNA assay for detecting minimal residual disease in non-small cell lung cancer patients receiving curative treatments. Thorac Cancer 2024;15:1095-102. [Crossref] [PubMed]
- Tian X, Liu X, Wang K, et al. Postoperative ctDNA in indicating the recurrence risk and monitoring the effect of adjuvant therapy in surgical non-small cell lung cancer. Thorac Cancer 2024;15:797-807. [Crossref] [PubMed]
- Tan AC, Lai GGY, Saw SPL, et al. Detection of circulating tumor DNA with ultradeep sequencing of plasma cell-free DNA for monitoring minimal residual disease and early detection of recurrence in early-stage lung cancer. Cancer 2024;130:1758-65. [Crossref] [PubMed]


