
Video-assisted mediastinocopic lymphadenectomy (VAMLA) for restaging clinical N2–3 non-small cell lung cancer (NSCLC) after neoadjuvant treatment
Highlight box
Key findings
• This study represents the first series of video-assisted mediastinoscopic lymphadenectomy (VAMLA) for restaging histologically proven clinical N2–3 (cN2–3) non-small cell lung cancer (NSCLC) after neoadjuvant therapy.
• We have demonstrated the same high diagnostic performance reported in the primary staging: sensitivity, 1 [95% confidence interval (CI): 0.72–1]; negative predictive value (NPV), 1 (95% CI: 0.89–1).
• The rate of persistent N2 after neoadjuvant therapy for the whole series was high (29.3%).
What is known and what is new?
• Mediastinal restaging with non-invasive imaging techniques is unreliable. For this reason, histological confirmation of objective tumour response after neoadjuvant therapy is recommended; endobronchial ultrasound-transbronchial needle aspiration after neoadjuvant therapy presents low accuracy. Its NPV varies from 20% to 78%; surgical techniques for restaging are more demanding and their efficacy is lower than primary staging. Its NPV ranges from 0.73 to 0.88; VAMLA and transcervical extended mediastinal lymphadenectomy (TEMLA) allow the dissection of all mediastinal nodal stations explored and the removal of all lymph nodes. Both operations are considered the surgical staging procedures with the highest accuracy. Before our study, only TEMLA was validated for restaging after neoadjuvant treatment with the highest sensitivity (0.85–1) and NPV (0.93–0.99).
• Our study certifies that VAMLA is a feasible and reliable method for restaging cN2–3 NSCLC treated with neoadjuvant treatment.
What is the implication, and what should change now?
• VAMLA should be included in restaging algorithms to select patients that would benefit from multidisciplinary approach that includes surgical resection after neoadjuvant therapy.
Introduction
The assessment of the objective tumour response after neoadjuvant therapy continues to be a diagnostic challenge. For this reason, the use of mediastinal downstaging as a criterion to select patients for operative treatment requires a reliable restaging method to assess pathologic tumour response before undergoing lung resection. According to current European Society of Thoracic Surgeons (ESTS) guidelines, histological confirmation of objective tumour response after neoadjuvant therapy is recommended. This confirmation can be done with endosonographic techniques but endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) after neoadjuvant therapy presents low accuracy; therefore, an invasive surgical technique is still recommended when the results of endoscopic procedures are negative (1).
Video-assisted mediastinoscopic lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA) allow the dissection and removal of all the lymph nodes (LNs) and fatty tissue of the examined mediastinal nodal stations. Both techniques are considered the surgical staging procedures with the highest sensitivity (0.85–1) and negative predictive value (NPV) (0.93–0.99) (2-5). However, when focused in restaging after neoadjuvant therapy, VAMLA has not been analyzed and TEMLA has reported a high sensitivity and NPV, 0.95 and 0.97, respectively (6).
After more than 10 years performing VAMLA (mostly for primary staging) (7), we present our preliminary results of mediastinal nodal restaging in patients with histologically proven clinical N2–3 (cN2–3) non-small cell lung cancer (NSCLC) treated with neoadjuvant treatment. The objective of this study is to evaluate the feasibility and diagnostic performance of VAMLA for restaging, and the rate of persistent N2–3 after neoadjuvant treatment. We present this article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-841/rc).
Methods
Patients
From a prospective database, all consecutive patients who underwent VAMLA from January 2010 to December 2023 were retrieved and analysed. Patients with histologically proven cN2-3 NSCLC, diagnosed by EBUS-TBNA, treated with neoadjuvant therapy, and restaged by VAMLA were selected for this study. Exclusion criteria are shown in Figure 1.
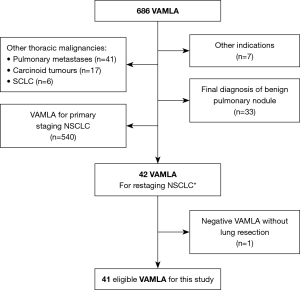
Study design
Prospective observational single-centre study to determine the diagnostic performance of VAMLA for mediastinal restaging of cN2–3 NSCLC after neoadjuvant therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics and Clinical Research Committee of Hospital Universitari Mútua Terrassa (EO/1520 29th April 2010) and individual patient consent was waived.
Endpoints
Primary endpoints were: to analyse the diagnostic performance of VAMLA for detecting persistent N2–3 disease in patients with cN2–3 NSCLC treated with neoadjuvant therapy, and the assessment of the prevalence of unsuspected persistent N2–3 disease after neoadjuvant treatment. Secondary endpoints were: diagnostic performance of integrated positron emission tomography (PET)-computed tomography (CT) and the whole staging protocol in restaging.
Preoperative work-up of patients with NSCLC
To assess the anatomic extent of lung cancer, posteroanterior and lateral chest X-rays, CT of the chest extending to the upper abdomen, and PET-CT were performed systematically. To further explore anatomic extent, bronchoscopy was performed in all patients. CT or magnetic resonance imaging of the brain (or both), abdominal ultrasonography, and bone scans were performed depending on symptoms or findings of the previous tests. Operability was assessed by medical history, physical examination, electrocardiogram and pulmonary function tests. Invasive staging of the mediastinum was indicated according to current ESTS guidelines (1). From 2016, EBUS-TBNA, endoscopic ultrasound-fine needle aspiration (EUS-FNA), and their combination could be performed in our hospital. Consequently, patients with NSCLC staged before 2016 underwent VAMLA directly and those staged after 2016, underwent EBUS-TBNA followed by VAMLA to validate negative results.
Restaging after induction therapy
At completion of neoadjuvant treatment, a new PET-CT scan was performed to rule out local, regional and distant progression of disease. Patients who, according to radiologic and metabolic criteria, showed stable disease or some response [as defined by Response Evaluation Criteria in Solid Tumours (RECIST) (8)] underwent invasive mediastinal restaging to select those who could undergo complete resection (9) defined as: microscopically proven free resection margins, systematic nodal dissection (SND) or lobe-specific SND, absence of extracapsular nodal involvement and microscopically proven negative highest mediastinal node. Invasive restaging protocol included EBUS-TBNA followed by validation of negative results with VAMLA.
EBUS-TBNA technique
EBUS-TBNA was performed using a flexible bronchoscope (BFUC180F; Olympus Optical Co., Ltd., Tokyo, Japan) with a distal probe capable of producing linear parallel scans of the mediastinal and peribronchial tissues and a working channel suited for the performance of TBNA under direct ultrasound guidance. General anaesthesia was performed by an anaesthesiologist using topical lidocaine spray and intravenous midazolam, propofol, and/or fentanyl according to standard recommendations (10). Patients were mechanically ventilated through a laryngeal mask. Contrary to primary staging, mediastinal restaging consisted of the selective sampling of the LNs that were positive in the initial staging. LNs with a short-axis diameter of ≥5 mm identified during the procedure were targeted under direct ultrasound visualization with a 21-gauge or 22-gauge cytology needle specially designed for EBUS-TBNA (NA-201SX-4022; Olympus Optical Co., Ltd.). During the EBUS-TBNA procedure, all pathologically proven mediastinal stations in the initial staging were sampled regardless of their size or their maximum standardized uptake value on the postinduction PET-CT. Negative nodal stations on EBUS-TBNA in the initial staging were not resampled except if they were suspicious on the postinduction PET-CT.
VAMLA technique
In the past 20 years, more than 3,000 mediastinoscopies were performed by thoracic surgeons in a dedicated thoracic surgery department mainly dealing with thoracic malignancies. VAMLA was introduced in the staging protocol of Thoracic Surgery Department as a natural progression of very thorough videomediastinoscopies. Our previous results were already published elsewhere (5,7). Contrary to the objective of mediastinoscopy, the objective of VAMLA is to remove all LNs and surrounding fatty tissue of all reachable mediastinal nodal stations. These are the right and left paratracheal, subcarinal, and hilar nodes (stations 2R, 2L, 4R, 4L, 7, 10R, and 10L) (11). We used the same technique described by Hürtgen et al. in 2002 (12). For this study, the following systematic exploration has been followed (Video 1): the first step is the complete excision of all LNs and fat of the subcarinal nodal station limited laterally by the main bronchi, anteriorly by the pulmonary artery, and posteriorly by the oesophagus. In most cases, this dissection included the upper part of the para-oesophageal nodes. The second step is the dissection of right inferior paratracheal LNs with the fatty tissue, from the lower margin of the brachiocephalic artery to the lower margin of the azygos vein and along the right main bronchus. The third and final step is the identification of the left recurrent laryngeal nerve and the removal of the left paratracheal nodes one by one. For left lung cancers, an extended cervical mediastinoscopy (ECM) was added to VAMLA to explore and to take biopsies of the subaortic and paraaortic LNs (stations 5 and 6) (13). During the procedure, each whole node or all fragments of one node were kept and labelled in one container to facilitate the counting of removed and involved LNs.
The anaesthesia, patient positioning, and instruments for VAMLA are the same for mediastinoscopy. Particularities for VAMLA include the spreadable videomediastinoscope that allows a larger operative field, an optimal exposure, and bimanual dissection; electrothermal tissue sealing facilitates the dissection of adhesions and fibrotic tissue minimizing haemorrhages and reducing the risk of injury to the left recurrent laryngeal nerve.
Lung resection
European Respiratory Society/ESTS guidelines on fitness for radical therapy were followed to assess patient’s operability (14). Patients with negative VAMLA underwent lung resection and lymphadenectomy of the remaining LNs according to the ESTS guidelines on intraoperative SND (15). The approach for the lung resection [video-assisted thoracoscopic surgery (VATS) or thoracotomy] depended on the surgeon’s preferences.
Statistical analysis
Patient demographic and clinical characteristics, operative results from VAMLA and lung resections, and oncological and pathological characteristics were collected. Categorical variables were expressed as absolute and relative frequencies, and continuous variables as means and standard deviations (SDs). The result of the SND (15) (this includes surgical verification of the nodal stations removed at VAMLA) performed during lung resection was considered the gold standard test to validate the negative results of VAMLA. Definitive pathologic positive results of VAMLA were considered true positive results of this study. Patients with negative VAMLA who did not undergo lung resection were excluded from analysis. Pathologic findings were reviewed and staging values were calculated using the standard formulas [including 95% confidence interval (CI)]: sensitivity = true positives/true positives + false negatives; specificity = true negatives/true negatives + false positives; NPV = true negatives/true negatives + false negatives; PPV = true positives/true positives + false positives; diagnostic accuracy = true positives + true negatives/true positives + true negatives + false positives + false negatives. The rate of persistent N2/3 disease after VAMLA and after tumour resection was analysed in the global series.
Results
A total of 686 patients underwent VAMLA in the study period; 540 patients with VAMLA for primary staging were excluded from this analysis. Other exclusions are shown in Figure 1. Forty-one patients (33 men, 8 women; median age 66 years, SD: 9.1 years) with histologically proven cN2–3 NSCLC treated with neoadjuvant treatment who underwent VAMLA for restaging were eligible for analysis. Most patients (n=33; 80.5%) underwent concomitant cisplatin-based chemotherapy and radical radiotherapy (mean 58.3±3.9 Gy). Other neoadjuvant therapies used in this series were: chemotherapy alone (n=2; 4.9%), immunotherapy preceded by chemoradiotherapy (n=2; 4.9%) or chemotherapy (n=2; 4.9%), tyrosine kinase inhibitor and immunotherapy (n=1; 2.4%) and, immunotherapy alone (n=1; 2.4%). In five patients with cN3 disease diagnosed in the primary staging, restaging PET-CT after neoadjuvant therapy showed mediastinal downstaging (ycN0) in all of them. VAMLA confirmed persistent mediastinal involvement in one patient. The remaining four patients underwent lung resection and SND that confirmed mediastinal downstaging. On final pathologic report, one patient showed complete pathologic response and three patients had some viable malignant cells in the primary tumour. In the remaining 36 patients with cN2, cN categories after neoadjuvant treatment (ycN) according to PET-CT were: ycN0 in 29 (80.5%), ycN1 in 1 (2.7%) and ycN2 in 6 (16.6%). Three patients had synchronous tumours, for a total of 51 NSCLC. Patient characteristics are shown in Table 1.
Table 1
| Variables | Values |
|---|---|
| Patients | 41 |
| Age (years) | 66±9.1 |
| Sex | |
| Male | 33 (80.5) |
| Female | 8 (19.5) |
| cTNM classification at primary staging | |
| cT0N2M0 | 1 (2.4) |
| cT1N2M0 | 11 (26.8) |
| cT2N2M0 | 11 (26.8) |
| cT3N2M0 | 5 (12.2) |
| cT4N2M0 | 5 (12.2) |
| cT2N3M0 | 2 (4.9) |
| cT3N3M0 | 1 (2.4) |
| cT4N3M0 | 2 (4.9) |
| cT2N2M1b | 1 (2.4) |
| cT2N2M1c | 1 (2.4) |
| cT4N2M1c | 1 (2.4) |
| Neoadjuvant treatments | |
| Chemoradiotherapy | 33 (80.5) |
| CDDP + VNR | 24 (72.7) |
| CBDCA + VNR | 7 (21.2) |
| CDDP + VP16 | 2 (6.1) |
| Chemotherapy + immunotherapy | 2 (4.9) |
| Chemoradiotherapy + immunotherapy | 2 (4.9) |
| Chemotherapy alone | 2 (4.9) |
| TKI and immunotherapy | 1 (2.4) |
| Immunotherapy alone | 1 (2.4) |
| Laterality | |
| Right side | 21 (51.2) |
| Left side | 17 (41.5) |
| Synchronous tumours (n=3) | |
| Right side (all lobes) | 1 (2.4) |
| Same lobe (right upper lobe) | 2 (4.9) |
| Tumour location (n=51†) | |
| Right upper lobe | 22 (43.1) |
| Middle lobe | 3 (5.9) |
| Right lower lobe | 10 (19.6) |
| Left upper lobe | 10 (19.6) |
| Left lower lobe | 6 (11.8) |
| Clinical T category after neoadjuvant therapy by PET-CT (n=41) | |
| T1 | 19 (46.3) |
| T2 | 14 (34.1) |
| T3 | 7 (17.1) |
| T4 | 1 (2.4) |
| Clinical N category after neoadjuvant therapy by PET-CT (n=41) | |
| N0 | 34 (82.9) |
| N1 | 1 (2.4) |
| N2 | 6 (14.6) |
| Final histologic type (n=41) | |
| Adenocarcinoma | 25 (61.0) |
| Squamous cell | 10 (24.4) |
| Large cell | 1 (2.4) |
| Pleomorphic cell | 1 (2.4) |
| Others | 4 (9.8) |
Values are presented as n, mean ± SD, or n (%). †, 3 patients with 11 synchronous tumours required 9 lung resections (2 patients had 2 synchronous tumours in the same lobe, 1 patient had 7 synchronous tumours in 3 lobes). cTNM, clinical tumour, node, and metastasis; CDDP, cisplatin; VNR, vinorelbine; CBDCA, carboplatin; VP16, etoposide; TKI, tyrosine kinase inhibitor; PET-CT, positron emission tomography-computed tomography; SD, standard deviation.
Results of restaging EBUS-TBNA
Twenty-nine patients (24 men, 5 women; median age 66 years, SD: 7.39 years) underwent EBUS-TBNA for restaging. A mean of 1 nodal station and 3 LNs per patient were sampled. A mean of 5.1±2.4 needle passes were performed. The nodal categories according to restaging PET-CT were ycN0 in 23 (80%) patients, ycN1 in 0 (0%) and ycN2 in 6 (20%). In 8 patients (27%), EBUS-TBNA was false negative in those nodal stations accessible to this technique and verified by VAMLA. Complications related to EBUS-TBNA occurred in 1 (3.4%) patient: minor bleeding that was treated with endoscopic measures.
VAMLA results
VAMLA was feasible in all patients. In six patients the exploration was reported as incomplete due to calcified or fibrotic nodes (four on the right inferior paratracheal station, one in the subcarinal station, and one in both subcarinal and right inferior paratracheal stations). The mean number of LN station explored was 3 (SD: 0.9) and the mean number of LNs resected per patient was 10 (SD: 6.1). Table 2 shows surgical results of restaging with VAMLA. VAMLA was negative in 31 (76%) patients and positive in 10 (24%) patients with eight cases of single-level N2 disease and two cases of multi-level N2 disease. Complication rate related to VAMLA was 4 (9.7%): 3 (7.3%) temporary and 1 (2.4%) permanent left recurrent laryngeal nerve palsies.
Table 2
| Variables | Mean | SD |
|---|---|---|
| Operative time (minutes) | 120 | 28.2 |
| Number of LN stations explored | 3 | 0.9 |
| Number of resected LNs | ||
| Total | 10 | 6.1 |
| Right inferior paratracheal | 5 | 3.4 |
| Left inferior paratracheal | 2 | 2.5 |
| Subcarinal | 3 | 1.7 |
| Days between end of neoadjuvant treatment and VAMLA | 57 | 103.68 |
VAMLA, video-assisted mediastinoscopic lymphademectomy; SD, standard deviation; LN, lymph node.
Lung resection results
Thirty-one patients with negative VAMLA underwent lung resection. Thoracotomy was performed in 22 (71%) patients and VATS in 9 (29%). Two VATS procedures were converted to thoracotomy. In 1 patient, this was due to the difficulty in the vascular dissection; and in the other to accidental bleeding during arterial dissection. Table 3 shows their clinical characteristics. Final pathologic findings from SND showed ypN0 in 27 (87.1%) patients, ypN1 in 2 (6.45%) patients and ypN2 in 2 (6.45%) patients (Figure 2). The positive mediastinal nodal stations were: one case in the right pulmonary ligament (station #9) and one case in the subaortic nodal station (station #5). For the purpose of this study, these two cases were considered true negative VAMLAs and false negative cases of the whole restaging protocol because these stations are located beyond the exploration range of VAMLA (Figure 2). The clinical characteristics of patients with persistent N2 disease after the complete restaging protocol are summarized in Table 4.
Table 3
| Variables | Values |
|---|---|
| Patients | 31 |
| Age (years) | 66±9.21 |
| Sex | |
| Male | 25 (80.6) |
| Female | 6 (19.4) |
| Days elapsed from VAMLA to lung resection | 14±13.5 |
| Laterality | |
| Right-sided tumours | 17 (54.8) |
| Left-sided tumours | 12 (38.7) |
| Synchronous tumours (n=2) | |
| Right side | 2 (6.5) |
| Type of lung resection (n=38†) | |
| Lobectomy | 24 (63.2) |
| Pneumonectomy | 1 (2.6) |
| Bilobectomy | 3 (7.9) |
| Segmentectomy | 9 (23.7) |
| Lobectomy and SVC resection | 1 (2.6) |
| Type of approach (n=31) | |
| VATS | 9 (29.0) |
| Conversion to thoracotomy | 2 (22.2) |
| Thoracotomy | 22 (71.0) |
| ypTNM (n=31) | |
| ypT0N0M0 | 12 (38.7) |
| ypT1aN0M0 | 7 (22.6) |
| ypT1bN0M0 | 4 (12.9) |
| ypT1cN0M0 | 4 (12.9) |
| ypT1bN1M0 | 2 (6.5) |
| ypT1aN2M0 | 1 (3.2) |
| ypT1bN2M0 | 1 (3.2) |
| Persistent pathological N2 disease after lung resection | 2 (6.5) |
Values are presented as n, mean ± SD, or n (%). †, 1 patient required 7 additional lung resections for synchronous tumours in all lobes of the right lung. VAMLA, video-assisted mediastinoscopic lymphademectomy; SVC, superior vena cava; VATS, video-assisted thoracic surgery; ypTNM, pathological TNM classification after neoadjuvant treatment; TNM, tumour, node, and metastasis; SD, standard deviation.
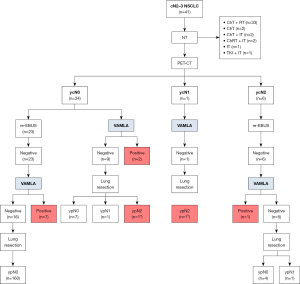
Table 4
| No. | Sex | Age (years) | Histology | cTNM† | ycTNM‡ | LN removed by VAMLA | ypN2§ |
|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | ADK | T2aN2M0 | T2aN0M0 | 14 | 7 |
| 2 | Male | 75 | SQCC | T4N2M0 | T2aN0M0 | 7 | 7, 8 |
| 3 | Female | 70 | ADK | T1a(7)N2M0 | T1a(2)N0M0 | 13 | 9 |
| 4 | Male | 48 | ADK | T2aN3M0 | T1bN0M0 | 15 | 4R (×3), 7 (×3) |
| 5 | Male | 66 | SQCC | T4N2M0 | T4N2M0 | 16 | 4R (×2) |
| 6 | Male | 66 | SQCC | T2aN2M0 | T1bN0M0 | 8 | 7 (×3) |
| 7 | Male | 61 | ADK | T2bN2M0 | T2aN0M0 | 8 | 4L (×2) |
| 8 | Female | 54 | ADK | T2bN2M0 | T2bN0M0 | 20 | 7 (×3) |
| 9 | Male | 62 | ADK | T1cN2M0 | T1cN0M0 | 9 | 4R (×2) |
| 10 | Female | 49 | NSCLC | T1bN2M0 | T1bN0M0 | 24 | 4L |
| 11 | Female | 58 | ADK | T2N2M1c | T1cN1M0 | 9 | 5 (×2) |
| 12 | Male | 66 | ADK | T3N2M0 | T3N0M0 | 9 | 4R |
†, cTNM by PET-CT and EBUS-TBNA before neoadjuvant treatment; ‡, cTNM by PET-CT after neoadjuvant treatment; §, in parenthesis the number of involved nodes when there were more than one. LN, lymph node; cTNM, clinical TNM; TNM, tumour, node, and metastasis; ycTNM, cTNM classification after neoadjuvant treatment; VAMLA, video-assisted mediastinoscopic lymphademectomy; ypN2, persistant N2 after induction therapy; ADK, adenocarcinoma; SQCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; PET-CT, positron emission tomography-computed tomography; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration.
Complications occurred in 4 (15.6%) patients: two patients presented atelectasis treated with bronchoscopic aspiration and antibiotics, and one of them required completion middle lobectomy due to persistent atelectasis; one patient presented a bleeding from the chest wall 24 hours after surgery that needed reintervention; one patient developed bronchopleural fistula that required surgical repair. Among the four patients with left recurrent laryngeal nerve palsy after VAMLA, one presented pneumonia.
Three patients presented with oligometastatic disease on primary staging. One had a single metastasis in one adrenal gland that was resected after restaging VAMLA was negative and prior to lung resection. The other two patients had two metastases each: one had two brain metastases that were treated with stereotactic radiosurgery; and the other had one adrenal metastasis that was resected and one brain metastasis that responded to systemic treatment and required no local therapy.
Study endpoints
Diagnostic performance of VAMLA for restaging cN2–3 NSCLC treated with neoadjuvant treatment was: sensitivity, 1 (95% CI: 0.72–1); specificity, 1 (95% CI: 0.89–1); PPV, 1 (95% CI: 0.72–1); NPV, 1 (95% CI: 0.89–1); and accuracy, 1 (95% CI: 0.91–1). Diagnostic performance of PET-CT to rule out persistent N2–3 after neoadjuvant treatment was: sensitivity, 0.08 (95% CI: 0.01–0.35); specificity, 0.82 (95% CI: 0.65–0.92); NPV, 0.68 (95% CI: 0.52–0.81); PPV, 0.17 (95% CI: 0.03–0.56); and accuracy, 0.61 (95% CI: 0.47–0.74). The restaging values of the whole restaging protocol, including PET-CT, EBUS-TBNA, VAMLA, ECM, and SND were: sensitivity, 0.83 (95% CI: 0.55–0.95); specificity, 1 (95% CI: 0.89–1); PPV, 1 (95% CI: 0.72–1); NPV, 0.93 (95% CI: 0.80–0.98); and accuracy, 0.95 (95% CI: 0.84–0.98) (Table 5).
Table 5
| Staging values | PET-CT | VAMLA | Restaging protocol† |
|---|---|---|---|
| Sensitivity (95% CI) | 0.08 (0.01–0.35) | 1 (0.72–1) | 0.83 (0.55–0.95) |
| Specificity (95% CI) | 0.82 (0.65–0.92) | 1 (0.89–1) | 1 (0.89–1) |
| NPV (95% CI) | 0.68 (0.52–0.81) | 1 (0.89–1) | 0.93 (0.80–0.98) |
| PPV (95% CI) | 0.17 (0.03–0.56) | 1 (0.72–1) | 1 (0.72–1) |
| Accuracy (95% CI) | 0.61 (0.47–0.74) | 1 (0.91–1) | 0.95 (0.84–0.98) |
†, restaging protocol includes: PET-CT, EBUS-TBNA, VAMLA, ECM, and SND performed during lung resection. PET-CT, positron emission tomography-computed tomography; VAMLA, video-assisted mediastinoscopic lymphadenectomy; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration; ECM, extended cervical mediastinoscopy; SND, systematic nodal dissection.
The persistent N2 rate for the whole series was 29.3% (10 cases identified at VAMLA and two cases at tumour resection).
Discussion
We present the first results of VAMLA for restaging histologically proven cN2–3 NSCLC after neoadjuvant therapy reported to date. Our main findings of this series were that VAMLA demonstrated the same performance reported in the primary staging (7), high sensitivity [1 (95% CI: 0.72–1)] and NPV [1 (95% CI: 0.89–1)], and a high rate of persistent N2 disease (29.3%).
The role of lung resection after neoadjuvant chemotherapy or chemoradiotherapy in patients with pathologically documented N2 disease remains controversial. Two randomized trials demonstrated that there was no overall survival (OS) benefit with surgical resection except for patients whose NSCLC had undergone downstaging from cN2 to pathologic N0 after neoadjuvant treatment (ypN0) (16,17). Currently, several phase II and phase III clinical trials have reported high tumour response rates and global survival benefits with neoadjuvant treatment with platinum-based chemotherapy plus immune checkpoint inhibitors (ICIs) in patients with locally advanced NSCLC (18-22). These trials have replaced the classical term “downstaging” with major pathological response (MPR) and pathological complete response (pCR) which have been considered as surrogate endpoints of OS and event free-survival (EFS). All of these trials report a correlation between the grade of pathologic response and short-term OS and EFS. Recently, Hines et al. (23) have conducted the first systematic review and meta-analysis of seven randomised controlled trials evaluating neoadjuvant ICIs for resectable NSCLC. They report a robust correlation between pCR and MPR with 1- and 2-year EFS but this correlation was not replicated in terms of OS, concluding that pCR and MPR are not yet adequate surrogates for OS in clinical trials. Moreover, none of these trials mandated invasive mediastinal restaging after neoadjuvant treatment, and, for this reason, there is no data regarding the value of this practice. In addition, survival rates of patients with persistent mediastinal nodal disease have not been reported, yet. In the light of the evidence of these preliminary results, it seems that the lack of pathologic response after neoadjuvant chemotherapy plus ICIs have a poor impact on EFS and OS (20,22,23). Therefore, invasive mediastinal restaging can continue being a useful method to provide pathological evidence of response after neoadjuvant therapy and, consequently, to select the best candidates for tumour resection.
According to the current ESTS guidelines on restaging, the same techniques used in primary staging can be used for mediastinal restaging with the premise that non-invasive imaging techniques are not accurate enough for mediastinal restaging. For this reason, histological confirmation of objective tumour response after neoadjuvant therapy is recommended (1). In the systematic review of restaging after neoadjuvant therapy conducted by de Cabanyes Candela et al. (24), they found that mediastinal nodal involvement has false negative and false positive rates of 33% and 33%, respectively, by CT, and 25% and 33%, respectively, by PET-CT. In our study, we have also obtained low restaging values of PET-CT (false negative and false positive rates of 31% and 83%, respectively), with a sensitivity of 0.08 (95% CI: 0.01–0.35) and NPV of 0.68 (95% CI: 0.52–0.81) confirming the poor reliability of this technique to evaluate the mediastinal nodal status after neoadjuvant therapy. Regarding the efficacy of endosonography methods in restaging, several studies showed that there is a high heterogeneity depending on the author. Jiang et al. (25) carried out a systematic review on endosonography with LN sampling for restaging the mediastinum reporting a pooled sensitivity for EBUS-TBNA and EUS-FNA of 65% and 73%, respectively. The NPV for EBUS-TBNA varies from 20% to 78% (26,27) indicating a high variability in efficacy and a lower performance of this technique in comparison with the performance reported in primary staging. In our study, with the data of negative EBUS-TBNA validated by VAMLA, we observed a false negative rate of 27%.
Table 6 summarizes the performance of the most representative series of surgical techniques for restaging the mediastinum and of the present series. Sensitivity and NPV range from 0.61 to 1 and 0.73 to 1, respectively (28-33). The use of a first mediastinoscopy for restaging is addressed in a small series. In this article, sensitivity of 0.81, NPV of 0.90 with a prevalence of persistent N2 after neoadjuvant therapy of 46% were reported (28). Theoretically, this approach could be a good strategy to perform an easier and safer mediastinoscopy due to the absence of adhesions in the mediastinum. Remediastinoscopy (reMS) does not differ much from a conventional mediastinoscopy. However, reMS is technically more demanding because of peritracheal adhesions, resulting in a lower accuracy in comparison with the first procedure. Although reMS is not a common procedure, several authors, including our team, have reported its feasibility and the following results for sensitivity and NPV: 0.61–0.74 and 0.73–0.88, respectively (29-31).
Table 6
| Parameters | Lardinois et al. (28) |
Marra et al. (29) |
De Waele et al. (30) |
Call et al. (31) |
Jaklitsch et al. (32) |
Zielinski et al. (33) |
Present series |
|---|---|---|---|---|---|---|---|
| Technique | ycMed | reMS | reMS | reMS | VATS | TEMLA | VAMLA |
| Patients | 24 | 104 | 104 | 83 | 70 | 78 | 41 |
| Sensitivity | 0.81 | 0.61 | 0.71 | 0.74 | 0.75 | 0.96 | 1 |
| Specificity | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| PPV | 1 | 0.85 | 1 | 1 | 1 | 0.98 | 1 |
| NPV | – | 0.88 | 0.73 | 0.79 | 0.76 | 1 | 1 |
| Accuracy | 0.91 | 1 | 0.84 | 0.87 | – | 0.98 | 1 |
ycMed, clinical restaging mediastinoscopy after induction therapy without a staging mediastinoscopy; reMS, repeat mediastinoscopy; VATS, video-assisted thoracoscopic surgery; TEMLA, transcervical extended mediastinal lymphadenectomy; VAMLA, video-assisted mediastinoscopic lymphademectomy; PPV, positive predictive value; NPV, negative predictive value.
Focusing on the use of transcervical lymphadenectomies for restaging after neoadjuvant therapy, only TEMLA has been validated by Zielinski et al. (33). The restaging values of EBUS-TBNA and/or EUS-FNA in 88 patients were compared with those of TEMLA performed to 78 patients. There was a significant difference between EBUS-TBNA/EUS-FNA and TEMLA for sensitivity (0.64 and 1; P<0.01) and NPV (0.82 and 1; P<0.01) in favour of TEMLA. Our preliminary results with the use of VAMLA for restaging are in concordance with the results from the team of Poland, with the maximal restaging values reported to date. However, we would like to highlight some differences between these procedures. VAMLA is an endoscopic technique performed through a videomediastinoscope, and TEMLA is an open procedure assisted by a videomediastinoscope or a videothoracoscope. The mean number of nodes removed differs with 10 (SD: 6.1) LNs in our series versus 27.9 (range, 10–46) in TEMLA. Finally, the range of exploration is quite different, TEMLA can explore all mediastinal nodal stations from supraclavicular to para-oesophageal, whereas VAMLA explores the right and left paratracheal and subcarinal nodes. With all of these considerations, and based with our results, we think that the performance of VAMLA and TEMLA is equal except in the aortopulmonary windows. Para-aortic and subaortic nodes cannot be reached with VAMLA and for this reason we have performed ECM in most patients with left-side tumours. Despite the use of ECM, we have one patient with persistent N2 in the subaortic station that was considered false negative of the whole staging protocol. The other false negative of the protocol in our series was in the pulmonary ligament nodal station that neither VAMLA nor TEMLA can reach.
Regarding complications, there are no other series of VAMLA for restaging to compare with our study. Previously published reports of surgical restaging procedures such as mediastinoscopy, reMS and VATS describe few complications, ranging from 0 to 4% (28-31). TEMLA for restaging reports no mortality and morbidity rate of 6.4% (33). The complication rate of our series is slightly higher (9.7%) in comparison with other surgical procedures. However, of the 4 patients with left recurrent nerve palsy, only one had it permanently. Only one patient presented pneumonia after VAMLA that was treated with antibiotics with complete resolution.
Study limitations
The main limitation of this study is that it was performed in a single centre and by a group of thoracic surgeons with wide experience in the surgical staging (7,13) and restaging (31) of the mediastinum. Although this series represents the first results of VAMLA to restage cN2–3 NSCLC treated with neoadjuvant treatment, this technique is performed in other institutions for primary staging and we believe that our results can be easily generalized after a short learning curve.
Conclusions
These preliminary results with VAMLA to restage cN2–3 NSCLC treated with neoadjuvant therapy demonstrated a high accuracy and a high rate of persistent N2 that might have passed undetected if restaging had been performed with imaging techniques, only. Therefore, VAMLA should be included in restaging algorithms to select patients that would benefit from multidisciplinary approach that includes surgical resection after neoadjuvant therapy.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-841/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-841/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-841/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-841/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics and Clinical Research Committee of Hospital Universitari Mútua Terrassa (EO/1520 29th April 2010) and individual patient consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- Turna A, Demirkaya A, Ozkul S, et al. Video-assisted mediastinoscopic lymphadenectomy is associated with better survival than mediastinoscopy in patients with resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:774-80. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Transcervical Extended Mediastinal Lymphadenectomy (TEMLA) for staging of non-small-cell lung cancer (NSCLC). Pneumonol Alergol Pol 2011;79:196-206. [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-Assisted Mediastinoscopic Lymphadenectomy for Staging Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Call S, Reig-Oussedik N, Obiols C, et al. Video-assisted mediastinoscopic lymphadenectomy (VAMLA): Mature results for staging non-small cell lung cancer with normal mediastinum. J Thorac Cardiovasc Surg 2024;168:1364-74. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2012;41:1043-6. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Nadal E, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924-33. [Crossref] [PubMed]
- Provencio M, Nadal E, González-Larriba JL, et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:504-13. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Cascone T, Leung CH, Weissferdt A, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med 2023;29:593-604. [Crossref] [PubMed]
- Cascone T, Awad MM, Spicer JD, et al. Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med 2024;390:1756-69. [Crossref] [PubMed]
- Hines JB, Cameron RB, Esposito A, et al. Evaluation of Major Pathologic Response and Pathologic Complete Response as Surrogate End Points for Survival in Randomized Controlled Trials of Neoadjuvant Immune Checkpoint Blockade in Resectable in NSCLC. J Thorac Oncol 2024;19:1108-16. [Crossref] [PubMed]
- de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer: prediction of pathologic stage. J Thorac Oncol 2010;5:389-98. [Crossref] [PubMed]
- Jiang L, Huang W, Liu J, et al. Endosonography with lymph node sampling for restaging the mediastinum in lung cancer: A systematic review and pooled data analysis. J Thorac Cardiovasc Surg 2020;159:1099-1108.e5. [Crossref] [PubMed]
- Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy--a prospective study. Eur J Cardiothorac Surg 2010;37:1180-4. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Lardinois D, Schallberger A, Betticher D, et al. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg 2003;75:1102-6. [Crossref] [PubMed]
- Marra A, Hillejan L, Fechner S, et al. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008;135:843-9. [Crossref] [PubMed]
- De Waele M, Serra-Mitjans M, Hendriks J, et al. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg 2008;33:824-8. [Crossref] [PubMed]
- Call S, Rami-Porta R, Obiols C, et al. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011;39:1022-7. [Crossref] [PubMed]
- Jaklitsch MT, Gu L, Demmy T, et al. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg 2013;146:9-16. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]


