
Hyperthermic intrathoracic chemotherapy in overcoming tyrosine kinase inhibitor resistance in a patient with malignant pleural effusion: a case report
Highlight box
Key findings
• Hyperthermic intrathoracic chemotherapy (HITHOC) implemented in a patient facilitated a higher drug concentration in the pleural cavity and thus effectively eliminated pleural metastatic lesions and contributed to reduced tumor burden.
• HITHOC improves the sensitivity of tumor cells to third-generation epidermal growth factor receptor tyrosine kinase inhibitors.
What is known and what is new?
• Resistance to targeted therapy inevitably occurs in patients with cancer and remains a key clinical challenge.
• HITHOC can effectively increase the drug concentration and efficacy within the pleural cavity, thereby reducing tumor burden and improving sensitivity to targeted therapy.
What is the implication, and what should change now?
• Patients with pleural metastasis can benefit from HITHOC.
• HITHOC can reduce tumor burden, thereby improving sensitivity of tumor cells to targeted therapy.
Introduction
Background
Lung cancer has an incidence of 23% and an overall 5-year survival of only 19%, constituting a global health crisis, with nearly half of new cases being diagnosed at the advanced stages (1). Among Asian patients, over 50% of lung cancer cases carry epidermal growth factor receptor (EGFR) mutations, and thus targeted therapy, mainly that involving EGFR tyrosine kinase inhibitors (TKIs), is particularly critical (2). However, acquired resistance to EGFR-TKIs inevitably occurs in patients, but the mechanism related to its emergence remains unclear (3). Malignant pleural effusion is commonly found in patients who develop resistance to EGFR-TKIs (4). This centrally involves the pleural cavity, which is located between the chest wall and lung, is anatomically defined as the “third space” (5).
Study rationale
Due to lack of blood circulation in the pleural cavity, traditional drug administration methods, primarily oral or intravenous, often result in insufficient drug concentration within the pleural cavity. Therefore, traditional therapy, consisting of oral or intravenous medication along with pleural cavity drainage, does not yield a satisfactory outcome. Hyperthermic intrathoracic chemotherapy (HITHOC), first reported in a review in 1997, has been applied in the treatment of patients with pleural disseminated lesion and/or malignant pleural effusions for over a quarter of a century (6-8). Perfusion at above-body temperature levels and the administration of the chemotherapy drug directly to the pleural cavity at a high concentration (relative to intravenous administration) can effectively eliminate both solid tumors in the pleural cavity and malignant pleural effusions.
Overview
We report a case in which HITHOC was applied in a patient with malignant pleural effusion and resistance to third-generation TKIs, which resulted in a significant decrease of tumor burden. From a clinical perspective, we also discuss the possible factors contributing to tumors developing resistance to TKIs and suggest possible managing strategies. We believe that HITHOC complements the limitations of oral and intravenous drug administration, thereby enhancing the treatment efficacy of therapy against metastatic lesions within the pleural cavity. We present this article in accordance with the CARE reporting checklist available at (https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1252/rc).
Case presentation
A 50-year-old Asian male attended hospital on November 17, 2020, due to sustained blood-tainted sputum and dull pain both in the chest and back lasting for a month. Computed tomography (CT) and positron emission tomography-CT (PET-CT) scans revealed a potentially malignant nodule in the anterior segment of the left upper lung lobe (27 mm × 17 mm × 21 mm), along with pleural metastasis (Figure 1). Biopsy confirmed adenocarcinoma of the lung, and blood tests indicated an elevated carcinoembryonic antigen (CEA) level of 53.28 ng/mL. The tumor carried the EGFR p.L858R mutation, as confirmed by DNA next-generation sequencing (NGS) of plasma cell-free DNA (cfDNA). The patient was started on oral osimertinib therapy, which led to the resolution of his symptoms (Figure 1). In routine follow-up sessions, sustained shrinkage was observed. However, a follow-up PET-CT scan on May 1, 2023, showed tumor enlargement (13 mm × 12 mm × 17 mm). Subsequently, the patient underwent wedge resection on May 7, 2023, at which time nodular thickening of the pleura was surgically removed. Postoperative pathology suggested that the tumor (15 mm × 14 mm × 13 mm) was moderately differentiated and consisted of acinar adenocarcinoma (70%), papillary adenocarcinoma (25%), and micropapillary adenocarcinoma (5%). Lymph node metastasis was positive, and tumor cell infiltration into the pleura was surgically removed. There was no vessel carcinoma embolus or neural or pleural invasion. NGS performed on the tumor specimen also indicated an EGFR p.L858R mutation. Thus, osimertinib treatment was continued with routine follow-up (Figure 2). On March 1, 2024, the CEA level was measured at 19.3 ng/mL, which rose to 26.1 ng/mL on March 26, 2024, suggesting possible drug resistance. Osimertinib was then replaced by another third-generation TKI, almonertinib, on April 1, 2024. However, the blood CEA level was observed to increase from 28.71 ng/mL on April 9, 2024, to 29.98 ng/mL on April 18, 2024, and from 36.8 ng/mL on May 16, 2024, to 60.4 ng/mL on May 28, 2024. By June 12, 2024, the CEA level was 227.4 ng/mL. On June 13, 2024, another PET-CT scan was performed, which indicated disease progression, including the thickening of nodular lesions in the pleura and malignant pleural effusion. These results suggested resistance to third-generation TKIs. Rebiopsy was performed on the pleura and indicated adenocarcinoma infiltration in the connective tissue. Subsequent NGS confirmed the EGFR p.L858R mutation, EGFR amplification, and TP53 p.V157F mutation of the tumor.
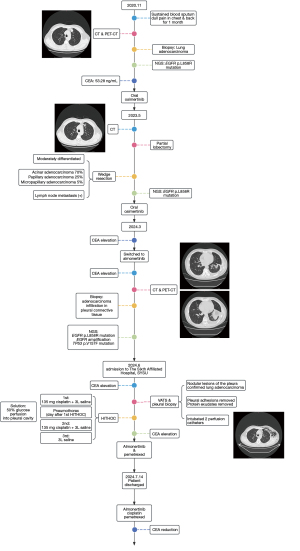
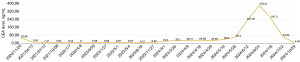
The patient was admitted to The Sixth Affiliated Hospital, Sun Yat-sen University on June 23, 2024. The CEA level was 376.47 ng/mL on June 24, 2024. A CT scan performed on June 25, 2024, indicated that the tumor was primarily localized in the left pleural cavity and that distal metastasis was absent. Video-assisted thoracic surgery (VATS) and pleural biopsy were performed on June 28, 2024, and intraoperative frozen-section pathological examination on the nodular lesions of the pleura confirmed the diagnosis of lung adenocarcinoma. During surgery, pleural adhesions were thoroughly released, and protein exudates were removed (Video 1). An upper perfusion catheter (fluid inflow) was placed in the second intercostal space along the midclavicular line, while a lower perfusion catheter (fluid outflow) was placed in the seventh intercostal space along the lateral chest wall (Figure S1). HITHOC was performed on days 1, 3, and 5 after the surgery; that is, on July 1, 3, and 5, 2024, respectively. The BR TRG-II hyperthermic perfusion intraperitoneal treatment system (Bright Medical Tech, Guangzhou, China) was applied for 60 minutes with a flow rate of 400 to 600 mL/min. The temperature was set at 46±0.1 ℃ during the perfusion, and 135 mg of cisplatin was added into 3 liters of saline and administered to the patient twice, while only 3 liters of saline were supplied the third time. The patient responded well to surgery and HITHOC, and the CEA level fell from 376.47 ng/mL on June 24, 2024, to 247.3 ng/mL on July 6, 2024, the day after the last HITHOC session. During HITHOC, the patient developed pneumothorax. We successfully resolved this by perfusing hyperosmolar 50% glucose solution to the patient’s pleural cavity. After HITOHC, oral almonertinib was continued, and intravenous pemetrexed was prescribed to the patient to further consolidate the effect of HITHOC on July 12, 2024. The patient was discharged on July 14, 2024. Further combination therapy included oral almonertinib, intravenous cisplatin, and pemetrexed (Figure 1). The CEA level continued to fall: on July 22, 2024, it was 64.52 ng/mL, and at the latest measurement on October 29, 2024, it was 4.65 ng/mL.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
In this case, we discovered that applying HITHOC to a patient harboring pleural metastatic lesions could significantly reduce tumor burden and improve sensitivity to third-generation TKIs.
Strengths and limitations
In this case report, we applied HITHOC to a patient harboring resistance to third-generation EGFR-TKIs and pleural oligometastatic lesion. We successfully reduced tumor burden and increased tumor sensitivity to third-generation TKIs. However, due to a relatively short follow-up, it remains unclear whether the patient can benefit from HITHOC and subsequent combination therapy. HITHOC requires specified medical instrumentation, limiting the accessibility of this therapy. Further clinical and mechanism research on HITHOC could further improve the clinical understanding of this therapy, including but not limited to the appropriate time for applying HITHOC and its efficacy.
Comparison with similar reports
Recent years have witnessed the emergence of numerous therapies for lung carcinoma. Among them, targeted therapy has been widely applied, as it can achieve long-term tumor regression with minimum side effects compared to other therapies such as chemotherapy. However, acquired resistance to targeted therapies inevitably emerges, and post-resistance treatments remain a challenge (3). Therefore, strategies for sustaining the sensitivity of the tumor to targeted therapies have considerable potential for improving the prognosis of patients. We believe that the following three factors may affect the emergence and development of resistance to targeted therapies: genetic alteration patterns, combinational medication, and tumor burden.
We speculate that complex genetic alteration patterns may contribute to the heterogenicity of tumors. In approaches in precision medicine, such as in targeted therapy, tumors with high levels of heterogenicity are more likely to develop resistance. The BENEFIT trial examined the correlation of genetic alterations and the efficacy of the first-generation TKI, gefitinib (9). The median progression-free survival (mPFS) for patients carrying the EGFR mutation alone was 13.2 months. In comparison, the mPFS for those carrying both the EGFR mutation and tumor-suppressor gene mutation (TP53, RB1, and PTEN) was 9.3 months. We also noticed that for patients carrying both the EGFR mutation and other driver gene mutations (MET, ERBB2, KRAS, RET, and ROS-1), their mPFS was only 4.7 months. We speculate that within tumor cells carrying multiple driver gene mutations, numerous proliferation signaling pathways can be activated simultaneously, and thus inhibiting the EGFR pathway alone is not sufficient for suppressing tumor growth and progression. Resistance to EGFR-TKIs within such cells develops in the same manner.
Targeted therapy can precisely eliminate tumor cells carrying certain driver gene mutations, thereby achieving both satisfactory overall effectiveness and a low level of side effects. However, as tumor subclones carrying sensitive genetic alternations gradually are eliminated by targeted therapy, the efficacy of such therapy decreases accordingly. When the tumor stops shrinking, the patient eventually arrives at so-called plateau stage. This phenomenon is commonly observed among patients receiving targeted therapy. Recent years have witnessed a series of research conducted on target therapy-based combinational therapy aimed at improving the effect of targeted therapy. Among these approaches, combining targeted therapy with chemotherapy is relatively mature. The FLAURA trial demonstrated that third-generation TKI osimertinib alone could achieve a progression-free survival (PFS) of 18.9 months among patients with advanced stage non-small cell lung cancer (NSCLC) (10). In the FLAURA2 trial, which examined the addition of chemotherapy, a PFS of 27.5 months was observed (11). As for the first-generation TKI, icotinib, the ICOMPARE trial reported that compared with an adjuvant targeted therapy for 1 year, extending this regimen to 2 years could achieve a better median disease-free survival (mDFS; 42.98 versus 32.89 months) (12). Moreover, the more recent ICTAN trial reported that the combination of oral icotinib with chemotherapy for 6 months yielded a better DFS and OS as compared to chemotherapy alone (13). We hypothesize that adjuvant chemotherapy sensitizes the tumor to icotinib, thereby allowing icotinib to effectively eliminate tumor in a shorter span of time, without negative distal effects.
We believe that compared with targeting genetic alteration patterns and applying combinational medications, relieving tumor burden contributes to sensitizing tumor to targeted therapy to a greater extent. A comparison analysis of the FLAURA trial and ADAURA trial strongly supports this opinion. In the FLAURA trial, after routine follow-up for 18 months, almost 50% of patients developed treatment resistance (median PFS 18.9 months) (10). In comparison, in the ADAURA trial, fewer than 10% of the patients developed resistance at the 18th month of regular follow-up (14). The key underlying reason for this may be the difference in tumor burden between the two trials. The tumor was removed in the ADAURA trial but remained in the FLAURA trial. A similar difference was observed for icotinib, which yielded a PFS of 11 months among patients with advanced-stage NSCLC receiving icotinib as first-line therapy; in contrast, NSCLC yielded 2 years of PFS among patients with early-stage NSCLC (15). Therefore, when there is a risk of resistance to targeted therapy, appropriate treatment to minimize tumor burden of the patient may be a critical factor influencing the sensitivity of tumor to targeted therapy, and therefore, the final outcome of the patient.
Interpretation of findings
The treatment process of this patient points to certain key areas that should be emphasized in patients resistant to targeted therapy. This includes not only pathological transformation, genetic typing, and patterns of progression but also the specific distribution of metastatic lesions. According to our understanding, metastatic lesions in lung cancer can be categorized into four major types: conventional metastasis, barrier metastasis, space metastasis, and barrier-plus-space metastasis. Conventional metastasis sites have a rich blood supply, enabling effective action of both intravenous and oral medications. However, treatment of intracranial metastases, which are challenging due to the blood-brain barrier, generally relies on small-molecule targeted drugs to ensure good effect. Meanwhile, for pleural metastasis, which located adjacent to the pleural cavity space, HITHOC can be used to improve treatment outcomes. Finally, leptomeningeal metastases, which involve the blood-brain barrier and the subarachnoid space, must be treated with a combination approach via high-dose TKIs and intrathecal chemotherapy to enhance therapeutic outcomes. As mentioned above, the pleural cavity is anatomically defined as the third space. Due to lack of blood circulation, the efficacy of drugs within this area is limited in traditional oral or intravenous drug administration methods. However, in HITHOC, through sustained high-concentration chemotherapy (e.g., cisplatin solution), “flushing” can be achieved, thereby effectively eliminating tumor cells. Notably, due to the isolation of the blood system, we can administer higher doses of chemotherapy via HITHOC as compared to traditional intravenous routes, without incurring greater risks to safety. An interesting phenomenon that was observed during treatment was the patient developing pneumothorax the first day after HITHOC and not on the day after surgery. We suspect that pleural metastatic lesions were eliminated by HITHOC, resulting in the integrity of the pleura being compromised and the subsequent development of pneumothorax.
Furthermore, NGS was performed after tumor progression. A sustained EGFR-TKI-sensitive genetic alteration was observed and thus indicated the continued efficacy of TKI therapy. Therefore, the combinational therapy of cisplatin, pemetrexed, and third-generation TKI almonertinib was prescribed to the patient by our medical team after HITHOC. Finally, after the patient was discharged, the tumor burden of the patient and CEA the level continued to decline.
In summary, applying HITHOC to patients with pleural oligometastasis can contribute to minimizing the tumor burden, thereby improving the sensitivity to targeted therapy and possibly reversing resistance.
Patient perspective
I am really grateful to the medical team at the Sixth-Affiliated Hospital of SYSU. They helped me regain sensitivity to third generation TKI. Now my quality of life was much better than before, when I was carrying those malignant pleural effusions. I had trouble even just breathing. Now I am on regular follow-ups by this medical team, and I am so glad to see that my CEA is maintained at low level. I am delighted to see that my experience of getting better may help people with similar dilemma.
Conclusions
For patients demonstrating resistance to targeted therapy and carrying pleural oligometastatic lesions, HITHOC can minimize tumor burden, thereby improving the sensitivity to targeted therapy and possibly restoring sensitivity to TKIs.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1252/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1252/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1252/coif). Y.Z.Z. reports funding from the Guangzhou Basic Research Project (No. 202201011326). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Liam CK, Pang YK, Poh ME. EGFR mutations in Asian patients with advanced lung adenocarcinoma. J Thorac Oncol 2014;9:e70-1. [Crossref] [PubMed]
- Zalaquett Z, Catherine Rita Hachem M, Kassis Y, et al. Acquired resistance mechanisms to osimertinib: The constant battle. Cancer Treat Rev 2023;116:102557. [Crossref] [PubMed]
- Kawamura T, Kenmotsu H, Kobayashi H, et al. Negative impact of malignant effusion on osimertinib treatment for non-small cell lung cancer harboring EGFR mutation. Invest New Drugs 2020;38:194-201. [Crossref] [PubMed]
- Charalampidis C, Youroukou A, Lazaridis G, et al. Pleura space anatomy. J Thorac Dis 2015;7:S27-32. [Crossref] [PubMed]
- Shirakusa T, Okabayashi K. Video-assisted thoracic surgery for lung cancer. Gan To Kagaku Ryoho 1997;24:520-4.
- Li H, Liu T, Sun Z, et al. New horizons in non-small-cell lung cancer patients with ipsilateral pleural dissemination (M1a): review of the literature. Ann Transl Med 2021;9:959. [Crossref] [PubMed]
- Migliore M, Nardini M. Does cytoreduction surgery and hyperthermic intrathoracic chemotherapy prolong survival in patients with N0-N1 nonsmall cell lung cancer and malignant pleural effusion? Eur Respir Rev 2019;28:190018. [Crossref] [PubMed]
- Wang Z, Cheng Y, An T, et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir Med 2018;6:681-90. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 2023;389:1935-48. [Crossref] [PubMed]
- Lv C, Wang R, Li S, et al. Randomized phase II adjuvant trial to compare two treatment durations of icotinib (2 years versus 1 year) for stage II-IIIA EGFR-positive lung adenocarcinoma patients (ICOMPARE study). ESMO Open 2023;8:101565. [Crossref] [PubMed]
- Wang SY, Long H, Li N, et al. Adjuvant icotinib of 12 months or 6 months versus observation following adjuvant chemotherapy for resected EGFR-mutated stage II–IIIA non-small-cell lung cancer (ICTAN, GASTO1002): A randomized phase 3 trial. J Clin Oncol 2024;42:abstr 8004.
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- He J, Su C, Liang W, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med 2021;9:1021-9. [Crossref] [PubMed]
(English Language Editor: J. Gray)


