
Efficacy and safety of first-line immunotherapy-based regimens for patients with extensive-stage small cell lung cancer: a systematic review and network meta-analysis
Highlight box
Key findings
• Our study demonstrated for the first time that adding anlotinib and benmelstobart to chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) compared with chemotherapy alone or chemotherapy plus immunotherapy, with an acceptable safety profile in patients with extensive-stage small cell lung cancer (ES-SCLC).
What is known and what is new?
• Immunotherapy plus chemotherapy is now the standard first-line treatment for ES-SCLC. ETER701 study has assessed the efficacy and safety of chemotherapy supplemented with anlotinib and benmelstobart (Anl/Ben/CT), yet there has been a gap in evidence-based medicine that definitively positions Anl/Ben/CT as the superior regimen due to the absence of comparative studies among diverse immunotherapy-based treatments.
• Our study introduces a network meta-analysis that synthesizes the most comprehensive randomized controlled trials (RCTs) to compare the efficacy and safety of 15 distinct regimens for ES-SCLC. It is proved that Anl/Ben/CT could be a new and clinically preferable first-line treatment option for ES-SCLC.
What is the implication, and what should change now?
• The findings underscore the importance of considering the Anl/Ben/CT regimen in clinical practice for the treatment of ES-SCLC patients. The demonstrated superiority of this regimen in enhancing survival outcomes without compromising safety underscores the need for a reevaluation of current treatment protocols.
Introduction
Small cell lung cancer (SCLC) is characterized as the most aggressive form of lung cancer, accounting for approximately 15% of all cases within the lung cancer spectrum (1). The epidemiologic data indicates that nearly two-thirds of patients with SCLC are diagnosed at an extensive stage (ES) (2). SCLC is characterized by a higher sensitivity to chemotherapy, thereby making chemotherapy with a platinum-based agent plus etoposide the standard first-line treatment for ES-SCLC over the past few decades (3,4). Nevertheless, the median survival of patients with ES-SCLC treated with standard first-line chemotherapy is only approximately 10 months, with a 5-year survival rate of only 7% (5).
Over recent years, immunotherapy, represented by targeting programmed cell death (ligand) 1 [PD-(L)1] and cytotoxic T lymphocyte-associated antigen-4, has made landmark progress in the area of cancer therapy (6,7). Multiple randomized controlled trials (RCTs) regarding combination regimens of immunotherapy plus chemotherapy versus chemotherapy alone have been widely explored in the first-line treatment of ES-SCLC (8,9). Phase III trial of IMpower133 showed that the addition of atezolizumab to chemotherapy resulted in significantly longer median overall survival (OS) (12.3 months vs. 10.3 months) than chemotherapy alone in ES-SCLC (3). Results from RCT of CASPIAN indicated that first-line durvalumab plus platinum-etoposide significantly prolonged OS compared to treatment with platinum-etoposide alone (10). Other representative RCTs include RATIONALE-312, ASTRUM-005, and CAPSTONE-1, etc. (11-13). Based on these RCT results, multiple combination regimens of distinct immune checkpoint inhibitors plus chemotherapy have been globally authorized for first-line treatment of ES-SCLC (14). Nevertheless, the combination of immunotherapy and chemotherapy treatment also inevitably yielded resistance, and improving long-term survival remains an unmet need. The result of the ETER701 study from the 2023 World Conference on Lung Cancer (WCLC), a phase III RCT comparing the efficacy and safety of the regimen with adding anlotinib to benmelstobart & chemotherapy (Anl/Ben/CT) versus chemotherapy alone, demonstrated that the four-agent regimen was dramatically superior to chemotherapy alone in terms of survival prolongation (15). It is noteworthy that the Anl/Ben/CT regimen demonstrates the most significant prolongation of survival compared to other contemporary first-line treatment modalities (15,16). The above trials consistently indicate that immunotherapy-based combination treatments have superior anti-tumor effects compared to chemotherapy alone, with a higher but acceptable incidence of adverse events Despite the promising results, a direct head-to-head comparison between the Anl/Ben/CT regimen and immunotherapy plus chemotherapy treatments remains elusive. This lack of direct evidence makes it difficult to conclusively determine if Anl/Ben/CT offers a distinct advantage over the immunotherapy combination chemotherapy approaches. Consequently, further research is warranted to clarify the relative efficacy of these treatment strategies.
In this paper, a network meta-analysis (NMA) incorporating the most comprehensive RCTs was conducted to evaluate the efficacy and safety of 15 varying regimens in ES-SCLC, with the aim of identifying the optimal regimen to assist in clinical decision-making. We present this article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-636/rc).
Methods
Search strategy and selection criteria
The review protocol was prospectively registered in PROSPERO (CRD42023481850). We systematically searched PubMed, Embase, Cochrane Library, and major international conferences to retrieve relevant RCTs published up to 28 December 2023. The detailed search strategies are described in Table S1. The inclusion criteria and exclusion criteria were as follows:
The inclusion criteria:
- Studies were RCTs in phase II or phase III.
- Eligible patients were newly diagnosed with treatment-naive histologically or cytologically documented ES-SCLC.
- RCTs that used immunotherapy-based combination treatment as first-line treatment settings.
- Any of the following outcomes: progression-free survival (PFS), OS, objective response rate (ORR), and grade 3 or higher adverse events, were available.
The exclusion criteria:
- Trials in which the treatment was administered as adjuvant or neoadjuvant therapy.
- RCTs that were based on overlapping patients.
Data extraction and quality assessment
Data extraction and quality assessment were independently conducted by two investigators (W.G.Z. and X.Y.Z.). The main information extracted from the original research included study ID, therapy regimens, and outcomes [e.g., PFS, OS, ORR, and grade 3 or higher adverse events (≥3 AEs)]. In this NMA, the Cochrane Risk of Bias Tool was utilized to assess the risk of bias in individual studies in Review Manager 5.3 software. The assessment included seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Sensitivity analysis
Sensitivity analysis was performed by excluding phase II RCTs.
Statistical analysis
The NMA was performed using a Markov Chain Monte Carlo simulation technique within the GEMTC and the JAGS package in R software (17). There were 150,000 sample iterations generated with 100,000 burn-ins and a thinning interval of 1 for PFS and OS. The thinning interval was increased to 10 to minimize auto-correlation for ORR and ≥3 AEs. Fixed-effects consistency model was used in this NMA to guarantee the model’s robustness. Deviance information criteria (DIC) were calculated to compare and evaluate the fixed and random effect models (18). The convergence adequacy (reaching a stable equilibrium distribution) was tested by visually inspecting the trace plots and estimating the values of the Brooks–Gelman–Rubin statistic (19). The ranking of each regimen was compared based on the surface under the cumulative ranking curve (SUCRA) (20). In this study, it was considered a statistical significance if the 95% confidence interval did not cross 1. All statistical analyses were conducted by R software (version 4.1.3) and Stata software (version 16.0).
Results
Clinical traits
As shown in Figure 1, the PubMed, Embase, Cochrane Library, and major international conferences were systematically and comprehensively retrieved. After a rigorous screening with inclusion and exclusion criteria, a final 12 RCTs were included in this NMA (11-13,15,21-28). The regimens included chemotherapy (CT), Anl/Ben/CT, serplulimab plus chemotherapy (Ser/CT), tislelizumab plus CT (Tis/CT), atezolizumab plus CT (Ate/CT), nivolumab plus CT (Niv/CT), adebrelimab plus CT (Ade/CT), toripalimab plus CT (Tor/CT), durvalumab plus CT (Dur/CT), pembrolizumab plus CT (Pem/CT), Ipi plus CT (Ipi/CT), concurrent Ipi plus CT (Con-Ipi/CT), phased Ipi plus CT (Pha-Ipi/CT), durvalumab plus tremelimumab plus CT (Dur/Tre/CT), and Niv plus Ipi plus CT (Niv/Ipi/CT). The characteristics of enrolled RCTs are described in Table 1. The risk of bias evaluation is shown in Figure S1.
Table 1
| Trial | Year | Phase | Clinical trial number | Sample size | Median age (years) | Sex | Treatment strategy | |
|---|---|---|---|---|---|---|---|---|
| Female | Male | |||||||
| RATIONALE-312 | 2023 | III | NCT0400571 | |||||
| Intervention arm | 227 | 63 | 41 | 186 | Tis 200 mg + Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C)-Tis 200 mg/3W maintenance | |||
| Control arm | 230 | 62 | 44 | 186 | Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C) | |||
| EXTENTORCH | 2023 | III | NCT04012606 | |||||
| Intervention arm | 223 | 62 | 40 | 183 | Tor 240 mg + Eto + Cis/Car/3W (4-6C) + Tor 240 mg maintenance | |||
| Control arm | 219 | 63 | 36 | 183 | Eto + Cis/Car/3W (4-6C) | |||
| ETER701 | 2023 | III | NA | |||||
| Intervention arm | 246 | 62 | 37 | 209 | Ben + Anl + Eto + Cis/Car/3W (4C)-Ben + Anl maintenance | |||
| Control arm | 247 | 63 | 40 | 207 | Eto + Cis/Car/3W (4C) | |||
| CAPSTONE-1 | 2022 | III | NCT03711305 | |||||
| Intervention arm | 230 | 62 | 46 | 184 | Ade 20 mg/kg + Car (AUC5) + Eto (100 mg/m²) (4-6C)-Ade 20 mg/kg maintenance | |||
| Control arm | 232 | 62 | 44 | 188 | Car (AUC5) + Eto 100 mg/m2 (4-6C) | |||
| ASTRUM-005 | 2022 | III | NCT04063163 | |||||
| Intervention arm | 389 | 63 | 72 | 317 | Ser 4.5 mg/kg + Car (AUC5) + Eto 100 mg/m2/3W (4C)-Ser 4.5 mg/kg maintenance | |||
| Control arm | 196 | 62 | 32 | 164 | Car (AUC5) + Eto 100 mg/m2/3W (4C) | |||
| CheckMate-451 | 2021 | III | NCT02538666 | |||||
| Arm 1 | 279 | 64 | 99 | 180 | CT (≤4C)-Niv 1 mg/kg + Ipi 3 mg/kg/3W-Niv 240 mg/2W maintenance | |||
| Arm 2 | 280 | 65 | 103 | 177 | CT (≤4C)-Niv 240 mg/2W maintenance | |||
| Arm 3 | 275 | 64 | 100 | 175 | CT (≤4C) | |||
| KEYNOTE-604 | 2020 | III | NCT03066778 | |||||
| Intervention arm | 228 | 64 | 76 | 152 | Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C)-Pem 200 mg maintenance | |||
| Control arm | 225 | 65 | 83 | 142 | Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C) | |||
| ECOG-ACRIN EA5161 | 2020 | Ⅱ | NCT03382561 | |||||
| Intervention arm | 80 | NA | NA | Niv 360 mg + Eto + Cis/Car/3W (4C)-Niv 240 mg/2W | ||||
| Control arm | 80 | NA | NA | Eto + Cis/Car/3W (4C) | ||||
| CASPIAN | 2019 | III | NCT03043872 | |||||
| Arm 1 | 268 | 63 | 66 | 202 | Dur 1,500 mg + Tre 75 mg + Car (AUC5-6)/Cis 75-80 mg/m2 + Eto 80–100 mg/m2/3W (4C)-Dur 1,500 mg maintenance | |||
| Arm 2 | 268 | 62 | 78 | 190 | Dur 1,500 mg + Car (AUC5-6)/Cis 75–80 mg/m2 + Eto 80–100 mg/m2/3W (4C)-Dur 1,500 mg maintenance | |||
| Arm 3 | 269 | 63 | 85 | 184 | Car (AUC5-6)/Cis 75–80 mg/m2 + Eto 80–100 mg/m2/3W (4C) | |||
| Impower133 | 2018 | III | NCT02763579 | |||||
| Intervention arm | 201 | 64 | 72 | 129 | Ate 1,200 mg + Car (AUC5) + Eto 100 mg/m2/3W (4C)-Ate 1,200 mg maintenance | |||
| Control arm | 202 | 64 | 70 | 132 | Car (AUC5) + Eto 100 mg/m2/3W (4C) | |||
| CA184-156 | 2016 | III | NCT01450761 | |||||
| Intervention arm | 478 | 62 | 161 | 317 | Ipi 10 mg/kg + Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C)-Ipi 10 mg/kg/12W maintenance | |||
| Control arm | 476 | 63 | 150 | 326 | Car (AUC5)/Cis 75 mg/m2 + Eto 100 mg/m2/3W (4C) | |||
| CA184-041 | 2013 | Ⅱ | NCT00527735 | |||||
| Arm 1 | 43 | 57 | 10 | 33 | Concurrent-Ipi 10 mg/kg + Pac 175 mg/m2 + Car (AUC6) (4-6C) | |||
| Arm 2 | 42 | 59 | 10 | 32 | Phased-Ipi 10 mg/kg + Pac 175 mg/m2 + Car (AUC6) (4-6C) | |||
| Arm 3 | 45 | 58 | 12 | 33 | Pac 175 mg/m2 + Car (AUC6) (4-6C) | |||
Ade, adebrelimab; Anl, anlotinib; Ate, atezolizumab; Ben, benmelstobart; Car, carboplatin; Cis, cisplatin; CT, chemotherapy; Dur, durvalumab; Eto, etoposide; Ipi, ipilimumab; NA, not available; Niv, nivolumab; Pac, paclitaxel; Pem, pembrolizumab; Ser, serplulimab; Tis, tislelizumab; Tor, toripalimab; Tre, tremelimumab.
NMA
PFS and OS
As depicted in Figure 2A, the network plots of PFS and OS were shown. All 12 RCTs included were available both in PFS and OS analyses involving 6,178 ES-SCLC individuals.
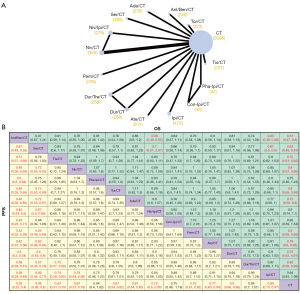
Regarding PFS, the NMA result showed that most immunotherapy-based combination regimens significantly reduced the risk of cancer progression versus chemotherapy alone [Anl/Ben/CT, Ser/CT, Tis/CT, Niv/CT, Tor/CT, Ade/CT, Niv/Ipi/CT, Pem/CT, Ate/CT, Dur/CT, Ipi/CT vs. CT: all hazard ratio (HRs) <1 and 95% CIs did not cross 1], with the exception of Pha-Ipi/CT (HR 0.64, 95% CI: 0.40–1.02), Con-Ipi/CT (HR 0.75, 95% CI: 0.48–1.18) and Dur/Tre/CT (HR 0.84, 95% CI: 0.70–1.01). Among immunotherapy-based regimens, significant prolongation of PFS was observed for ES-SCLC with Anl/Ben/CT compared to the others (all HR <1 and 95% CIs did not cross 1) (Figure 2B). Besides, Ser/CT could be a superior regimen following Anl/Ben/CT (Ser/CT vs. the other regimens except for Anl/Ben/CT: all HRs <1 and most 95% CIs did not cross 1). A pronounced PFS prolongation was also observed in both Tis/CT (HR 0.74, 95% CI: 0.58–0.95) and Niv/CT (HR 0.78, 95% CI: 0.64–0.96) when compared with Ipi/CT. Notably, Tis/CT was superior to the three-drug regimen of Dur/Tre/CT (HR 0.75, 95% CI: 0.57–0.99).
Regarding OS, similar results to PFS were obtained, that is, most immunotherapy-based regimens were superior in comparison with CT alone (all HR <1 and most 95% CI did not cross 1) (Figure 2B). Among immunotherapy-based regimens, Anl/Ben/CT exhibited superiority over the others in extending OS (all HR <1), especially relative to Niv/Ipi/CT (HR 0.68, 95% CI: 0.49–0.95) and Ipi/CT (HR 0.65, 95% CI: 0.48–0.88) (Figure 2B). Similar to its efficacy in PFS prolongation, Ser/CT was inferior only to Anl/Ben/CT in prolonging OS among all immunotherapy-based regimens. With specific regard, Ser/CT was demonstrated to be significantly superior to Niv/Ipi/CT (HR 0.7, 95% CI: 0.51–0.97) and Ipi/CT (HR 0.67, 95% CI: 0.50–0.90). Additionally, Ade/CT was observed to be more favorable than Ipi/CT (HR 0.77, 95% CI: 0.59–1.0). (Figure 2B).
ORR
As shown in Figure 3A, ORR was available in 11 RCTs, involving 14 regimens and 5,691 ES-SCLC patients. When compared with CT, most immunotherapy-based treatments can provide patients with a higher ORR [Anl/Ben/CT, Ser/CT, Niv/CT, Pem/CT, Dur/CT vs. CT: all odds ratio (ORs) >1 and 95% CIs did not cross 1] (Figure 3B). Among all immunotherapy-based regimens, Anl/Ben/CT exhibited higher ORR versus the other regimens, e.g., in comparison with Ade/CT (OR 1.76, 95% CI: 1–3.14), Con-Ipi/CT (OR 2.61, 95% CI: 1.02–6.76), Ate/CT (OR 2.6, 95% CI: 1.46–4.66), Dur/Tre/CT (OR 2.16, 95% CI: 1.26–3.71), and Ipi/CT (OR 2.18, 95% CI: 1.33–3.59) (Figure 3B). Significantly higher ORR was observed for Ser/CT when compared with Ate/CT (OR 2.04, 95% CI: 1.15–3.59), and Ipi/CT (OR 1.71, 95% CI: 1.06–2.74). Additionally, Niv/CT showed an increase in ORR in ES-SCLC versus Con-Ipi/CT (OR 2.62, 95% CI: 1–6.93), Ate/CT (OR 2.6, 95% CI: 1.41–4.84), Dur/Tre/CT (OR 2.16, 95% CI: 1.21–3.86), and Ipi/CT (OR 2.18, 95% CI: 1.29–3.74) (Figure 3B).
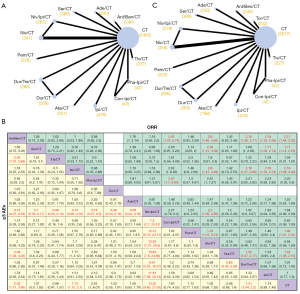
≥3 AEs
All the 12 RCTs included were available for the NMA of ≥3 AEs (Figure 3C). Compared with CT alone, ≥3 AEs were higher for 5 immunotherapy-based regimens, including Anl/Ben/CT (OR 2.03, 95% CI: 1.11–3.86), Niv/CT (OR 1.73, 95% CI: 1.12–2.7), Pha-Ipi/CT (OR 2.43, 95% CI: 1–6.07), Niv/Ipi/CT (OR 13.52, 95% CI: 8.66–21.63), and Dur/Tre/CT (OR 1.51, 95% CI: 1.04–2.2) (Figure 3B). Of importance, Niv/Ipi/CT resulted in significantly higher ≥3 AEs than other immunotherapy-based regimens (all OR >1 and 95% CIs did not cross 1) (Figure 3B). Besides, regarding Anl/Ben/CT, it did not significantly increase the incidence of ≥3 AEs compared with other immunotherapy-based regimens except Tis/CT (OR 2.36, 95% CI: 1.01–5.69) and Dur/CT (OR 2.08, 95% CI: 1.03–4.33) (Figure 3B). For Ser/CT, no significant differences in ≥3 AEs were identified, either compared with the other immunotherapy-based regimens or with CT (Figure 3B).
Rank probabilities
As shown in Figure 4A and Figure S2, it was showed the Bayesian rank probabilities of all comparable therapies in terms of PFS, OS, ORR, and ≥3 AEs. Significantly, the probability of Anl/Ben/CT ranking first was the highest among all regimens, regardless of PFS (98.9%), OS (41.4%), or ORR (23.5%) (Figure 4A). In contrast, CT had the highest probability of ranking last in both PFS (82.1%) and OS (32.1%) (Figure S3). With respect to Ser/CT, the probability of PFS (78.6%) and OS (27.7%) being ranked second is the highest (Figure 4A). Regarding ≥3 AEs, ranking first and second with the highest probability were Niv/Ipi/CT (99.9%) and Pha-Ipi/CT (47.3%), respectively.
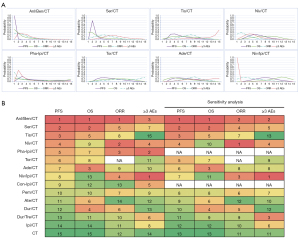
Furthermore, the ranking of each regimen was evaluated based on the SUCRA. The results are generally consistent with the NMAs calculated by HRs. Anl/Ben/CT ranked first in both PFS, OS, and ORR among 15 regimens (Figure 4B). Ser/CT ranked second in PFS and OS, and Niv/CT ranked second in ORR. Regarding safety, ranked first and second for ≥3 AEs were Niv/Ipi/CT and Pha-Ipi/CT, respectively.
Sensitivity analyses
The sensitivity analysis was performed by excluding Phase II RCTs in order to examine the reliability and robustness of the NMA. The results of the sensitivity analysis were nearly identical to the originals (Figure 4B).
Consistency assessment
It is demonstrated that the fit of the consistency model was similar to that of the inconsistency model (Table S2). Figures S3-S6 show the trace plot and the Brooks-Gelman-Rubin diagnostic plot, indicating the excellent stability of the model convergence.
Discussion
To the best of our knowledge, our study is currently the NMA that incorporates the most comprehensive RCT involving immunotherapy for ES-SCLC. This NMA demonstrated for the first time that Anl/Ben/CT is the most efficacious regimen for extending survival in ES-SCLC, highlighting its potential as a preferred first-line treatment option for this patient population.
Currently, the standard first line of care for ES-SCLC is chemotherapy combined with immunotherapy (14). Adding immunotherapy to chemotherapy in the first-line treatment of ES-SCLC is approved by the Food and Drug Administration based on multiple RCTs. IMpower133, a multinational, phase III RCT, has evaluated the synergistic effect of the addition of atezolizumab to chemotherapy in the first-line treatment of ES-SCLC (29). It was shown that the median OS was 12.3 months and 10.3 months in the atezolizumab group and the placebo group, respectively (29). The primary objective of the CAPSTONE-1 study was to evaluate the effectiveness and safety of incorporating adebrelimab into conventional chemotherapy regimens as first-line treatment for ES-SCLC (13). As expected, a significantly improved OS (15.3 months vs. 12.8 months) was obtained, and the safety profile was acceptable (13). ASTRUM-005 is a phase III RCT evaluating the efficacy of the programmed cell death protein 1 (PD-1) inhibitor serplulimab plus chemotherapy compared with placebo plus chemotherapy for ES-SCLC in the first-line setting (12). The median OS was significantly longer in the serplulimab group (15.4 months) than in the placebo group (10.9 months) (12). Out of all regimens of immunotherapy combined with chemotherapy, serplulimab plus chemotherapy yielded the longest median OS (12). It remains uncertain whether the combination of serplulimab and chemotherapy offers a superior therapeutic outcome compared to alternative regimens, given the absence of RCTs directly assessing the comparative efficacy of these treatment approaches. To identify the optimal regimen, Zhang et al. compared the efficacy of varying combination strategies by NMA, demonstrating that Ser/CT was optimal (30). Consistently, our NMA demonstrated that Ser/CT was optimal in prolonging both PFS and OS with acceptable ≥3 AEs among immunotherapy-combination chemotherapy regimens.
In fact, the use of immunotherapy in conjunction with chemotherapy, despite its benefits, can result in the development of resistance, which is a persistent challenge for achieving sustained long-term survival. Enhancing long-term survival rates in this context continues to be an area where further advancements are required (6). Differing from other tumors, the tumor microenvironment (TME) of SCLC is mostly of a cold TME-enriched phenotype (a.k.a. the immunosuppressive microenvironment), which is featured by more Tregs, more exhausted CD8+ T-cells, and fewer activated CD8+ T-cells, and markedly attenuates the efficacy of immunotherapy (31). Angiogenesis and vascularization play essential roles in the development of a cold TME (32). Tumor vessel normalization and TME reprogramming can transform cold tumors into hot tumors, thereby potentially exerting synergistic anti-tumor effects with immunotherapy (32). Vascular abnormalities stem from the increased levels of proangiogenic factors (VEGFR, PDGFR, and FGFR) and proangiogenic factors (33). The antiangiogenic drug anlotinib targets angiogenesis pathways such as VEGFR, FGFR, and PDGFR (34). Expectedly, anlotinib can inhibit tumor angiogenesis and normalize blood vessels, thereby reprogramming the immunosuppressive TME into an immunostimulatory TME characterized by increased immune cell extravasation and enhanced antigen presentation function (33). Moreover, clinical trials have substantiated the synergistic impact of co-administering anlotinib with immunotherapy in the treatment of non-SCLC (35). The antitumor effects of anlotinib have been deemed significant, and its safety profile has been found to be acceptable in the context of SCLC (36). Based on the sufficient evidence above, ETER701 has explored the efficacy and safety of adding anlotinib to the combination of immunotherapy with chemotherapy, with the result showing a significant prolongation of OS compared to its control chemotherapy alone (19.3 vs. 11.9 months) (15). Up to now, Anl/Ben/CT is the regimen that yields the historically longest OS for ES-SCLC (15). No RCT has yet compared Anl/Ben/CT with other combination regimens, particularly with Ser/CT. Therefore, no evidence-based medicine is available indicating that Anl/Ben/CT is superior to other regimens despite its median OS being the longest. In this study, we conducted the NMA by enrolling all immunotherapy-based RCTs in first-line therapy, and the results showed that Anl/Ben/CT was optimal concerning PFS, OS, and ORR. Regarding safety, notwithstanding the third ranking for ≥3 AEs, HR-based NMA results showed no significant difference in ≥3 AEs for Anl/Ben/CT compared with other immunotherapy-based regimens, except for Tis/CT and Dur/CT. Collectively, our study for the first time confirmed that Anl/Ben/CT is historically the most efficacious regimen with tolerable and manageable safety profile for ES-SCLC, and provided evidence for its use as a new preferred option in the first-line setting.
Several limitations must be taken into account in this study. Firstly, some RCTs incorporated in this research were sourced from conference reports, notably the ETER701 study presented at the 2023 WCLC and 2024 European Lung Cancer Congress, wherein subgroup analysis results were not accessible, thereby precluding the execution of subgroup analysis in the NMA. Therefore, it is still uncertain whether the efficacy of each regimen across the prespecified subgroups is consistent with the overall analysis. Secondly, the chemotherapy regimens among the included RCTs were not exactly identical, and we merged multiple chemotherapy regimens into one regimen to facilitate NMA in this study, potentially causing bias. Thirdly, our study confirmed that anlotinib can significantly enhance the antitumor effect in the regimen of PD-L1 inhibitor benmelstobart plus chemotherapy, whereas it requires corresponding RCTs to investigate whether anlotinib can also enhance the efficacy of regimens of other PD-(L)1 inhibitor plus chemotherapy. In addition, our study did not include non-English language research, which may introduce a potential bias in the analysis.
Conclusions
Adding anlotinib to the regimen of benmelstobart plus chemotherapy significantly improved PFS and OS compared with chemotherapy alone or chemotherapy plus immunotherapy, with an acceptable safety profile in patients with ES-SCLC. In conclusion, Anl/Ben/CT could be the new and clinically preferable first-line treatment option for ES-SCLC.
Acknowledgments
The study was submitted in abstract form to the World Lung Cancer Congress (EP.13B.01).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-636/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-636/prf
Funding: This work was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-636/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oronsky B, Reid TR, Oronsky A, et al. What's New in SCLC? A Review. Neoplasia 2017;19:842-7. [Crossref] [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol 2009;27:4787-92. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol 2023;9:419-29. [Crossref] [PubMed]
- Cai Z, Gu X, Xie J, et al. Safety and efficacy of thoracic radiotherapy combined with chemo-immunotherapy in patients with extensive-stage small cell lung cancer: a multicenter retrospective analysis. Transl Lung Cancer Res 2023;12:1987-2000. [Crossref] [PubMed]
- Dawkins JBN, Webster RM. The small-cell lung cancer drug market. Nat Rev Drug Discov 2020;19:507-8. [Crossref] [PubMed]
- Yang G, Sun H, Sun N, et al. Efficacy and safety comparison of PD-1 inhibitors vs. PD-L1 inhibitors in extensive-stage small-cell lung cancer: a retrospective comparative cohort study. J Thorac Dis 2022;14:4925-37. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Cheng Y, Fan Y, Zhao Y, et al. OA01.06 First-Line Chemotherapy With or Without Tislelizumab for Extensive-Stage Small Cell Lung Cancer: RATIONALE-312 Phase 3 Study. J Thorac Oncol 2023;18:S46.
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs. Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin 2023;73:620-52. [Crossref] [PubMed]
- Cheng Y, Yang R, Chen J, et al. OA01.03 Benmelstobart with Anlotinib plus Chemotherapy as First-line Therapy for ES-SCLC: A Randomized, Double-blind, Phase III Trial. J Thorac Oncol 2023;18:S44.
- Cheng Y, Chen J, Zhang W, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med 2024;30:2967-76. [Crossref] [PubMed]
- Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One 2014;9:e115065. [Crossref] [PubMed]
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. [Crossref] [PubMed]
- Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat 1998;7:434-55.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol 2020;38:9000.
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369-79. [Crossref] [PubMed]
- Owonikoko TK, Park K, Govindan R, et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J Clin Oncol 2021;39:1349-59. [Crossref] [PubMed]
- Cheng Y, Liu Y, Zhang W, et al. LBA93 EXTENTORCH: A randomized, phase III trial of toripalimab versus placebo, in combination with chemotherapy as a first-line therapy for patients with extensive stage small cell lung cancer (ES-SCLC). Ann Oncol 2023;34:S1334.
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Zhang T, Li W, Diwu D, et al. Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis. Front Immunol 2023;14:1197044. [Crossref] [PubMed]
- Liu Q, Zhang J, Guo C, et al. Proteogenomic characterization of small cell lung cancer identifies biological insights and subtype-specific therapeutic strategies. Cell 2024;187:184-203.e28. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Su Y, Luo B, Lu Y, et al. Anlotinib Induces a T Cell-Inflamed Tumor Microenvironment by Facilitating Vessel Normalization and Enhances the Efficacy of PD-1 Checkpoint Blockade in Neuroblastoma. Clin Cancer Res 2022;28:793-809. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Yu L, Hu Y, Xu J, et al. Multi-target angiogenesis inhibitor combined with PD-1 inhibitors may benefit advanced non-small cell lung cancer patients in late line after failure of EGFR-TKI therapy. Int J Cancer 2023;153:635-43. [Crossref] [PubMed]
- Yu L, Xu J, Qiao R, et al. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer Med 2023;12:5372-83. [Crossref] [PubMed]



