
Intraoperative longitude-latitude-depth three-dimensional localization of pulmonary nodules
Highlight box
Key findings
• The longitude-latitude-depth (LLD) method achieved a higher accuracy (96.59%) in localizing small pulmonary nodules compared to computed tomography (CT)-guided hook-wire localization. It was associated with significantly reduced localization time, postoperative complications, hospital stay, and cost. The LLD method is noninvasive, time-saving, and safer during surgery.
What is known and what is new?
• Preoperative CT-guided localization methods are widely used to localize pulmonary nodules, but they are invasive and involve risks such as pneumothorax, hemoptysis, and prolonged recovery.
• This novel LLD method uses 3D coordinates based on natural anatomical reference lines to localize pulmonary nodules with greater accuracy and fewer complications, providing a noninvasive, intraoperative alternative to CT-guided localization.
What is the implication, and what should change now?
• The LLD method offers a safer and more efficient option for localizing pulmonary nodules in surgery. Adoption of this method could reduce complications, shorten hospital stays, and lower healthcare costs and thus could become the new standard in pulmonary nodule localization during sublobar resections.
Introduction
Lung cancer is the leading cause of cancer death worldwide (1,2). With the widespread application of computed tomography (CT) screening, the detection of early small pulmonary nodules and lung cancer have dramatically increased (3). Lobectomy was previously recommended as the standard surgical procedure for stage IA (≤3 cm) lung cancer (4). However, two multicenter, randomized phase II clinical trials in Japan (JCOG0802) and North America have reported that sublobar resection, classified as wedge resection and segmentectomy, as being comparable or even superior to lobectomy in terms of survival in patients with stage IA (tumor diameter ≤2 cm) tumor (5,6). During sublobar resection via video-assisted thoracoscopic surgery (VATS), especially wedge resection, the most difficult procedures involve identifying pulmonary nodules and subsequently providing an adequate surgical margin. Although pulmonary nodules with dominant solid components or large diameter (≥2 cm) can be palpated easily and nodules with pleural indentation sign can be observed during surgery, localizing smaller nodules with dominant ground glass opacity (GGO) remains challenging. If these small pulmonary nodules cannot be accurately determined, extended resection or thoracotomy need to be performed. The widely used preoperative nodule localization methods include CT-guided hook-wire, microcoil, and electromagnetic navigation bronchoscopy localization. However, these methods are invasive and can lead to complications, such as pneumothorax (7).
Preoperative CT three-dimensional (3D) reconstruction is a simple noninvasive method for localizing pulmonary nodules. However, it is based on inflated lungs, but a nodule location may differ from that in a collapsed lung during surgery. Therefore, 3D reconstruction is inaccurate in nodule localization during surgery, especially in wedge resection (8). There are also a few other noninvasive intraoperative methods of nodule localization that are performed according to several anatomical lines during natural lung collapse in VATS; these include clock dial integrated positioning, the theodolitic method, or the combination of CT 3D reconstruction and the natural line of the deflated lung (9-11). However, existing intraoperative localization methods typically rely on a limited number of natural anatomical lines and two-dimensional parameters, which can may not fully account for the complex three-dimensional structure of the lung, leading to imprecision in nodule localization. In this paper, we describe a novel intraoperative nodule localization method, the longitude-latitude-depth (LLD) 3D localization method (or Qilu localization method), which integrates multiple natural longitudinal and horizontal lines and depth (distance to pleura) during lung deflation. By considering both the vertical and horizontal anatomical planes of the lung, as well as the depth of the nodule, the LLD method provides a more comprehensive 3D approach to localization. Furthermore, the LLD method leverages the natural deflation of the lung during surgery, making it a noninvasive and practical approach that avoids the complications typically associated with invasive localization techniques. With its ability to better account for the lung’s natural anatomical structures and dynamic deflation during surgery, the LLD method enhances the surgeon’s ability to identify and remove small, difficult-to-localize nodules. This, in turn, can reduce the need for extended resections or thoracotomy, leading to faster recovery times, lower hospitalization costs, and improved patient outcomes. In this study, we retrospectively compared our intraoperative LLD localization method to the preoperative CT-guided hook-wire localization method. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1170/rc).
Methods
Selection of patients
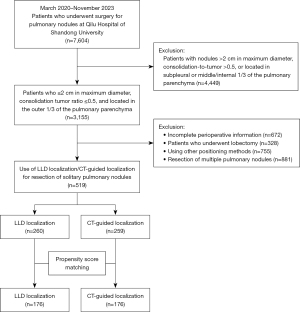
Patients who received LLD localization or CT-guided hook-wire localization followed by VATS at the Qilu Hospital of Shandong University between March 2020 and November 2023) were retrospectively included. This study was conducted according to the tenets of the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of the Qilu Hospital of Shandong University (No. KYLL-202008-023). All patients provided informed consent for the use of the clinical data. Patients who had resection of a solitary peripheral pulmonary nodule with a maximum diameter ≤2 cm, a consolidation tumor ratio ≤0.5, and location in the outer one-third of the pulmonary parenchyma were included. Participants who had resection of multiple pulmonary nodules, were undergoing lobectomy, had subpleural or middle/internal one-third nodules, or had undergone other localization methods were not included. The detailed process of participant inclusion is detailed in Figure 1.
Perioperative information
Patient data, sourced from the Department of Thoracic Surgery at Qilu Hospital of Shandong University, encompassed various parameters: age, sex, smoking history, body mass index (BMI), American Society of Anesthesiologists (ASA) score, operative method (segmentectomy or wedge resection), tumor attributes (location, diameter, CT values), minimum tumor distance from lung surface, localization duration, needle-carrying time, estimated blood loss during the procedure, day of removal of the chest tube, postoperative Numerical Rating Scale (NRS) pain score, complications following the localization, postoperative length of stay (POS), overall hospitalization cost, and pathological data. Localization complications included pain (NRS >4), pneumothorax, hemothorax, or hemoptysis. NRS pain scores were evaluated by nurses 24 and 48 hours postsurgery. Arterial blood samples were used for monitoring postoperative oxygen and carbon dioxide levels, with the oxygenation index calculated from nasal cannula oxygen concentration and arterial oxygen partial pressure.
The formula for calculation was as follows:
Propensity score matching (PSM)
PSM was used to evenly distribute confounders between groups, improving the accuracy of comparisons. The variables for PSM included demographic information (age, sex, BMI, smoking history, ASA physical status), GGO status, operation method, and pathology information (tumor location and depth, maximum tumor diameter, and histological category). The caliper value was selected as 0.2.
CT-guided hook-wire localization
Patients underwent preoperative localization in the CT room on the operation day. A chest CT scan determined the puncture location, angle, and depth, with major blood vessels, bullae, and interlobar fissures being avoided. After local anesthetic administration, the hook-wire system was inserted near the parietal pleura under guided angle and plane. The introducer accessed the lesion without penetration to prevent tumor spread. The hook wire was then released, and a final CT scan confirmed its precise location relative to the nodule. Complications postpuncture and localization time were noted. The barbed wire’s distal end was excised near the skin surface, and the patients were transferred for VATS.
Intraoperative LLD localization method (Qilu localization method)
After general anesthesia, one-lung ventilation, and the establishment of two thoracoscopic portals (camera and operating portals), the localization of pulmonary nodules was initiated when the lung naturally collapsed. Given the 3D structure of the lung, the nodules were also localized in three dimensions. First, the projection point on the lung surface was identified, which corresponds to the point on the pleura that is closest to the nodule, as visualized on the CT scan, with the nodule located vertically to this point. The distance from the projection point to pulmonary nodule measured on CT was defined as the depth (depth dimension). The actual depth was a slightly less than this distance because of the collapse of lung. Lung deflation causes the tissue to shift, which can result in the nodule being closer to the lung surface than initially indicated on the inflated CT scan.
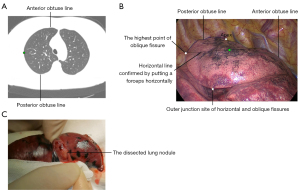
As is known, any location on the earth can be precisely determined by latitude and longitude coordinates. Due to the fact that a collapsed or deflated lung during surgery has many naturally formed horizontal and longitudinal lines, the projection point can be accurately localized by the relative distance to these reference horizontal lines (relative latitude coordinate: latitude dimension) and the relative distance to these reference longitudinal lines (relative longitude coordinate: longitude dimension). Based on our experience, adding more anatomical reference lines can improve the accuracy of pulmonary nodule localization. Therefore, we included the following natural horizontal lines and longitudinal lines as a reference. The horizontal lines from up to down included the apex, the highest point of oblique fissure, inferior margin of the azygos vein (used for a right pulmonary nodule near the mediastinal surface), inferior margin of the aortic arch (used for a left pulmonary nodule near the mediastinal surface), the highest point of the horizontal fissure (only used in the right lung), the outer junction site of the horizontal fissure and oblique fissure (only used in right lung), the lowest point of the horizontal fissure (only used in the right lung), the inferior pulmonary vein, the highest point of the diaphragm, the lowest point of the oblique fissure, and the lowest point of the lung (Figure 2). The longitudinal lines from the anterior hilum to the posterior hilum included the anterior hilar line, anterior acute angle line formed by the anterior mediastinal surface and costal surface, anterior obtuse line (foremost line) formed by gravity during lung deflation (turning into the acute angle after lung collapse), the vertical line of the outer junction point of the horizontal fissure and oblique fissure (only used in the right lung), the oblique fissure, the posterior obtuse line (rearmost line) formed by gravity during lung deflation (turning into the acute angle after lung collapse), the posterior acute angle line formed by the posterior mediastinal surface and costal surface, and the posterior hilar line (Figure 3).
In our nodule localization practice, we first determine the depth of pulmonary nodule and the projection point on the lung surface (depth dimension) on the CT slice showing the largest diameter. Second, the latitude coordinate (latitude dimension) is determined by the ratio of the vertical distance from the projection point level to the adjacent horizontal line above and below through measurement of the vertical distance on CT (or by direct counting of the slices on CT scan slice). If a nodule is extremely close to a horizontal line, the latitude dimension is determined by the vertical distance to that line. Subsequently, we identify these horizontal lines during lung deflation and determine the latitude dimension in the surgery. Notably, whether this horizontal line is truly horizontal can be confirmed by placing the forceps horizontally in the thorax as a reference. Third, the longitude coordinate (longitude dimension) is determined by the ratio of the surface distance from the projection point to adjacent longitudinal line (shown as a point on the CT scan slice) one side and the other side, as measured on the same CT slice used for the depth dimension, ensuring that the reference point is consistently aligned with the nodule’s position on the same imaging plane, thereby maintaining accuracy in spatial relationships across the three-dimensional coordinates. We subsequently identify these longitudinal lines in the deflated lung during the surgery and determined the longitude dimension. If a nodule is extremely close to a longitudinal line, the longitude-dimension can also be determined by the surface distance from the projection point to the adjacent line on CT. However, the actual distance during lung collapse as measured on CT is around two-thirds’ the distance during lung inflation. Notably, the vertical line, if applied, can be confirmed by using the lower part of an acute obtuse angle as a reference because it is nearly vertical. Finally, the intersection site of latitude dimension and longitude dimension is identified as the projection point and confirmed by palpation if possible. The localized point is then marked by electric cauterization and the localization duration is recorded.
Here, we present a typical case of our intraoperative LLD localization method. CT revealed an 8-mm pulmonary nodule that had grown over the previous year. We first, determined the projection point (green point in Figure 4A) and the depth dimension (about 1.4 cm from lung surface) on CT. Second, the latitude dimension was determined as a horizontal line of the highest point of the oblique fissure (or the horizontal line of the midpoint between the apex and the outer junction site of the horizontal and oblique fissures) on CT and then confirmed by placing a forceps horizontally during surgery. Finally, the longitude dimension was determined to be located about the midpoint between the anterior obtuse line and posterior obtuse line by measuring the distance on the lung surface on CT, and then the midpoint of the previous horizontal line between anterior and posterior obtuse line was determined as the projection point which was confirmed by palpation during surgery (Figure 4B). After sublobar resection, the pulmonary nodule was identified to be exactly beneath the localized projection point (Figure 4C).
Surgical procedures
All patients first underwent thoracoscopic segmentectomy or wedge resection. Following the identification of the pulmonary nodule using either LLD or CT-guided hook-wire localization, the distance between the mark and the actual projection point of the nodule was measured intraoperatively. The mark refers to the point made on the lung surface with electric cauterization after the pulmonary nodule was located by LLD method during surgery or the hook-wire placed under CT guidance preoperatively. The actual projection point is the point on the lung surface that is perpendicular to the nodule, identified within the resected pulmonary tissue specimen. A successful localization was defined as having a distance of ≤1 cm between the mark and the nodule, while one of >1 cm was recorded as a failure. The nodules were all ≥2 cm or more than the diameter of the nodule from the surgical margin of the specimen. If nodules could not be identified within the resected specimen after the mark was placed, extended resection was performed to ensure complete removal of the tumor and surrounding tissue.
Statistical analysis
In this study, statistical data were analyzed using SPSS 25 (IBM Corp., Armonk, NY, USA). After PSM, continuous variables were compared using the Wilcoxon/Mann-Whitney test or Student t test, while categorical variables were analyzed with the Pearson chi-square or Fisher exact test. Two-sided P values of <0.05 were considered statistically significant.
Results
Patient characteristics
During the study period, a total of 7,604 patients with pulmonary nodules underwent surgery at Qilu Hospital of Shandong University. After screening, 519 patients that met the criteria, with complete clinicopathological data and follow-up, were included for analysis. The detailed flowchart for the cohort’s population screening procedure is illustrated in Figure 1, and patient characteristics before and after PSM are shown in Table 1. Before matching, there were no significant differences in age, sex, BMI, smoking history, ASA score, tumor location, tumor diameter, or operation method between the two groups. GGO status, operation method, and histological category showed significant differences between the two groups. After PSM, none of the variables were significantly different. The probability density distributions before and after PSM are detailed in Figure S1.
Table 1
| Variable | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| CT-guided localization (n=259) | LLD localization (n=260) | P value | CT-guided localization (n=176) | LLD localization (n=176) | P value | ||
| Age (years), median (IQR) | 55.00 (48.00, 60.00) | 55.00 (44.75, 61.25) | 0.84 | 55.00 (47.00, 60.00) | 55.00 (44.00, 61.00) | 0.92 | |
| Sex, n (%) | 0.73 | 0.73 | |||||
| Female | 178 (68.73) | 175 (67.31) | 119 (67.61) | 122 (69.32) | |||
| Male | 81 (31.27) | 85 (32.69) | 57 (32.39) | 54 (30.68) | |||
| BMI (kg/m2), median (IQR) | 24.50 (22.43, 26.41) | 24.79 (22.66, 26.72) | 0.39 | 24.41 (22.45, 26.58) | 24.87 (22.84, 26.70) | 0.49 | |
| Smoking history, n (%) | 0.79 | 0.88 | |||||
| Nonsmoker | 219 (84.56) | 222 (85.38) | 151 (85.80) | 150 (85.23) | |||
| Smoker | 40 (15.44) | 38 (14.62) | 25 (14.20) | 26 (14.77) | |||
| ASA score, n (%) | 0.81 | 0.53 | |||||
| I | 102 (39.38) | 99 (38.08) | 74 (42.05) | 72 (40.91) | |||
| II | 155 (59.85) | 160 (61.54) | 100 (56.82) | 104 (59.09) | |||
| III | 2 (0.77) | 1 (0.38) | 2 (1.14) | 0 (0.00) | |||
| Location, n (%) | 0.11 | 0.96 | |||||
| LUL | 38 (14.67) | 36 (13.85) | 24 (13.64) | 24 (13.64) | |||
| LLL | 57 (22.01) | 68 (26.15) | 44 (25.00) | 47 (26.70) | |||
| RUL | 51 (19.69) | 51 (19.62) | 36 (20.45) | 34 (19.32) | |||
| RML | 5 (1.93) | 15 (5.77) | 5 (2.84) | 3 (1.70) | |||
| RLL | 108 (41.70) | 90 (34.62) | 67 (38.07) | 68 (38.64) | |||
| GGO, n (%) | <0.001 | 0.89 | |||||
| pGGO | 37 (14.29) | 88 (33.85) | 35 (19.89) | 34 (19.32) | |||
| mGGO | 222 (85.71) | 172 (66.15) | 141 (80.11) | 142 (80.68) | |||
| Diameter (mm), median (IQR) | 10.00 (8.00, 12.50) | 10.00 (8.00, 13.00) | 0.52 | 10.00 (8.00, 12.00) | 10.00 (8.00, 13.00) | 0.86 | |
| Depth (mm), median (IQR) | 12.00 (7.00, 19.00) | 9.50 (6.00, 15.00) | <0.001 | 11.00 (5.00, 17.00) | 10.50 (7.00, 16.00) | 0.97 | |
| Operation method, n (%) | 0.89 | 0.50 | |||||
| Wedge resection | 43 (16.60) | 42 (16.15) | 23 (13.07) | 28 (15.91) | |||
| Segmentectomy | 216 (83.40) | 218 (83.85) | 153 (86.93) | 148 (84.09) | |||
| Pathology, n (%) | <0.001 | 0.94 | |||||
| PGL | 50 (19.31) | 93 (35.77) | 43 (24.43) | 45 (25.57) | |||
| MIA | 83 (32.05) | 85 (32.69) | 61 (34.66) | 58 (32.95) | |||
| IAC | 126 (48.65) | 82 (31.54) | 72 (40.91) | 73 (41.48) | |||
CT, computed tomography; LLD localization, longitude-latitude-depth 3D localization; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; GGO, ground-glass opacity; pGGO, pure ground-glass opacity; mGGO, mixed ground glass-opacity; PGL, precursor glandular lesions; MIA, minimally invasive adenocarcinoma; IAC, invasive adenocarcinoma cancer.
Perioperative outcomes
A comparison of perioperative outcomes between the patients who underwent CT-guided localization and those who underwent LLD localization is shown in Table 2. Compared to the CT-guided localization group, the LLD localization group showed a significant improvement in localization duration (5 vs. 18 min), estimated intraoperative blood loss (37.5 vs. 55 mL), chest tube removal time (3 vs. 4 days), NRS score (3 vs. 4), oxygenation index on postoperative day 1 (POD1) (464.63 vs. 373.17 mmHg) and POD2 (460.97 vs. 382.92 mmHg), postoperative hospitalization time (5 days vs. 6 days), total hospitalization cost (CNY ¥39,764.25 vs. CNY ¥48,458.41), and failure rate of localization (3.41% vs. 8.52%). Furthermore, LLD localization was absolutely superior to CT-guided localization in terms of needle-carrying time (0 vs. 81 min) and lack of localization complications.
Table 2
| Perioperative outcomes | CT-guided localization (n=176) | LLD localization (n=176) | P value |
|---|---|---|---|
| Localization duration (min), median (IQR) | 18.00 (15.00, 21.00) | 5.00 (5.00, 6.00) | <0.001 |
| Waiting time for operation (min), median (IQR) | 81.00 (57.00, 105.00) | 0 (0.00, 0.00) | <0.001 |
| Localization complications, n (%) | |||
| Pain | 8 (4.55) | 0 (0.00) | 0.01 |
| Hemothorax | 6 (3.41) | 0 (0.00) | 0.04 |
| Pneumothorax | 8 (4.55) | 0 (0.00) | 0.01 |
| Hemoptysis | 12 (6.82) | 0 (0.00) | <0.001 |
| Estimated blood loss (mL), median (IQR) | 55.00 (35.00, 75.00) | 37.50 (30.00, 55.00) | <0.001 |
| Chest tube removal (days), median (IQR) | 4.00 (3.00, 5.00) | 3.00 (3.00, 4.00) | <0.001 |
| NRS score, median (IQR) | |||
| POD1 | 5.00 (4.00, 5.00) | 3.00 (3.00, 4.00) | <0.001 |
| POD2 | 3.00 (3.00, 4.00) | 3.00 (3.00, 3.00) | <0.001 |
| Oxygenation index (mmHg), median (IQR) | |||
| POD1 | 373.17 (360.97, 380.48) | 464.63 (429.26, 495.12) | <0.001 |
| POD2 | 382.92 (368.29, 392.68) | 460.97 (433.53, 490.24) | <0.001 |
| POS (days), median (IQR) | 6.00 (5.00, 7.00) | 5.00 (4.00, 6.00) | <0.001 |
| Hospitalization cost (CNY ¥), median (IQR) | 48,458.41 (43,345.85, 55,053.93) | 39,764.25 (36,279.69, 44,504.82) | <0.001 |
| Failed to localization, n (%) | 15 (8.52) | 6 (3.41) | 0.043 |
CT, computed tomography; LLD localization, longitude-latitude-depth 3D localization; IQR, interquartile range; NRS, numerical rating scale; POD, postoperative day; POS, postoperative length of stay.
Discussion
Because of its comparable prognosis and simpler procedures relative to segmentectomy (12-15), wedge resection is widely performed for small pulmonary nodules (≤2 cm diameter and GGO ratio ≥0.5). However, GGO-dominant nodules can be difficult to localize when they are not palpable or visible, making accurate localization the most critical step in wedge resection. Moreover, even during segmentectomy, accurate nodule localization is also helpful to ensure the correct intersegmental plane in order to prevent dissection failure. To overcome the difficulty in intraoperative localization of small GGO-dominant nodules, we developed a novel intraoperative LLD method using relative 3D coordinates: depth to the nearest pleura (depth dimension), latitude relative to horizontal lines (latitude dimension), and longitude relative to longitudinal lines (longitude dimension). This method can achieve 96.59% accuracy in localizing small pulmonary nodules during lung deflation in surgery. Although widely used, CT-guided hook-wire localization is invasive and carries the risk of complications (7). In our studies, we found that our LLD localization was noninvasive and caused no related complications, while CT-guided hook-wire localization caused more complications. Additionally, LLD localization also reduces both localization time and hospitalization costs compared to CT-guided hook-wire, making it more time- and cost-efficient. Finally, CT-guided hook-wire localization, which performed in the CT room, has a longer needle-carrying time than does LLD localization and can be immediately followed by sublobar resection. This extended time increases the risk of surgery and the likelihood of pulmonary complication and decoupling (16).
During LLD localization, we prioritize depth dimension, latitude dimension, and longitude dimension sequences. The depth dimension is crucial as it determines both the CT projection point and the surgical margin during sublobar resection, particularly in wedge resection (12). The latitude dimension is the second step since the lung’s vertical diameter during deflation closely matches that seen on CT during inflation. Surgeons can accurately determine the horizontal location of pulmonary nodules during surgery by counting CT slices and calculating the relative distance (17). The longitude dimension is the final and most challenging step due to the difference in lung shape between deflation during surgery and inflation on CT. Additionally, the lung is concentrically contracted around the hilum during surgery, and so the contraction ratio of the lateral lung is slightly greater more than that of the hilar region. This is also the main reasons for the failure of traditional non-invasive localization methods. Lung collapse can alter the position of the nodule relative to the lung surface. During lung deflation, the natural anatomical landmarks shift, which can affect the precision of the localization process. This issue is particularly problematic for small or deep nodules located near the periphery of the lungs, as their relative position may change significantly during deflation. In such cases, the projection point may no longer align accurately with the resected tissue, leading to localization failure. Therefore, the exclusive use of anterior obtuse line and posterior obtuse line cannot precisely localize the longitude dimension of pulmonary nodules, as indicated in previous research (10,11). In our study, we included multiple natural longitudinal lines, especially the oblique fissure and outer junction of fissures as references and determined the relative longitudinal coordinate by selecting two adjacent natural longitudinal lines (shown as a point on CT) as references and measuring the distance from the projection point to those two adjacent lines on CT. Thus, the longitude dimension can be more accurately determined during the surgery. In addition to direct LLD localization, we also developed additional techniques to enhance accuracy. Once the relative 3D coordinates are determined, careful palpation of nodules with elevated CT values in targeted areas can help to minimize interference from small bronchi, thus improving the chances of directly localizing the pulmonary nodule. Additionally, in cases of multiple pulmonary nodules, visible pleural indentation, dominant solid components, or other easily confirmed nodules can serve as additional references for localizing smaller nodules during surgery. Moreover, when pulmonary nodules are near the oblique fissure, horizontal fissure, or diaphragm, their planes can serve as projection points for localization instead of the lung surface. Multiple natural horizontal or longitudinal lines can also be used repeatedly to enhance accuracy when localizing a single pulmonary nodule. Furthermore, uncommon anatomical structures such as the intersegmental fissure in the lung can also serve as references. Finally, complete lung deflation is crucial for accurate nodule localization. Asking the anesthetist for early one-lung ventilation can save time during deflation.
Although the LLD method is an effective and non-invasive technique for precise intraoperative localization, there are still some challenges in its clinical application. One of the primary challenges of implementing the LLD method in clinical practice is the learning curve associated with the technique. The accurate identification of anatomical reference points, as well as the precise measurement of depth, latitude, and longitude dimensions, requires significant experience and skill. Surgeons unfamiliar with this method may initially struggle with the consistency and accuracy of the localization process, which could affect surgical outcomes. In addition, certain patient factors, such as poor lung function, obesity, or extensive pleural adhesions, may interfere with the ability to accurately locate nodules using the LLD method. In patients with severe lung collapse or when there is limited lung deflation, the anatomical landmarks may shift unpredictably, reducing the reliability of the method. Therefore, given the challenges outlined above, several avenues for future research remain. One key direction for future studies should be the integration of the LLD method into a broader range of clinical scenarios, as well as further validation in different patient populations. The applicability of the LLD method to different patient populations, including those with central or multiple pulmonary nodules, and the challenges associated with using this method in such cases should be further investigated. Future research could also explore combining the LLD method with preoperative imaging and planning tools, such as 3D printing or virtual reality modeling, to enhance preoperative accuracy and provide intraoperative guidance. This could further improve the real-time decision-making during surgery, offering surgeons a more intuitive interface for navigating complex anatomical structures.
Conclusions
Our initial experience in LLD localization method indicates that it can provide satisfactory accuracy in pulmonary nodule localization. Compared to CT-guided hook-wire localization, it is noninvasive, time-saving, cost-effective, and complication free, making it a practicable, safe, and effective technique with promising potential for localizing small pulmonary nodules during surgery.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1170/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1170/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1170/prf
Funding: This work was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1170/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the tenets of the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of the Qilu Hospital of Shandong University (No. KYLL-202008-023). All patients provided informed consent for clinical data usage.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Qiu B, Ji Y, He H, et al. Three-dimensional reconstruction/personalized three-dimensional printed model for thoracoscopic anatomical partial-lobectomy in stage I lung cancer: a retrospective study. Transl Lung Cancer Res 2020;9:1235-46. [Crossref] [PubMed]
- Zhou C, Li X, Li W, et al. Clock dial integrated positioning combined with single utility port video-assisted thoracoscopic surgery: a new localization method for lung tumors. J Thorac Dis 2021;13:1143-50. [Crossref] [PubMed]
- Zhang T, Li Y, Feng C, et al. Clinical application of theodolitic method for locating small pulmonary nodules in video assisted thoracoscopic surgery. Medical Journal of Chinese People’s Liberation Army 2019;12:1043-6.
- Zhao X, Lu H, Zhang Z. Preliminary Study of CT Three-dimensional Reconstruction Combined with Ground Glass Nodules of Natural Lung Collapse in Thoracoscopic Pulmonary Segmental Resection. Zhongguo Fei Ai Za Zhi 2021;24:683-9. [Crossref] [PubMed]
- Altorki N, Wang X, Damman B, et al. Lobectomy, segmentectomy, or wedge resection for peripheral clinical T1aN0 non-small cell lung cancer: A post hoc analysis of CALGB 140503 (Alliance). J Thorac Cardiovasc Surg 2024;167:338-347.e1. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Chiang XH, Lu TP, Hsieh MS, et al. Thoracoscopic Wedge Resection Versus Segmentectomy for cT1N0 Lung Adenocarcinoma. Ann Surg Oncol 2021;28:8398-411. [Crossref] [PubMed]
- Isaka T, Nagashima T, Adachi H, et al. Wedge resection vs. segmentectomy for lung cancer measuring ≤ 2 cm with consolidation tumor ratio > 0.25. Front Oncol 2023;13:1253414. [Crossref] [PubMed]
- Wang L, Sun D, Gao M, et al. Computed tomography-guided localization of pulmonary nodules prior to thoracoscopic surgery. Thorac Cancer 2023;14:119-26. [Crossref] [PubMed]
- Park S, Lee SM, Kim W, et al. Computer-aided Detection of Subsolid Nodules at Chest CT: Improved Performance with Deep Learning-based CT Section Thickness Reduction. Radiology 2021;299:211-9. [Crossref] [PubMed]




