
Lobectomy plus lobe-specific lymphadenectomy as the minimum standards of curative resection for hypermetabolic clinical stage IA non-small cell lung cancer
Highlight box
Key findings
• Patients undergoing standard lobectomy (vs. sublobar resection) and lymphadenectomy that met at least lobe-specific nodal dissection (LSND) criteria (vs. lymphadenectomy that failed the LSND criteria) for hypermetabolic clinical stage IA (cIA) non-small cell lung cancer (NSCLC) had lower lung cancer-specific cumulative incidence of death and cumulative incidence of tumor recurrence.
What is known and what is new?
• Three recent randomized controlled trials (JCOG 0802/WJOG4607L, CALGB140503, and DRKS00004897) have proven the oncological non-inferiority of segmentectomy or sublobar resection compared to lobectomy in peripheral cT1a-bN0M0 NSCLC. However, there are limited studies assessing the feasibility of sublobar resection for hypermetabolic cIA tumors.
• In this study, lobectomy was proved superior to sublobar resection as a curative-intent procedure for hypermetabolic cIA NSCLC, owing to a lower risk of lung cancer-specific mortality and tumor recurrence. Lymphadenectomy that met at least the LSND criteria contributed to more accurate N staging and more favorable long-term outcomes. However, systemic nodal dissection was not associated with additional oncological benefits.
What is the implication, and what should change now?
• Whether sublobar resection can be deemed an acceptable procedure for hypermetabolic cIA NSCLC requires further evidence. Given that adherence to the LSND criteria has resulted in more accurate pathological N staging and improved survival outcomes, lobectomy combined with LSND should be regarded as the minimum standard for curative resection in hypermetabolic early-stage NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related mortality in China and globally (1,2). A surge in the incidence of patients diagnosed with early-stage non-small cell lung cancer (NSCLC) is observed, for whom surgical resection stands as the soundest choice of treatment (3). One of the most intricate and commonly disputed issues in lung cancer surgery revolves around the definition of “complete resection”. In an era, replete with numerous screen-detected pulmonary malignancies, the discourse pertains to the query of whether lobectomy invariably constitutes the apt procedure for early-stage NSCLC and what defines adequate lymph node (LN) assessment.
Three modern randomized controlled trials (JCOG 0802/WJOG4607L, CALGB140503, and DRKS00004897) have demonstrated the oncological non-inferiority of segmentectomy or sublobar resection to lobectomy in peripheral NSCLC up to 2 cm (4-6). The attainment of adequate margins and high-quality LN examinations proved crucial and undeniably bolstered the success of sublobar resection in those meticulously planned trials (7). It is noteworthy that while heightened tumor metabolic activity serves as a reliable indicator of occult nodal metastasis and an increased risk of postoperative recurrence, a preoperative positron emission tomography-computed tomography (PET-CT) scan was not obligatory for patient enrollment in all three trials. Given the complexity of intraoperatively evaluating LN status in routine clinical practice, there remains an understanding gap about whether sublobar resection is also suitable for hypermetabolic NSCLC. Evidence from several retrospective analyses comparing lobectomy and sublobar resection for hypermetabolic NSCLC is inconclusive (8-10). Furthermore, limited studies have delved into the role of lymphadenectomy in this specific population at a notably high risk of encountering unexpected nodal metastasis. We postulate that lobectomy coupled with lobe-specific nodal dissection (LSND) would correlate with improved prognosis and should be considered the minimum standards for curative-intent resections.
In this study, we examined patients undergoing pulmonary resection for hypermetabolic clinical stage IA (cIA) NSCLC at the National Cancer Center (Beijing, China). The primary aim was to determine whether sublobar resection could achieve comparable long-term outcomes (tumor recurrence and lung cancer-specific death) compared to lobectomy. The secondary aim was to investigate the relationship between lymphadenectomy extent and oncological outcomes, including the risk of pathologic nodal upstaging, recurrence-free survival (RFS), tumor recurrence and lung cancer-specific death. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-804/rc).
Methods
Patient cohort
This retrospective analysis was performed on patients undergoing surgical resection for cIA NSCLC from November 2013 to October 2019 at the National Cancer Center (Beijing, China). A two-step screening was performed to generate the study cohort (Figure 1). The optimal cut-off point on the receiver operating characteristic (ROC) curve assessing the discriminative power of the maximum standardized uptake value (SUVmax) in predicting aggressive pathology was determined as the threshold for hypermetabolic tumors. Tumors meeting any of the following criteria would be defined as possessing aggressive pathology: (I) tumors with nodal involvement, (II) tumors with lymphovascular invasion, (III) poorly differentiated tumors. Calculation was performed on patients who underwent lobectomy and systemic nodal dissection (SND) (n=404).
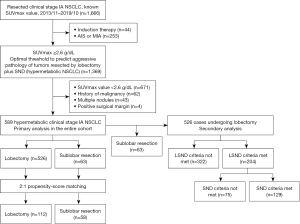
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board (IRB) at the National Cancer Center approved this study (No. NCC3692) and patient written consent was waived due to the retrospective nature of the study.
Radiologic evaluation and surgical procedures
Preoperative contrast-enhanced thin-section CT scan using 64-detector-row CT scanners (Lightspeed Ultra, GE Healthcare, Chicago, IL, USA; Toshiba, Tokyo, Japan) and a whole-body PET-CT scan (Discovery 690; GE Healthcare) within 30 days before surgery were mandatory for all patients included in the study cohort. The acquisition of 18F-fluorodeoxyglucose (FDG) PET-CT data followed the standard procedure of the Department of Nuclear Medicine/PET-CT Center in National Cancer Center (11). Clinical stage was determined based on the 8th edition of the tumor-node-metastasis (TNM) classification system (12). Clinical node-negative disease was defined when there was no suspicion of LN metastasis neither on CT (node diameter <1 cm on the short-axis) nor on PET (SUVmax <2.5 g/dL). In cases where there were discrepancies between the interpretations of the two investigators and the original diagnostic reports, consensus was reached to resolve the discrepancies.
Given the observational nature of this study, surgeons determined the surgical approach, extent of resection, and strategy of lymphadenectomy based on each patient’s preoperative evaluation and actual intraoperative findings. Intraoperative SND and LNSD were defined according to the European Society for Thoracic Surgeons (ESTS) guidelines (13) (Appendix 1).
Follow-up
Patients in the study cohort were followed up from the day of surgery. Routine follow-up was conducted every 3 months for the first 2 years, every 6 months for the subsequent 2 years, and annually thereafter. Physical examination, lung cancer biomarker testing, and chest CT scans were mandatory for each follow-up round. For patients with suspicious clinical symptoms and/or meaningful examination results, further imaging examinations, such as PET-CT scan, brain magnetic resonance imaging (MRI) and bone scintigraphy, blood testing [e.g., minimal residual disease (MRD)] and/or LN biopsy were performed as needed. Locoregional recurrence was defined as tumor relapse occurring at the resection margins, at the ipsilateral pleura, or within LNs at stations 1–14. Distant recurrence was defined as tumor relapse in the contralateral lung or at extrathoracic sites. Lung cancer-specific survival (LCSS) and RFS were periods from the date of resection until death from lung cancer (obtained from electronic medical records or through a telephone interview) or diagnosis of recurrence (the day of imaging examination). For survivors without recurrence, LCSS and RFS were censored on the last day of survival confirmation. Otherwise, patients were censored at the time of the last follow-up. Survival information was updated through January 2024.
Analysis scheme
The primary data analyses focused on the between-group comparison of survival and relapse outcomes, performed in a competing risk framework. For recurrence, death from any cause without recurrence was considered a competing event. For lung cancer-specific death, death from causes other than lung cancer or from unknown causes was considered a competing event. Cumulative incidence of recurrence (CIR) and lung cancer-specific cumulative incidence of death (LC-CID) were calculated to compare the probability of recurrence and lung cancer-specific death between patients who underwent lobectomy and sublobar resection. To better understand the surgical indications of this special patient population, a machine learning investigation was performed to examine the prognostic importance of tumor-related and treatment-related variables.
The secondary data analyses focused on lymphadenectomy’s prognostic value. Analyses were conducted within a subgroup of patients who underwent lobectomy (n=526). A logistic regression analysis was conducted to identify risk factors for pathologic nodal upstaging. RFS was then assessed using the Cox proportional hazard model. CIR and LC-CID were compared between patients who met the LSND criteria and those who did not, and between patients who met the LSND criteria and those with SND.
Statistical analysis
Patient characteristics were reported as medians with interquartile ranges, means with standard deviations, or frequencies with percentages. Between-group comparisons were performed with the Mann-Whitney U test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables. The progression of propensity-score matching is illustrated in Appendix 1. Prognostic differences in the CIR and LC-CID were tested with the Gray method. Multivariable competing risk models for lung cancer-specific death and tumor recurrence were adjusted for variables with a P value <0.1 in the univariable analyses using a backwards selection strategy. A machine learning model based on the random forest algorithm was utilized to investigate variable importance (VIMP). Relevant information is available in Appendix 1. The univariable models for pathologic nodal upstaging and RFS included interested tumor- and surgery-related covariations based on the literature and clinical significance, and covariates with a P value <0.1 were then adjusted in the subsequent multivariable models. All significance tests were two-sided, and a P value less than 0.05 was considered statistically significant. Unless otherwise specified, complete-case analyses were performed. Statistical analyses were performed with R software (version 4.3.2).
Results
Participants
We classified tumors with SUVmax ≥2.6 g/dL as hypermetabolic NSCLC, as it demonstrated the optimal discriminative power in predicting aggressive pathology (Figure S1). In total, 589 patients were included. Table 1 summarizes patients’ demographic characteristics and tumor-related features. Before matching, 18 of 20 covariates were unbalanced [absolute standardized mean difference (ASMD) ≥0.1], except for the presence of ground-glass opacity (GGO) component and tumor differentiation. Patients undergoing lobectomy were younger, more often females, had a lower age-adjusted Charlson Comorbidity Index and performed better in spirometry. Tumors resected with lobectomy had higher SUVmax values, increased sizes, and higher rates of lymphovascular invasion and nodal metastasis. As a result of matching, only the number of examined LNs and LN stations had significant differences between the two groups. Significantly more locoregional recurrences (locoregional only) occurred after sublobar resection than lobectomy (17.5% vs. 5.7%; P<0.001); However, the incidence of distant recurrence was comparable between the two groups (19.0% vs. 16.7%, P=0.74). Margin information was available for 41 patients within the sublobar group. Larger tumors exhibited a higher likelihood of having insufficient margins (Table S1).
Table 1
| Characteristics | All study patients (n=589) | Propensity-matched patients (n=170) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LR group (n=526) | SR group (n=63) | ASMD | P value | LR group (n=112) | SR group (n=58) | ASMD | P value | ||
| Age (years) | 60 (54, 66) | 66 (62, 74) | 0.796 | <0.001 | 66 (61, 72) | 66 (61, 72) | 0.037 | 0.85 | |
| Male sex | 266 (50.6) | 43 (68.2) | 0.380 | 0.01 | 62 (55.4) | 39 (67.2) | 0.253 | 0.18 | |
| Smoking history | 206 (39.2) | 29 (46.0) | 0.138 | 0.36 | 51 (45.5) | 27 (46.6) | 0.020 | >0.99 | |
| aCCI | 0.613 | <0.001 | 0.162 | 0.40 | |||||
| 0–2 | 420 (79.8) | 31 (49.2) | 67 (59.8) | 30 (51.7) | |||||
| ≥3 | 106 (20.2) | 32 (50.8) | 45 (40.2) | 28 (48.3) | |||||
| FEV1 (%) (n=575) | 85 (73, 96) | 83 (65, 95) | 0.205 | 0.29 | 83 (75, 94) | 83 (63, 95) | 0.198 | 0.54 | |
| DLCO (%) (n=555) | 94 (78, 111) | 87 (72, 101) | 0.357 | 0.02 | 95 (76, 110) | 87 (75, 100) | 0.354 | 0.06 | |
| CEA (mg/dL) | 2.6 (1.6, 4.2) | 3.1 (1.8, 5.0) | 0.230 | 0.33 | 2.7 (1.7, 4.1) | 2.8 (1.6, 4.1) | 0.010 | 0.90 | |
| Maximum tumor diameter (cm) | 2.1 (1.7, 2.5) | 1.7 (1.5, 2.3) | 0.435 | <0.001 | 1.8 (1.5, 2.2) | 1.7 (1.5, 2.3) | 0.014 | 0.95 | |
| GGO component | 98 (18.6) | 11 (17.5) | 0.031 | 0.96 | 19 (17.0) | 10 (17.2) | 0.007 | >0.99 | |
| SUVmax (g/dL) | 5.1 (3.7, 7.2) | 4.3 (3.4, 6.0) | 0.168 | 0.03 | 4.8 (3.6, 6.1) | 4.2 (3.3, 5.9) | 0.060 | 0.26 | |
| Histopathology | 0.31 | 0.75 | |||||||
| Adenocarcinoma | 478 (90.9) | 55 (87.3) | 0.107 | 104 (92.9) | 52 (89.7) | 0.105 | |||
| Squamous | 33 (6.3) | 7 (11.1) | 0.154 | 7 (6.2) | 5 (8.6) | 0.084 | |||
| Others | 15 (2.8) | 1 (1.6) | 0.101 | 1 (0.9) | 1 (1.7) | 0.064 | |||
| Low grade/undifferentiated tumor | 66 (12.6) | 8 (12.7) | 0.004 | <0.001 | 15 (13.4) | 7 (12.1) | 0.046 | >0.99 | |
| Tumor size (cm) | 2.0 (1.6, 2.5) | 1.7 (1.4, 2.2) | 0.435 | 0.01 | 1.8 (1.5, 2.2) | 1.7 (1.3, 2.2) | 0.150 | 0.34 | |
| Pleural invasion | 165 (31.4) | 17 (27.0) | 0.168 | 0.57 | 31 (27.7) | 16 (27.6) | 0.140 | >0.99 | |
| Lymphovascular invasion | 80 (15.2) | 3 (4.8) | 0.930 | 0.04 | 13 (11.6) | 2 (3.45) | 0.228 | 0.14 | |
| No. of LNs harvested | 16 (11, 21) | 6 (1, 12) | 1.369 | <0.001 | 15 (12, 19) | 6 (1, 12) | 1.237 | <0.001 | |
| No. of LN stations sampled | |||||||||
| N1 | 2 (2, 3) | 1 (0, 2) | 1.091 | <0.001 | 2 (2, 3) | 1 (0, 2) | 0.915 | <0.001 | |
| N2 | 3 (3, 4) | 2 (1, 3) | 0.683 | <0.001 | 3 (2, 4) | 2 (1, 3) | 0.507 | 0.001 | |
| Pathological stage | 0.19 | 0.55 | |||||||
| IA | 303 (57.6) | 45 (71.4) | 0.306 | 73 (65.2) | 41 (70.7) | 0.121 | |||
| IB | 122 (23.2) | 11 (17.5) | 0.151 | 27 (24.1) | 10 (17.2) | 0.182 | |||
| II | 52 (9.9) | 3 (4.8) | 0.241 | 8 (7.1) | 3 (5.2) | 0.089 | |||
| III | 49 (9.3) | 4 (6.4) | 0.122 | 4 (3.6) | 4 (6.9) | 0.131 | |||
| Adjuvant chemotherapy | 162 (30.8) | 15 (23.8) | 0.73 | 39 (34.8) | 17 (29.3) | 0.121 | 0.46 | ||
| Tumor recurrence | 0.002 | 0.003 | |||||||
| Locoregional only | 30 (5.7) | 11 (17.5) | 0.310 | 8 (7.1) | 11 (19.0) | 0.302 | |||
| Distant | 88 (16.7) | 12 (19.0) | 0.059 | 7 (6.2) | 10 (17.2) | 0.291 | |||
| Any death | 0.001 | 0.002 | |||||||
| Lung cancer-specific death | 54 (10.3) | 17 (27.0) | 0.377 | 9 (8.0) | 16 (27.6) | 0.437 | |||
| Other/unknown death | 32 (6.1) | 4 (6.5) | 0.011 | 9 (8.0) | 2 (3.4) | 0.251 | |||
Data are presented as number (%) or median (25th and 75th percentiles). LR, lobar resection; SR, sublobar resection; ASMD, absolute standardized mean difference; aCCI, age-adjusted Charlson Comorbidity Index; FEV1, forced expiratory volume in 1 second; DLCO, diffusion capacity of the lungs for carbon monoxide; CEA, carcinoembryonic antigen; GGO, ground-glass opacity; SUV, standardized uptake value; LN, lymph node.
LC-CID and CIR analysis: lobectomy vs. sublobar resection
Based on a median follow-up of 70 months, sublobar resection was associated with significantly higher LC-CID (5-year LC-CID, 20.7% vs. 7.8%, P<0.001) and CIR (5-year CIR, 38.2% vs. 21.6%, P=0.004) than lobectomy (Figure 2A,2B). After matching, the absolute between-group differences in CIR and LC-CIR were more significant (Figure 2C,2D). In the comparison that included three procedures, wedge resection showed significantly higher 5-year CIR and 5-year LC-CID than both lobectomy (adjusted P<0.001 for CIR and <0.001 for LC-CID, respectively) and segmentectomy (adjusted P=0.01 for CIR and 0.03 for LC-CID, respectively). Segmentectomy also had higher CIR (23.2% vs. 14.8%, adjusted P=0.79) and LC-CID (13.0% vs. 6.5%, adjusted P=0.36) compared with lobectomy (Figure S2A,S2B).
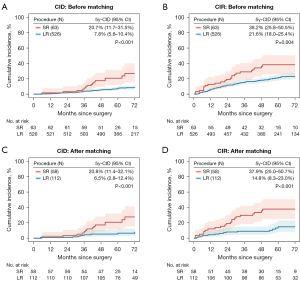
Competing risks regression analysis
Table S2 shows the results of the univariable competing risk regression model. Male, higher carcinoembryonic antigen (CEA) level (≥5 vs. <5 ng/mL), pure-solid CT appearance, increasing SUVmax value, sublobar resection [vs. lobectomy, hazard ratio (HR) =3.27, 95% confidence interval (CI): 1.90–5.62, P<0.001; HR =1.95, 95% CI: 1.24–3.06, P=0.004, respectively], higher stages, pleural invasion and lymphovascular invasion were risk factors significantly associated with both lung cancer-specific death and tumor recurrence. Poorly differentiated tumors and adjuvant chemotherapy were significant risk factors for recurrence but not lung cancer-specific death. When segmentectomy and wedge resection were taken into analysis separately, wedge resection but not segmentectomy was significantly associated with increased risk of lung cancer-specific death and tumor recurrence (HR =4.83, 95% CI: 2.64–8.85, P<0.001; HR =4.04, 95% CI: 2.05–7.97, P<0.001, respectively).
Table 2 shows the results of the final multivariable model, independent risk factors for lung cancer-specific death and recurrence were higher CEA level and sublobar resection (vs. lobectomy, HR =3.29, 95% CI: 1.82–5.94, P<0.001; HR =2.43, 95% CI: 1.49–3.98, P<0.001, respectively). Increasing SUVmax values and higher stages were also independently associated with postoperative recurrence. Similar as the results in the univariable model, wedge resection but not segmentectomy was the significant risk factor for lung cancer-specific death and tumor recurrence (Table S3).
Table 2
| Covariate | Lung cancer-specific death | Tumor recurrence | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Female sex (vs. male) | 0.75 (0.45–1.27) | 0.29 | 0.90 (0.63–1.30) | 0.58 | |
| CEA level (≥5 vs. <5 ng/mL) | 1.81 (1.09–3.01) | 0.02 | 1.89 (1.32–2.72) | <0.001 | |
| CT appearance | |||||
| GGO | Reference | Reference | |||
| Pure-solid nodules | 2.62 (0.98–7.01) | 0.06 | 1.50 (0.86–2.60) | 0.15 | |
| SUVmax (per 1 SUV increase) | 1.03 (0.97–1.10) | 0.28 | 1.05 (1.01–1.10) | 0.03 | |
| Extent of resection | |||||
| Lobectomy | Reference | Reference | |||
| Sublobar resection | 3.29 (1.82–5.94) | <0.001 | 2.43 (1.49–3.98) | <0.001 | |
| Pathological stage | |||||
| IA | Reference | Reference | |||
| IB | 0.80 (0.33–1.96) | 0.63 | 1.42 (0.76–2.68) | 0.27 | |
| II | 1.30 (0.46–3.67) | 0.61 | 1.68 (0.85–3.30) | 0.14 | |
| III | 1.29 (0.49–3.40) | 0.61 | 2.46 (1.29–4.69) | 0.001 | |
| Pleural invasion | 1.74 (0.81–3.74) | 0.16 | 1.26 (0.75–2.09) | 0.38 | |
| Lymphovascular invasion | 1.73 (0.92–3.24) | 0.09 | 1.38 (0.87–2.17) | 0.17 | |
| Receipt of adjuvant chemotherapy | 0.85 (0.46–1.58) | 0.60 | 1.31 (0.87–1.97) | 0.19 | |
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CT, computed tomography; GGO, ground-glass opacity; SUV, standardized uptake value.
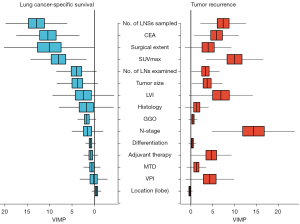
VIMP for LCSS and tumor recurrence
Figure 3 illustrates the results of the VIMP analysis. In the left part of the figure, the VIMP values are listed descendingly according to their importance in predicting LCSS based on a random survival forest. Three of the five most predictive variables for LCSS were directly related to surgical extent: the number of examined LN stations, surgical procedure (lobectomy or sublobar resection), and the number of examined LNs. As illustrated in the right part of the figure, the pathological N stage displayed the highest VIMP value, while the number of examined LN stations remained the treatment-related variable that contributed the most to predicting postoperative recurrence.
Prognostic role of different extents of lymphadenectomy
Table S4 shows the clinicopathological differences of 526 patients undergoing lobectomy included in the secondary analysis. Of these, 204 (38.8%) underwent at least LSND and 322 (61.2%) underwent lymphadenectomy that failed to meet the LSND criteria. LSND correlated with a significantly higher rate of nodal upstaging (23.2% vs. 15.0%, P=0.01), mainly due to the increased rate of N2 disease (Table S4). Younger age, increasing tumor size and LSND were associated with increased odds of pathological nodal upstaging (Table 3). Lymphadenectomy that met the LSND criteria resulted in a significantly lower CID than lymphadenectomy that did not (5-year LC-CID, 6.2% vs. 10.8%, P=0.03) (Figure 4A). Although not statistically significant, patients with LSND also had a lower CIR than those without LNSD (Figure 4B). Similar results were observed in the pN0 subgroup (Figure 4C,4D). Cox proportional hazard model showed that LSND was significantly correlated with improved RFS (Table S5). However, a more thorough lymphadenectomy strategy, i.e., SND, did not exhibit additional survival benefit over LSND (Figure S3).
Table 3
| Covariate | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (per 1-year increase) | 0.96 (0.94–0.98) | <0.001 | 0.95 (0.93–0.98) | <0.001 | |
| Female (vs. male) | 1.11 (0.71–1.72) | 0.64 | – | – | |
| Smoking status | |||||
| Never | Reference | – | – | ||
| Former/current | 0.90 (0.57–1.42) | 0.65 | – | – | |
| CEA level (≥5 vs. <5 ng/mL) | 1.01 (0.98–1.04) | 0.48 | – | – | |
| Tumor location | |||||
| Right upper lobe | Reference | – | – | ||
| Right middle lobe | 0.84 (0.32–2.18) | 0.72 | – | – | |
| Right lower lobe | 1.04 (0.54–2.01) | 0.90 | – | – | |
| Left upper lobe | 1.15 (0.60–2.23) | 0.67 | – | – | |
| Left lower lobe | 0.98 (0.55–1.74) | 0.94 | – | – | |
| Focality | |||||
| Multifocal | Reference | – | – | ||
| Unifocal | 1.11 (0.50–2.46) | 0.97 | – | – | |
| CT appearance | |||||
| GGO | Reference | – | – | ||
| Pure-solid nodules | 1.60 (0.85–3.00) | 0.15 | – | – | |
| Maximum tumor diameter (per 1 cm increase) | 1.88 (1.22–2.91) | 0.005 | 0.95 (0.53–1.81) | 0.95 | |
| SUVmax (per 1 SUV increase) | 1.07 (1.01–1.14) | 0.03 | 1.07 (1.00–1.14) | 0.06 | |
| Histopathological type | |||||
| Adenocarcinoma | Reference | – | – | ||
| Squamous | 0.78 (0.29–2.08) | 0.51 | – | – | |
| Others | 1.09 (0.30–3.95) | 0.89 | – | – | |
| Tumor size (per 1 cm increase) | 2.10 (1.48–2.97) | 0.001 | 2.19 (1.35–3.55) | <0.001 | |
| Extent of lymphadenectomy | |||||
| LSND criteria met | Reference | – | – | ||
| LSND criteria not met | 0.58 (0.37–0.91) | 0.02 | 0.55 (0.34–0.87) | 0.01 | |
OR, odds ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CT, computed tomography; GGO, ground-glass opacity; SUV, standardized uptake value; LSND, lobe-specific nodal dissection.
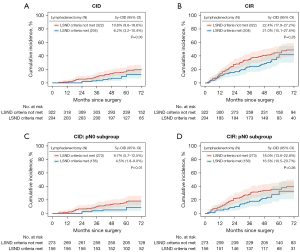
Discussion
Given the remarkable performance of sublobar resection with safe resection margins and thorough LN assessments in three randomized controlled trials, it seems safe to conclude that high-quality segmentectomy, or even wedge resection could achieve non-inferior survival outcomes for carefully-selected patients compared to lobectomy. However, whether sublobar resection could also achieve similar outcomes for highly aggressive tumors [e.g., tumors with spread through air spaces (STAS), and hypermetabolic tumors] was still questionable (4,14). Unfortunately, none of those trials included information on such issues.
This study is one of the few studies to compare sublobar resection and lobectomy for hypermetabolic NSCLC (8-10). The criteria we identified for hypermetabolic tumors was comparable to that reported in other studies, and it showed satisfactory predictive sensitivity and specificity for predicting aggressive pathology. According to our primary analysis, sublobar resection was significantly associated with a higher risk of postoperative recurrence and a reduced chance of survival. While sublobar resection had a similar risk of developing distant recurrences compared to lobectomy, the risk of locoregional recurrence was three times that of lobectomy. Wedge resection was an independent risk factor for lung cancer-specific death and tumor recurrence. As an explanation, nearly 40% of wedge resection cases in this study were omitted from the mediastinal LN examination. Patients undergoing wedge resection may experience an undesirable outcome due to low-quality lymphadenectomy plus insufficient resection margins (15,16). Segmentectomy, although not a significant risk factor, had a 5-year CID and CIR about one-fold higher than lobectomy.
Two prior studies, on the other hand, supported the potential indication for segmentectomy in treating hypermetabolic tumors (9,10). Kamel et al. reported that patients undergoing segmentectomy for hypermetabolic cIA lung adenocarcinomas had similar RFS to their matched counterparts undergoing lobectomy (9). However, the 5-year LCSS of segmentectomy was lower than that of lobectomy (83% vs. 92%, P=0.56). Another investigation by Handa et al. (10) reported that there was no survival difference between complex segmentectomy and so-called “location-adjusted” lobectomy for hypermetabolic cIA NSCLC. The discrepancy among these retrospective comparisons could be attributed in large part to the different indications of segmentectomy (whether it was a curative resection for generally healthy patients or a compromised resection for medically unfit patients). It was noteworthy that the 5-year LC-CID and CIR of lobectomy in this study were as low as 6.5% and 14.8% after propensity-score matching. Such performance of lobectomy was very close to that performed for STAS-positive T1 lung adenocarcinoma reported by Eguchi et al. (17), in which study the LC-CID and CIR of segmentectomy was double that of lobectomy. Accordingly, we consider lobectomy the preferred procedure for hypermetabolic stage IA NSCLC.
Our secondary analysis demonstrated that LSND was associated with significantly higher odds of pathological nodal upstaging. This was mainly attributed to the higher rate of mediastinal nodal metastasis. Patients who underwent at least LSND had significantly decreased LC-CID than patients who did not. While similar comparisons about CIR were underpowered to detect significant advantages, adherence to the LSND criteria was independently associated with better RFS. Our findings were consistent with several recent analyses conducted among stage IA NSCLC patients based on population-based or multicenter database (18,19). Smeltzer et al. reported that more stringent nodal staging quality strata resulted in increased survival benefits (20). The results of the VIMP analysis in this study, showing that the number of examined LN stations was a significant predictor of both LCSS and tumor recurrence, were in line with their conclusions. As an explanation, there may be a lower probability of missing undetected mediastinal nodal metastases when all stations within LSND drainage regions were examined. Since patients without LSND were less likely to receive adjuvant therapy, there was a nonnegligible possibility for some patients to miss necessary adjuvant therapy due to pN understaging (18).
Interestingly, we found no prognostic inferiority of LSND compared with SND, a theoretically more thorough criteria that requires nodal evaluation outside the lobe-specific drainage regions. A study by Handa et al. (21) concluded that SND had a superior prognosis over LSND for patients with clinical T2-3N0-1M0 hypermetabolic NSCLC. However, the definition of lobe-specific LN regions they adopted simply referred to the superior mediastinum for tumors of the upper lobe, and the inferior mediastinum for those of the lower lobe. A more recent study by Huang et al. demonstrated comparable 5-year overall survival and disease-free survival between patients who undergoing LSND and SND for stage I NSCLC (22). As a matter of fact, the incidence of T1 NSCLC having N2 disease that skips lobe-specific stations is extremely low (23-25). We observed only one case of single N2 metastasis outside the lobe-specific stations among 129 patients with SND, which may explain why SND had no survival advantage over LSND. In this regard, we strongly recommend LSND strategies as the minimum requirement for an adequate lymphadenectomy.
The strength of this study is that it identified a practical definition of hypermetabolic early-stage NSCLC, on the basis of which comprehensive clinicopathological and follow-up data have been collected, and an in-depth investigation into the surgical extent has been conducted. These findings may provide insight into surgical management for this specific spectrum of NSCLC.
As a retrospective study based on clinical practice at a single center, there are limitations associated with it in this study. A primary limitation of this study was the limited number of sublobar cases, which was underpowered for subgroup analyses based on the surgical procedure and the extent of lymphadenectomy performed. Secondly, we could not differentiate between compromised sublobar resection and intentional sublobar resection. Few patients who undergo lobectomy would omit the mediastinal LN examination and adjuvant therapy if necessary. Comparatively, compromised patients with sublobar resection tended to receive insufficient lymphadenectomy and no adjuvant chemotherapy. Such selection bias could have been avoided in a randomized trial. Moreover, margin distance information was unavailable, which has hampered in-depth investigations into the role of surgical extents.
Conclusions
In conclusion, our analysis demonstrated that compared with lobectomy, sublobar resection was oncologically less effective for hypermetabolic cIA NSCLC. Adherence to the LSND criteria led to more accurate pN staging and a more favorable prognosis. Lobectomy plus lobe-specific LN dissection should be considered as the minimum requirement for curative-intent surgery in this patient population.
Acknowledgments
The authors thank all staff in the National Cancer Center for their support during this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-804/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-804/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-804/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-804/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the National Cancer Center (No. NCC3692), and the requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Expert Consensus Panel. The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2023;166:637-54. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Stamatis G, Leschber G, Schwarz B, et al. Survival outcomes in a prospective randomized multicenter Phase III trial comparing patients undergoing anatomical segmentectomy versus standard lobectomy for non-small cell lung cancer up to 2 cm. Lung Cancer 2022;172:108-16. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Kneuertz PJ, Ferrari-Light D, Altorki NK. Sublobar Resection vs Lobectomy for Stage IA Non-Small Cell Lung Carcinoma-Takeaways From Modern Randomized Trials. Ann Thorac Surg 2024;117:897-903. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:1100-8. [Crossref] [PubMed]
- Kamel MK, Rahouma M, Lee B, et al. Segmentectomy Is Equivalent to Lobectomy in Hypermetabolic Clinical Stage IA Lung Adenocarcinomas. Ann Thorac Surg 2019;107:217-23. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Complex Segmentectomy for Hypermetabolic Clinical Stage IA Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;113:1317-24. [Crossref] [PubMed]
- Tao X, Li N, Wu N, et al. The efficiency of (18)F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2020;47:1209-19. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Swanson SJ, White A. Sublobar resections for lung cancer: Finally, some answers and some more questions? J Surg Oncol 2023;127:269-74. [Crossref] [PubMed]
- Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg 2018;156:394-402. [Crossref] [PubMed]
- Heiden BT, Eaton DB Jr, Chang SH, et al. Association Between Surgical Quality Metric Adherence and Overall Survival Among US Veterans With Early-Stage Non-Small Cell Lung Cancer. JAMA Surg 2023;158:293-301. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Subramanian MP, Eaton DB Jr, Heiden BT, et al. Lobe-specific lymph node sampling is associated with lower risk of cancer recurrence. JTCVS Open 2024;17:271-83. [Crossref] [PubMed]
- Gabryel P, Skrzypczak P, Campisi A, et al. Predictors of Long-Term Survival of Thoracoscopic Lobectomy for Stage IA Non-Small Cell Lung Cancer: A Large Retrospective Cohort Study. Cancers (Basel) 2023;15:3877. [Crossref] [PubMed]
- Smeltzer MP, Faris NR, Ray MA, et al. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80-7. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Systematic Versus Lobe-Specific Mediastinal Lymphadenectomy for Hypermetabolic Lung Cancer. Ann Surg Oncol 2021;28:7162-71. [Crossref] [PubMed]
- Huang CC, Tang EK, Shu CW, et al. Comparison of the Outcomes between Systematic Lymph Node Dissection and Lobe-Specific Lymph Node Dissection for Stage I Non-small Cell Lung Cancer. Diagnostics (Basel) 2023;13:1399. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Deng HY, Zhou J, Wang RL, et al. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol 2020;27:472-80. [Crossref] [PubMed]
- Abughararah TZ, Jeong YH, Alabbood F, et al. Lobe-specific lymph node dissection in stage IA non-small-cell lung cancer: a retrospective cohort study. Eur J Cardiothorac Surg 2021;59:783-90. [Crossref] [PubMed]


