
Metachronous development of L858R and T790M EGFR mutations following ALK inhibitor therapy in stage IV lung adenocarcinoma: a case report
Highlight box
Key findings
• Metachronous development of T790M and L858R epidermal growth factor receptor (EGFR) co-mutations following anaplastic lymphoma kinase (ALK) inhibitor therapy in stage IV lung adenocarcinoma and personalized treatment strategies.
What is known and what is new?
• ALK and EGFR mutations are key drivers in non-small cell lung cancer (NSCLC) and are generally mutually exclusive. Many studies described resistance to ALK inhibitors involves ALK-dependent mutations or bypass pathways like neuregulin 1-human epidermal growth factor receptor 3-EGFR. Concurrent ALK and EGFR mutations are rare, occurring in 0.9–1.3% of NSCLC cases.
• This is the first case showing EGFR L858R/T790M mutations emerging after ALK inhibitor therapy, with concurrent analysis using next-generation sequencing. The findings reveal tumor heterogeneity, clonal evolution, and the role of ALK inhibitors in promoting genomic instability, emphasizing the need for personalized therapeutic strategies to address temporal and spatial tumor heterogeneity.
What is the implication, and what should change now?
• The case underscores the need for continuous monitoring of tumor genetic profiles through re-biopsy or liquid biopsy, particularly when new lesions appear during targeted therapy. This approach is important for distinguishing between true drug resistance and the development of a new, distinct tumor with different driver mutations.
• A paradigm shifts towards routine re-biopsy or molecular profiling of new lesions in patients undergoing targeted therapy is essential to optimize personalized treatment strategies. Additionally, greater reliance on non-invasive methods like liquid biopsy could be invaluable, especially in elderly or frail patients, to better capture the dynamic nature of cancer evolution without the need for direct tissue sampling.
Introduction
A metachronous malignant neoplasm is defined as the occurrence of a second primary malignancy in the same patient, diagnosed at least six months after the initial tumor (1). It can be challenging to distinguish metachronous lung cancer from intrapulmonary metastasis, particularly when relying solely on histopathological results (2). Nevertheless, the identification of driver mutations at the genetic level through next-generation sequencing (NGS) can facilitate the differentiation between the two and the planning of an appropriate treatment plan (3).
Tumor heterogeneity describes the differences that were observed between tumors of the same type in different patients, as well as the differences between a primary tumor and a secondary tumor in a single individual, or among the cells within a single tumor. It has a number of potential causes, which can be classified into intrinsic and extrinsic factors. Intrinsic factors include genomic instability, epigenetic changes, plastic gene expression and alterations in signal transduction. Extrinsic factors encompass the tumor microenvironment, comprising tumor blood vessels, inflammatory cells and mesenchymal cells, which collectively contribute to an uneven surrounding environment (4). The spatial and temporal heterogeneity of tumors can help to elucidate the mechanisms underlying the acquisition of drug resistance due to tumor dynamic changes and the emergence of new cancers (5).
The dynamic nature of cancer and the development of polyclonal drug resistance highlight the growing importance of re-biopsies and liquid biopsies, such as blood, bronchoalveolar lavage fluid (BALF), cerebrospinal fluid. In this case study, we present the case of a patient with an anaplastic lymphoma kinase (ALK) rearrangement who initially showed improvement with ALK inhibitor treatment. However, a newly developed mass was observed, and a biopsy of the lesion revealed no ALK mutation, but the presence of L858R and T790M epidermal growth factor receptor (EGFR) mutations. This is the first reported case demonstrating the emergence of EGFR L858R and T790M mutations following ALK inhibitor therapy, analyzed using NGS. This case study particularly emphasizes the critical importance of rebiopsy and a thorough understanding of the genetic evolution of cancer and the mechanisms underlying drug resistance over time (6,7). We present this article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1071/rc).
Case presentation
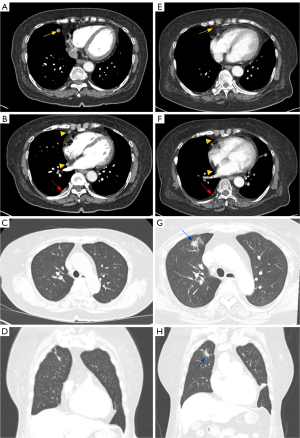
A 73-year-old East Asian woman was admitted to the Pulmonology Department of Chungnam National University Hospital in November 2022 for evaluation of an abnormal chest computed tomography (CT) scan. She had no respiratory symptoms like cough or dyspnea. Her medical history included only hypertension, with no other significant underlying diseases, and she had no history of smoking or a family history of cancer. The chest CT revealed a 1.1-cm spiculated nodule in right middle lobe (RML), as well as multiple pleural nodules with mild pleural thickening and fibrotic lesions in right upper lobe (RUL) (Figure 1A-1D).
A linear endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed on the enlarged lymph node 7. Pathologic results showed metastatic adenocarcinoma with ALK rearrangement with wild type EGFR mutation and NGS showed echinoderm microtubule-associated protein-like 4 (EML 4)-ALK fusion. Positron emission tomography (PET)-CT revealed pleural, multiple enhancing nodules with lymph nodes metastasis, leading to a diagnosis of stage IV lung cancer (T2N2M1a).
Targeted mutation chemotherapy with alectinib was initiated and continued for a period of one and a half years. Follow-up chest CT scans during treatment with alectinib demonstrated a reduction in the lung mass and pleural metastatic lesions. In July 2024, the chest CT, the previously observed main mass in the RML, subpleural nodules, and pleural metastasis were no longer visible (Figure 1E,1F). However, CT revealed a newly developed peribronchial consolidation with ground-glass opacity in the anterior segment of the RUL (Figure 1G,1H). She underwent radial EBUS-guided transbronchial lung biopsy (TBLB) to obtain tissue and culture of the lesion. The pathologic analysis confirmed adenocarcinoma, revealing the absence of an ALK rearrangement and the presence of newly identified EGFR mutation, which was not detected in the initial primary tumor (Figure 2A-2D). Furthermore, NGS of the second tumor identified an EGFR L858R and T790M co-mutation, along with a TP53 L194R mutation.

To perform a detailed analysis of the two cancers, we conducted a comparative assessment of the raw data obtained from targeted gene sequencing NGS, provided by GC Genome (Korea), for biopsies collected in 2022 and 2024 (Figure 3; Table S1). Targeted NGS, a focused approach for detecting specific genetic alterations, is highly effective in identifying clinically relevant mutations. In the 2022 biopsy, four copy number variants (CNVs), two single nucleotide variants (SNVs), and two fusions were detected. In contrast, the 2024 biopsy revealed a substantial increase in genetic alterations, with 59 CNVs, 190 SNVs, and no fusions identified (Figure 3A,3B). Visualization of the top 20 mutations (Figure 3C) demonstrated that the 2022 sample contained an ALK-EML4 rearrangement without any detectable EGFR mutations. Conversely, the 2024 sample exhibited SNVs and CNVs in ALK and EML4 but lacked the ALK-EML4 rearrangement. Notably, EGFR mutations, along with numerous additional mutations absent in the 2022 sample, were detected in the 2024 biopsy. These findings suggest that the genetic profiles of the tumors from 2022 and 2024 are distinct, reflecting significant molecular evolution over time.

The patient was finally diagnosed with metachronous lung cancer with an EGFR L858R/T790M mutation, which had developed following a primary lesion with ALK rearrangement. As the previously identified lesions (Figure 4A-4C) had all improved, the patient was responding well to the ALK tyrosine kinase inhibitor. Since there was no evidence of newly enlarged lymph nodes or other lesions outside the RUL (Figure 4D,4E), the patient was considered to have localized disease. Therefore, it was decided to continue the ALK inhibitor while administering stereotactic body radiotherapy (SBRT) to the newly developed RUL lesion. Subsequent to SBRT, the follow-up chest CT scan demonstrated a considerable reduction in the dimensions of the RUL lesion, with only residual scarring (Figure 4F-4H). Furthermore, PET-CT imaging demonstrated the absence of any hypermetabolic lung lesion. Figure 4I illustrates the dynamic changes in cancer lesions observed during the treatment period. The yellow line represents the ALK rearrangement, which significantly decreased following treatment with the ALK inhibitor, Alectinib. In contrast, the blue line represents the emergence of the EGFR mutation (L858R/T790M) in the RUL, which gradually increased over time. Following SBRT to the RUL, the newly developed lesion showed a marked decrease in size, leaving only residual scarring with no metabolic activity observed on PET-CT. She has remained well with no evidence of recurrence to date. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Discussion
Our case is distinguished by three significant characteristics that set it apart from other cases in the field. First, it was observed that a single patient exhibited both mutually exclusive EGFR and ALK mutations. Secondly, it is rare and noteworthy that a new EGFR mutation emerged following the use of an ALK inhibitor targeting the original ALK mutation. This highlights the complexity of tumor heterogeneity, clonal evolution, and selective pressure. To our knowledge, this is the first reported case demonstrating the emergence of EGFR L858R and T790M mutations following ALK inhibitor therapy, analyzed using NGS. Lastly, the concurrent de novo occurrence of both L858R and T790M mutations in a patient who had not previously received EGFR inhibitor treatment is remarkable.
EGFR and ALK mutations are key drivers in non-small cell lung cancer (NSCLC), and they are generally considered mutually exclusive (8). The occurrence of concomitant EGFR and ALK mutations within the same tumor lesion is rare, reported in only 0.9–1.3% of NSCLC patients (9,10). Cases in which different tumors harbor distinct EGFR and ALK mutations, as observed in our patient, are likely even less common and remain poorly documented. Also, this case demonstrates the emergence of distinct driver mutations in separate tumor clones over time, reflecting temporal and spatial tumor heterogeneity rather than concurrent dual oncogenic alterations. Treatment decisions in such scenarios can be particularly challenging, as most reported cases involve concurrent mutations within a single tumor lesion. In a case that is similar in some respects to ours, Yorozuya et al. reported a case with EGFR and ALK mutations in distinct lung lesions occurring simultaneously, underscoring the complexity of treatment. The initial surgical lung biopsy revealed stage IIB (pT3N0M0) EGFR-mutant adenocarcinoma, and a subsequent biopsy one month later identified a separate tumor as stage IA1 ALK-mutant adenocarcinoma. Gefitinib was initiated immediately after the first surgical biopsy. However, 11 months later, disease progression occurred in the ALK-mutant adenocarcinoma, prompting a third surgical biopsy. Treatment was then switched to alectinib, which subsequently led to the progression of the EGFR-mutant adenocarcinoma. Subsequently, alternating therapy with two tyrosine kinase inhibitors (TKIs) every 2 months was administered, resulting in a prolonged progression-free survival (PFS) of 39 months. This case highlights the need for individualized strategies in managing dual oncogenic drivers (11).
In our patient, re-biopsy for new lesion showed an EGFR mutated lung cancer, which raised the possibility of switching or adding to an EGFR-TKI. However, the lesion was confined to the RUL and the patient had demonstrated a favorable response to ALK inhibitors for existing lesions. Also, Won et al. reported that ALK inhibitors appear to be effective for patients with co-alterations (12). Wang et al. demonstrated that alectinib was more effective than EGFR-TKIs, with patients achieving partial remission and a PFS of 16 months, continuing to derive ongoing benefits (13). Hu et al. reported that when using EGFR-TKI the cancer progressed but using alectinib combined with SBRT led to a partial response (PR), with a ≥30% reduction in the maximum diameter of tumor target lesions maintained for at least 4 weeks (14). Consequently, we administered SBRT to the newly developed lesion in the RUL while maintaining alectinib treatment, which has effectively managed the patient’s condition without any further cancer recurrence to date.
The EGFR T790M mutation is primarily known as an acquired resistance mechanism, occurring in 50–60% of patients who develop resistance to first- and second-line EGFR-TKI therapy (15,16). However, the de novo T790M mutation is exceptionally rare, detected in only 2.59% of NSCLC patients harboring EGFR mutations (17). Although the precise prevalence is unknown, it is clear that the concurrent de novo occurrence of T790M and L858R mutations are extremely rare in EGFR-TKI treatment-naïve patients.
Several possibilities can be considered based on known resistance mechanisms. Resistance mechanisms can be classified into two main categories: ALK-dependent mechanisms (such as mutations in the ALK tyrosine kinase domain or amplification of the ALK gene) and ALK-independent mechanisms (including activation of bypass signaling pathways like EGFR, HER2, or MET, drug efflux pumps, or epithelial-to-mesenchymal transition) (6,18-20). In ALK-dependent mechanisms, continuous exposure to ALK inhibitors may select for resistant clones, complicating treatment (21). Patients with ALK rearrangement are typically treated with second-generation inhibitors like brigatinib or alectinib, but resistance mutations such as G1202R or I1171N often necessitate switching to lorlatinib, a third-generation inhibitor capable of overcoming multiple resistance mutations and penetrating the blood-brain barrier (18,22). Resistance process may also promote genomic instability, leading to new driver mutations through clonal selection and drug-induced genetic and epigenetic reprogramming (23). In ALK-independent mechanism, other studies have reported resistance emerging as a result of cell line prolonged exposure to, or patient treatment with, alectinib, with mechanisms associated with amplification of EGFR, HER2 and other receptors. Tan et al. showed that the mechanisms of resistance to second-generation ALK inhibitors, such as alectinib and ceritinib, using the H3122 NSCLC cell line harboring the EML4-ALK variant 1 fusion. The study revealed that prolonged treatment with these inhibitors did not lead to ALK kinase domain mutations, which are a common resistance mechanism for first-generation inhibitors like crizotinib. Instead, resistance was driven by the activation of alternative receptor tyrosine kinase (RTK) pathways, particularly the NRG1-HER3-EGFR axis (24). Jang et al. established the patient-derived NSCLC cell line, DFCI032, which harbors both EML4-ALK and activated EGFR. Study demonstrated that dual ALK/EGFR inhibitors, compound 7c, effectively target both ALK and EGFR, including the T790M mutation, preventing adaptive resistance (25). Similarly, Tani et al. highlighted that EGFR bypass signaling through TGFα overexpression induces alectinib resistance, proposing alectinib-afatinib combination therapy as a potential solution (26).
The cross-reactivity of TKIs between RTKs, particularly ALK and EGFR, is an important factor in resistance mechanisms. Structural overlap in the ATP-binding sites of ALK and EGFR may allow limited inhibitory effects of certain TKIs, such as alectinib, on EGFR. However, this cross-reactivity is typically insufficient to produce significant clinical activity against EGFR-driven tumors. Conversely, EGFR TKIs, such as osimertinib, are not known to exhibit substantial activity against ALK rearrangements. In this case, selective pressure from ALK inhibition and underlying genomic instability may have facilitated the emergence of EGFR mutations, highlighting the interplay between ALK and EGFR pathways in resistance development.
Although the exact mechanism underlying the emergence of an EGFR co-mutation following ALK inhibitor therapy in our case remains unclear, several plausible scenarios can be proposed. First, the primary tumor was already genetically heterogeneous, with the fastest-growing, ALK-rearranged components accounting for the lesions that were first identified in RML and treated. A more slowly growing subpopulation with EGFR driver mutations colonized RUL. Second, the second tumor may have evolved from the first, with treatment using an ALK inhibitor causing the selection of a tumor negative for ALK rearrangement. Third, an unforeseen mechanism, such as tumor instability, may have contributed to the emergence or selection of the new EGFR mutation following ALK inhibitor therapy.
However, the first scenario, which assumes that the tumor was genetically heterogeneous from the beginning with both ALK-rearranged and EGFR-mutant components, appears less likely given that no EGFR mutations were detected in the 2022 NGS dataset (Figure 3). Instead, the subsequent emergence of an EGFR-mutant lesion suggests that temporal tumor heterogeneity likely developed due to selective pressure exerted by ALK inhibitor therapy. This therapeutic pressure may have suppressed ALK-dependent tumor clones, facilitating the proliferation of sub-clones harboring alternative driver mutations, such as EGFR. Moreover, the emergence of 154 new mutations and the loss of ALK rearrangement observed in the 2024 dataset (Figure 3A) further support the hypothesis that alectinib drove clonal selection under therapeutic pressure, ultimately facilitating tumor evolution. Unlike previous studies that utilized patient-derived or established NSCLC cell lines to investigate resistance mechanisms (24-26), our study analyzed NGS data collected at two distinct time points (2022 and 2024) following ALK inhibitor therapy. This approach enabled a direct evaluation of both temporal and spatial tumor heterogeneity, providing clinically relevant insights into the evolution of resistance, including the emergence of new driver mutations such as EGFR. These findings underscore the complexity of managing patients with evolving tumor heterogeneity and highlight the necessity of further research into resistance mechanisms and optimized therapeutic strategies targeting multiple pathways.
The evolving nature of tumors underscores the critical importance of re-biopsy in exploring resistance mechanisms and genetic changes. This is exemplified by a reported case in which an ALK-rearranged lung adenocarcinoma progressed to express an EGFR L858R mutation, while the original ALK rearrangement was lost following treatment with ALK inhibitors (27). Additionally, histologic transformation from adenocarcinoma to small cell carcinoma or squamous cell carcinoma has also been observed during treatment (28-30). In our case, if the treatment regimen had been changed without performing a biopsy—under the assumption that the new lesion represented resistance to the ALK inhibitor—the EGFR co-mutated lesion in the RUL might not have responded effectively to the new therapy. Additionally, a critical window of opportunity for effective treatment could have been missed. This highlights the importance of conducting re-biopsy, even in patients receiving targeted therapies based on known mutations, when new lesions emerge. Re-biopsy is essential to assess tumor heterogeneity, identify potential drug resistance, and detect new mutations, ensuring that treatment strategies remain optimized for evolving disease conditions.
While re-biopsy is essential, it is not always feasible, especially in elderly or frail patients, and obtaining sufficient tissue can often be challenging. Additionally, it may pose a significant financial burden. However, liquid biopsies are ease of sampling, repeatability, and reduced invasiveness (31-33). Liquid biopsies analyzing circulating tumor cells (CTCs), cell free DNA (cfDNA) or extracellular vesicles (like exosomes) from body fluids (e.g., blood, urine, pleural effusions) or BALF have shown promising advantages of noninvasiveness and accessibility (31,34-36). Many studies described the usefulness and high detection quality about liquid biopsy. For example, the pooled diagnostic performance of CTCs for detecting lung cancer showed a sensitivity of 0.72 [95% confidence interval (CI): 0.65–0.79] and specificity of 0.96 (95% CI: 0.91–0.98) (37). Zhang et al. (38) and Kim et al. (31) showed soluble programmed death ligand 1 (sPD-L1) correlated level of PD-L1 expression in NSCLC. Oh et al. (39) and Cheng et al. (40) showed sPD-L1 is predictive and prognostic biomarker in cancer. Qiu et al. (41) and Luo et al. (42) described cfDNA for EGFR mutation has a high diagnostic accuracy. The sensitivity of cfDNA has been shown to be 67.4% and 62%, respectively, and the specificity of 93.5% and 95.9%, respectively. In our case, the patient was in a suitable condition for rebiopsy via bronchoscopy, which successfully detected the new EGFR mutation. Therefore, EGFR testing using cfDNA from blood was not performed. However, in patients for whom rebiopsy is challenging, liquid biopsy techniques, such as EGFR detection through cfDNA analysis from blood, could be a useful alternative. However, liquid biopsy has some limitation like lack of standardization, easily fragility, and low concentration of some molecules in the body fluid (32). Ongoing research aims to utilize liquid biopsies to better understand tumor heterogeneity and predict lung cancer progression and changes in characteristics.
Lung cancer remains a heterogeneous and complex disease, with its mechanisms of resistance not fully understood. Conducting further research on this rare molecular shift is essential for providing optimal treatment for patients. In particular, evaluating new lesions through rebiopsy is crucial; thus, the continued development of diagnostic techniques that do not require direct tissue acquisition is equally important. Ultimately, this case reinforces the need for personalized approaches, adaptability, and regular monitoring in cancer therapeutic strategies, as tumors evolve over time. The dynamic transformation of the tumor highlights the critical importance of customizing treatment plans based on the unique characteristics of each patient’s tumor and their response to therapy.
Conclusions
This case presents a rare instance of tumor evolution in NSCLC, where an initially ALK-rearranged lung adenocarcinoma treated with an ALK tyrosine kinase inhibitor subsequently developed metachronous cancer with EGFR L858R/T790M co-mutations. These findings underscore the critical importance of re-biopsy in understanding tumor evolution and reflect the emergence of distinct driver mutations in separate tumor clones over time, illustrating temporal and spatial tumor heterogeneity. Such insights emphasize the necessity of personalized treatment approaches tailored to each patient and the significance of continuous molecular assessment in developing effective targeted therapies.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1071/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1071/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1071/coif). H.Y.K. is an employee of NGeneS Inc., a for-profit company. This affiliation has been disclosed, and no direct financial support or involvement from NGeneS Inc. has influenced the study’s design, data collection, analysis, nor manuscript preparation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors have no conflicts of interest to declare. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karthikeyan VS, Sistla SC, Srinivasan R, et al. Metachronous multiple primary malignant neoplasms of the stomach and the breast: report of two cases with review of literature. Int Surg 2014;99:52-5. [Crossref] [PubMed]
- Murphy SJ, Harris FR, Kosari F, et al. Using Genomics to Differentiate Multiple Primaries From Metastatic Lung Cancer. J Thorac Oncol 2019;14:1567-82. [Crossref] [PubMed]
- Hu Y, Ren S, Chen C, et al. Metachronous primary lung adenocarcinomas harboring distinct KRAS mutations. Thorac Cancer 2020;11:2018-22. [Crossref] [PubMed]
- Sun XX, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin 2015;36:1219-27. [Crossref] [PubMed]
- Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15:81-94. [Crossref] [PubMed]
- Tabbò F, Reale ML, Bironzo P, et al. Resistance to anaplastic lymphoma kinase inhibitors: knowing the enemy is half the battle won. Transl Lung Cancer Res 2020;9:2545-56. [Crossref] [PubMed]
- Scheffler M, Wiesweg M, Michels S, et al. Rebiopsy in advanced non-small cell lung cancer, clinical relevance and prognostic implications. Lung Cancer 2022;168:10-20. [Crossref] [PubMed]
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014;20:1383-92. [Crossref] [PubMed]
- Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer 2012;77:460-3. [Crossref] [PubMed]
- Yorozuya T, Nagano Y, Chiba H, et al. Repeated treatment with gefitinib and alectinib in a patient with multiple EGFR-mutant and ALK-mutant lung adenocarcinomas: A case report. Respir Med Case Rep 2021;32:101378. [Crossref] [PubMed]
- Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol 2015;26:348-54. [Crossref] [PubMed]
- Wang H, Zhu S, Li Z, et al. Lung adenocarcinoma with EGFR 19Del and an ALK rearrangement benefits from alectinib instead of an EGFR-TKI: A case report. Medicine (Baltimore) 2022;101:e30316. [Crossref] [PubMed]
- Hu H, Tan S, Xie M, et al. Case report: Concomitant EGFR mutation and ALK rearrangement in non-small cell lung cancer. Front Pharmacol 2023;14:1167959. [Crossref] [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 2006;12:5764-9. [Crossref] [PubMed]
- Chhouri H, Alexandre D, Grumolato L. Mechanisms of Acquired Resistance and Tolerance to EGFR Targeted Therapy in Non-Small Cell Lung Cancer. Cancers (Basel) 2023;15:504. [Crossref] [PubMed]
- Panda GS, Noronha V, Shah D, et al. Treatment pattern and outcomes in de novo T790M-mutated non-small cell lung cancer. Ecancermedicalscience 2022;16:1385. [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- McCoach CE, Le AT, Gowan K, et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin Cancer Res 2018;24:3334-47. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Bhang HE, Ruddy DA, Krishnamurthy Radhakrishna V, et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat Med 2015;21:440-8. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Vander Velde R, Yoon N, Marusyk V, et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat Commun 2020;11:2393. [Crossref] [PubMed]
- Tan AC, Vyse S, Huang PH. Exploiting receptor tyrosine kinase co-activation for cancer therapy. Drug Discov Today 2017;22:72-84. [Crossref] [PubMed]
- Jang J, Son JB, To C, et al. Discovery of a potent dual ALK and EGFR T790M inhibitor. Eur J Med Chem 2017;136:497-510. [Crossref] [PubMed]
- Tani T, Yasuda H, Hamamoto J, et al. Activation of EGFR Bypass Signaling by TGFα Overexpression Induces Acquired Resistance to Alectinib in ALK-Translocated Lung Cancer Cells. Mol Cancer Ther 2016;15:162-71. [Crossref] [PubMed]
- Leung JKC, Kwok WC, Leung ACF, et al. Emerging EGFR-Mutated Subclones in a Patient With Metastatic ALK-Rearranged Lung Adenocarcinoma Treated With ALK-Targeted Therapy: A Case Report. JTO Clin Res Rep 2023;4:100542. [Crossref] [PubMed]
- Lin X, Qiu G, Li F, et al. Case Report: A Rare Case of Metachronous Multiple Primary Lung Cancers in a Patient With Successful Management by Switching From Anti-PD-1 Therapy to Anti-PD-L1 Therapy. Front Immunol 2021;12:683202. [Crossref] [PubMed]
- Shao Y, Zhong DS, Guan SS. Histologic transformation of lung adenocarcinoma to squamous cell carcinoma after chemotherapy: two case reports. Transl Cancer Res 2020;9:388-93. [Crossref] [PubMed]
- Woo CG, Son SM, Lee HC, et al. Histologic Changes in Non-Small Cell Lung Cancer under Various Treatments: A Comparison of Histology and Mutation Status in Serial Samples. Cancer Res Treat 2022;54:737-43. [Crossref] [PubMed]
- Kim SY, Park D, Sun P, et al. Prognostic and predictive significance of soluble programmed death ligand 1 in bronchoalveolar lavage fluid in stage IV non-small cell lung cancer. Transl Lung Cancer Res 2024;13:1888-906. [Crossref] [PubMed]
- Casagrande GMS, Silva MO, Reis RM, et al. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int J Mol Sci 2023;24:2505. [Crossref] [PubMed]
- An HJ, Chon HJ, Kim C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. Int J Mol Sci 2021;22:9414. [Crossref] [PubMed]
- Zhou J, Zhao C, Zhao J, et al. Re-biopsy and liquid biopsy for patients with non-small cell lung cancer after EGFR-tyrosine kinase inhibitor failure. Thorac Cancer 2019;10:957-65. [Crossref] [PubMed]
- de Sousa VML, Carvalho L. Heterogeneity in Lung Cancer. Pathobiology 2018;85:96-107. [Crossref] [PubMed]
- Kim IA, Hur JY, Kim HJ, et al. Extracellular Vesicle-Based Bronchoalveolar Lavage Fluid Liquid Biopsy for EGFR Mutation Testing in Advanced Non-Squamous NSCLC. Cancers (Basel) 2022;14:2744. [Crossref] [PubMed]
- Zhao Q, Yuan Z, Wang H, et al. Role of circulating tumor cells in diagnosis of lung cancer: a systematic review and meta-analysis. J Int Med Res 2021;49:300060521994926. [Crossref] [PubMed]
- Zhang J, Gao J, Li Y, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer 2015;6:534-8. [Crossref] [PubMed]
- Oh SY, Kim S, Keam B, et al. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep 2021;11:19712. [Crossref] [PubMed]
- Cheng S, Zheng J, Zhu J, et al. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int J Biol Markers 2015;30:e364-8. [Crossref] [PubMed]
- Qiu M, Wang J, Xu Y, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2015;24:206-12. [Crossref] [PubMed]
- Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014;4:6269. [Crossref] [PubMed]


