
Pathological complete response and long-term survival by pembrolizumab based immunochemotherapy in epidermal growth factor receptor mutated non-small cell lung cancer post-tyrosine kinase inhibitor failure: a case report and tumor microenvironment analysis
Highlight box
Key findings
• A patient with stage IVa epidermal growth factor receptor (EGFR) L858R-mutated non-small cell lung cancer (NSCLC) achieved a pathological complete response (pCR) and prolonged survival after failing multiple lines of EGFR-tyrosine kinase inhibitor (TKI) therapy, following treatment with pembrolizumab-based immunochemotherapy (ICT). Tumor microenvironment analysis showed high programmed cell death 1 ligand 1 (PD-L1) expression on tumor cells and increased infiltration of immune cells, particularly PD-1+ CD8+ T cells and PD-L1+ macrophages, in the tumor area compared to the stroma.
What is known and what is new?
• EGFR-mutated NSCLC typically responds well to EGFR-TKIs, but resistance is common. Immune checkpoint inhibitors (ICIs) have shown promise in various cancers, though their role in EGFR-mutated NSCLC is less defined.
• This case report demonstrates that pembrolizumab-based ICT can achieve pCR and long-term survival in EGFR-mutated NSCLC post-TKI failure, suggesting a potential role for this approach in this patient population.
What is the implication, and what should change now?
• The findings suggest that ICT may be a valuable treatment option for EGFR-mutated NSCLC patients who have progressed on TKIs. This could lead to reconsideration of treatment strategies, potentially integrating ICIs with chemotherapy earlier in the treatment sequence for these patients. Further prospective studies are needed to validate these findings and identify predictive biomarkers for response.
Introduction
Lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations represents a distinct subset of non-small cell lung cancer (NSCLC) that typically responds well to EGFR-tyrosine kinase inhibitors (TKIs) (1-3). However, resistance to these targeted therapies is a common challenge, leading to disease progression and a poor prognosis for affected patients. The emergence of immune checkpoint inhibitors (ICIs) has revolutionized the treatment landscape for various malignancies (4,5), yet their role in EGFR-mutated NSCLC remains less defined due to the presumed molecularly driven nature of these tumors.
Recent studies have suggested that EGFR-mutated NSCLC may exhibit a more robust immune response than previously thought, potentially making them candidates for immunotherapy (6,7). The combination of ICIs with conventional chemotherapy has shown promise in enhancing antitumor activity by not only directly targeting tumor cells but also modulating the tumor immune microenvironment (TIME) to foster an immune-permissive milieu (8-10).
This case report describes a patient with EGFR L858R-mutated advanced NSCLC who experienced disease progression following multiple lines of EGFR-TKI therapy. The patient subsequently received pembrolizumab in combination with chemotherapy, achieving a pathological complete response (pCR) and prolonged survival. The investigation of the tumor microenvironment (TME) revealed a significant infiltration of immune cells and elevated expression of programmed cell death 1 ligand 1 (PD-L1), supporting the rationale for this therapeutic approach. This case underscores the potential of immunochemotherapy (ICT) in EGFR-mutated NSCLC and contributes to the growing body of evidence suggesting that these patients may benefit from immunotherapeutic strategies, particularly when integrated with conventional cytotoxic agents. We present this case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-711/rc).
Case presentation
Patient information
In September 2019, a 74-year-old female patient with no history of smoking presented with symptoms of shortness of breath, cough, and hemoptysis. Bronchoscopic lung biopsy was performed for her and she was diagnosed with stage IVa (cT4N2M1a) lung adenocarcinoma located in the right upper lobe, accompanied by multiple metastases in the ipsilateral lung, as well as involvement of the pleura and mediastinal lymph nodes. Subsequent next-generation sequencing (NGS) identified an EGFR p.L858R mutation in exon 21 and a TP53 p.R248L mutation in exon 7.
Commencing on October 11, 2019, the patient was administered icotinib, a first-generation EGFR-TKI (125 mg orally, three times daily) in conjunction with pemetrexed [500 mg/m2 intravenously (IV), on day 1 of a 21-day cycle] and nedaplatin (80 mg/m2 IV, on day 1 of a 21-day cycle) as first-line therapy. This regimen resulted in a radiologic partial response (PR) after 2 months. Nevertheless, following six cycles of treatment, disease progression was observed. At this juncture, adequate pleural effusion was obtained for repeat NGS, which did not reveal any new genetic mutations. Beginning on April 28, 2020, the patient commenced second-line treatment with amonertinib, third-generation EGFR-TKI (110 mg orally, once daily) in combination with anlotinib, a TKI that can effectively inhibit multiple kinases such as vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR) (12 mg orally, once daily for 2 weeks, followed by a 1-week drug-free period, with each cycle lasting 3 weeks) for a duration of 3 months. Computed tomography (CT) scans indicated stable disease (SD) at the sixth week; however, disease progression was observed after 3 months. Consequently, the patient underwent a comprehensive examination, which included whole-body positron emission tomography (PET)-CT, cranial magnetic resonance imaging (MRI), and tumor re-biopsy. The disease persisted at stage IVa (cT4N2M1a) without evidence of extra-thoracic metastases, and the EGFR T790M mutation was identified via NGS. Consequently, a treatment regimen comprising osimertinib (80 mg orally, once daily) in combination with bevacizumab (350 mg IV, on day 1 of a 21-day cycle) was initiated on August 3, 2020. Regrettably, disease progression was observed 2 months later (Figures 1,2A).

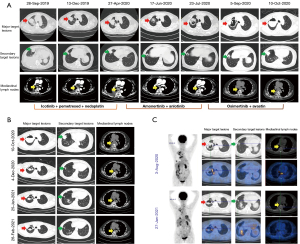
Recent studies have confirmed the efficacy of chemotherapy combined with ICIs with or without anti-VEGF antibodies among those with resistance to EGFR-TKIs (8,9,11). As the CT scans showed a cavitary lesion, additional anti-VEGF antibody would make it prone to pulmonary hemorrhage(12). So, an ICI based ICT was considered for this patient, consisting of pembrolizumab (200 mg IV on day 1 of a 3-week cycle) with pemetrexed (500 mg/m2 IV on day 1 of a 3-week cycle) and carboplatin (dosage calculated as area under the curve (AUC) (5 mg/mL·min) × (creatinine clearance + 25), administered IV on day 1 of a 3-week cycle) since October 10, 2020. Following two cycles of this treatment regimen, a chest CT scan indicated a PR, with a significant reduction in multiple tumor lesions and mediastinal lymph nodes. Upon completion of four cycles of ICT, the patient underwent a PET-CT scan for further evaluation. Compared to the initial assessment conducted in August 2020, there was a significant reduction in multiple tumor lesions, the disappearance of certain cavities, and a marked attenuation in glucose metabolism (Figures 1,2B,2C).
At this juncture, a multidisciplinary consultation was convened for the patient. Following a comprehensive evaluation of the medical team’s recommendations and the patient’s preferences, a “naked eye 3D thoracoscopic right upper lobectomy resection” was performed on March 3, 2021. Histopathological examination of the resected specimen revealed extensive inflammation and fibrosis, comprising 95% of the tissue, and necrosis, accounting for 5%, with no viable cancer cells detected. Furthermore, there was an absence of intravascular tumor emboli, nerve bundle involvement, pleural invasion, and lymph node metastases (0/5). According to the Mandard tumor regression grading system (13), it was classified as grade 1, indicating a pCR. The patient experienced an uneventful recovery and was discharged without any complications related to the surgical procedure. Postoperatively, she underwent regular adjuvant therapy with pembrolizumab and pemetrexed every 3 to 4 weeks. Follow-up chest CT scans were performed every 3 months. The patient has maintained a favorable physical condition, exhibiting progression-free survival (PFS) for over 40 months, with no indications of disease advancement observed (Figure 1).
TME exploration
As it reported, EGFR mutations in NSCLC promote the development of a non-inflammatory TIME, resulting in limited infiltration of immune cells in tumor tissues (14,15). EGFR-TKIs can change the quantity of immune cells, initially increasing the levels of CD8+ T cells and dendritic cells in TME while inhibiting Foxp3 regulatory T cells (Tregs) and M2-like polarization (7,16). To investigate the characteristics of the TIME, we performed multiplex immunofluorescence (mIF) staining using 4',6-diamidino-2-phenylindole (DAPI), pan-cytokeratin (pan-CK), CD45, CD8, CD68, programmed death receptor 1 (PD-1), and PD-L1 on biopsied tumor samples obtained post-progression on targeted therapy, as well as on samples from surgical resections (Figure 3). For the biopsied tumor tissues, five distinct tumor and stromal regions were randomly selected for subsequent statistical analysis. As illustrated in Figures 3A-3C,4A,4B, the tumor regions predominantly consisted of pan-CK+ tumor cells, with minimal infiltration by immune cells. In the stromal regions, CD45+ immune cells constituted 30–40% of all nucleated cells, exhibiting a significantly higher density compared to the tumor regions. The distribution of CD68+ macrophages was approximately similar to that of CD45+ immune cells. However, there was no significant difference in the distribution of CD8+ T cells between the tumor and stromal regions (Figure 4B). Notably, more than half of the tumor cells expressed PD-L1 across all tumor regions (Figure 4C). The proportion of PD-1 positive CD8+ T cells and PD-L1 positive CD68+ macrophages was significantly higher in the tumor area compared to the stromal area (Figure 4D). In the resected sample, there was a substantial infiltration of CD45+ immune cells, CD8+ T lymphocytes, CD68+ macrophages, and immature tertiary lymphoid structures (TLSs), with no viable tumor cells detected (Figure 3D,3E).


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Currently, the use of EGFR-TKI as neoadjuvant therapy has demonstrated limited pathological regression, with the major pathological response (MPR; defined as viable tumor cells constituting less than 10%) ranging from 9% to 22%, and only a few instances of pCR have been observed (10,17-21). The case presented here is a remarkable example of a patient with advanced NSCLC and EGFR L858R mutation achieving a pCR following treatment with pembrolizumab combined with chemotherapy, despite progressive disease under multiple lines of targeted therapy. This case underscores the potential of ICIs in reshaping the treatment paradigm for EGFR-mutated lung cancer, particularly when integrated with conventional chemotherapy.
It was previously believed that NSCLC patients with EGFR sensitive mutations should avoid ICI treatment, and they were typically excluded from large, multicenter, phase III clinical trials involving ICI-based immunotherapy (22). The use of pembrolizumab in EGFR-mutated lung cancer has been a subject of debate due to the predominantly molecularly driven nature of these tumors (23-25). However, recent studies have indicated that ICT could serve as a palliative treatment option for patients experiencing progression on EGFR-TKIs (26). The Impower 150 trial was the first to suggest that patients with EGFR-sensitive NSCLC might benefit from a combination of atezolizumab, bevacizumab, and chemotherapy. A Phase II trial investigating the combination of toripalimab (an anti-PD-1 antibody) with chemotherapy reported a median PFS of 7.0 months and an overall survival of 23.5 months as second-line treatment for EGFR-mutated NSCLC, supporting its application in those population (8). Similarly, the ORIENT-31 study demonstrated that the combination of sintilimab, IBI305, and chemotherapy resulted in a median PFS of 6.9 months with good tolerability (9). In recent KEYNOTE-789 (27) and CheckMate-722 (28) trials, the results shown no superiority of chemotherapy combined with pembrolizumab or nivolumab over chemotherapy alone in EGFR-mutated NSCLC patients who have progressed on EGFR-TKIs. Although the required number of PFS events in CheckMate-722 was not reached, the study found that patients receiving nivolumab with chemotherapy had an improved OS (19.4 vs. 15.9 months) than chemo-alone. Those differences in outcomes may be attributed to the heterogeneity of EGFR-mutated NSCLC, the strategy of EGFR-TKI selection, and the distinct immune microenvironment.
In our case, combining economic considerations and therapeutic efficacy, the patient was administered with icotinib in conjunction with pemetrexed and nedaplatin as the first-line treatment and achieved a PR initially. However, disease progression was observed after 6 months. The third generation EGFR-TKI seems not sensitive for her despite positive T790M mutation. Therefore, a combination of ICI with chemotherapy was recommended. Unexpectedly, the patient responded well. In contrast to prior studies, this female patient underwent surgical intervention after four cycles of ICT, enabling an assessment of pathological tumor regression and the TME. Pathological analysis indicated that the resected tumor bed was predominantly composed of immune cells and fibroblasts, with an absence of viable tumor cells. To explore potential factors associated with the efficacy of immunotherapy, mIF analysis of re-biopsied tumor tissue was performed to investigate the characteristics of TME. The results demonstrated a high PD-L1 expression on tumor cells and abundant immune cell infiltration in stroma region, which may be due to various previous treatments altered tumor heterogeneity, leading to increased immune cell infiltration and PD-L1 expression in TME (29). As previous studies revealed (30), EGFR-TKIs could increase the infiltration of CD8+ T cells and dendritic cells in TIME while inhibiting Foxp3 Tregs and M2-like polarization (7). As for PD-L1 expression, the outcomes after TKI treatment remain controversial (15). EGFR pathway can alter tumor PD-L1 expression levels through various intracellular and extracellular mechanisms. Sensitive EGFR-TKIs treatment could downregulate tumor PD-L1 expression, while the PD-L1 expression level in EGFR-TKI-resistant tumors may increase. Amplification of mesenchymal-epidermal transition (MET), hepatocyte growth factor (HGF), and EGFR T790M could also upregulate tumor PD-L1 expression through signaling pathways such as PI3K/Akt, MAPK, and NF-κB (31).
Notably, there was a higher proportion of PD-1+ CD8+ lymphocytes and PD-L1+ macrophages within the tumor regions compared to the stromal areas. CD8+ lymphocytes can directly kill cells and are generally considered key participants in tumor-specific immune responses. High expression of PD-1 induces apoptosis in CD8+ lymphocytes. An increase in the number of PD-1+ CD8+ lymphocytes often indicates that the immune response within the TME is in a suppressed state, inhibiting the anti-tumor effects of lymphocytes and making it difficult to effectively eliminate tumor cells (15). PD-L1 is an inhibitory molecule expressed on the surface of tumor cells and certain immune cells, which can inhibit cellular activity by binding to the PD-1 receptor. Similarly, the increase in PD-L1+ macrophages also reflect the immune evasion mechanisms within TME (15). These findings suggest a functionally impaired immune infiltration, which may contribute to the ineffectiveness of EGFR-TKI therapy (32). The subsequent application of ICT following EGFR-TKI treatment appeared to successfully restore impaired immune function and enhance immune infiltration, as the CD45+ immune cells, CD8+ T lymphocytes, CD68+ macrophages, and TLSs were substantially infiltrated in the resected samples. These findings support the notion that, despite EGFR mutations, certain lung cancers can respond dramatically to immunotherapy, especially when combined with chemotherapy to enhance the immune response.
The spatial distribution of immune cells is another important indicator. Some studies have shown that the extracellular matrix (ECM) can both promote the migration of immune cells and enhance their tumor-killing effects, as well as inhibit the activation of immune cells, which may indicate the tumor’s impact on and destructive capability against surrounding tissues (33). At the same time, the tumor-to-stroma ratio of immune cells can be used to reflect the status of the TME and assess treatment responses and prognosis. The relationship between immune cells and stroma can reflect the immune status of the TME and serves as an important metric for evaluating tumor immune evasion, immune suppression, and immune activation. During the treatment process, the tumor environment can recruit suppressive immune cells such as Tregs and tumor-associated macrophages (TAMs) through various pathways, leading to the occurrence of resistance events (34). The ratio of immune cells to tumor-stroma not only reveals the biological characteristics of the tumor, but also helps guide clinical decisions and personalized treatment strategies. What unfortunate was that we were unable to obtain tumor samples prior to TKI treatment for TME assessment, and thus unable to conduct dynamic comparative analysis of the TME before and after targeted therapy.
This case provides valuable insights for future treatment strategies in EGFR-mutated lung cancer. The success of pembrolizumab in combination with chemotherapy suggests that this approach should be considered in patients with progressive disease under targeted therapy, particularly those with evidence of an active immune response as indicated by TME analysis. TME has significant clinical implications in guiding anti-tumor therapies, and exploring how EGFR mutations alter TME, as well as the effects of targeted therapies on the immune microenvironment, may help optimize treatment strategies and provide new therapeutic approaches for EGFR-TKI resistance. Additionally, the identification of predictive biomarkers that can stratify patients likely to benefit from this combination is crucial for optimizing treatment outcomes.
While this case is encouraging, it is based on a single patient and should be interpreted with caution. Larger prospective studies are needed to validate these findings and to identify the specific characteristics of patients who are most likely to benefit from this treatment approach. Additionally, further research is required to understand the long-term outcomes, including the durability of response and potential for recurrence, in patients achieving a pCR with this regimen.
Conclusions
In conclusion, this case report highlights the potential of combining pembrolizumab with chemotherapy in achieving a pCR in a patient with EGFR-mutated lung adenocarcinoma who had progressed on multiple lines of targeted therapy. The exploration of the TME further elucidates the complex interplay between the immune system and tumor cells, providing a rationale for the observed response. Future studies should aim to corroborate these findings and refine the selection criteria for patients most likely to benefit from this innovative treatment approach.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-711/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-711/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-711/coif). W.L. serves as an unpaid Associate Editor-in-Chief of Translational Lung Cancer Research from May 2024 to April 2025. All authors report grants from the National Nature Science Foundation of China (No. 82203004) and the China Postdoctoral Science Foundation (No.2022M710896). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Isomoto K, Haratani K, Hayashi H, et al. Impact of EGFR-TKI Treatment on the Tumor Immune Microenvironment in EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res 2020;26:2037-46. [Crossref] [PubMed]
- Jia Y, Li X, Jiang T, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int J Cancer 2019;145:1432-44. [Crossref] [PubMed]
- Jiang T, Wang P, Zhang J, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther 2021;6:355. [Crossref] [PubMed]
- Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:1167-79. [Crossref] [PubMed]
- Bian D, Sun L, Hu J, et al. Neoadjuvant Afatinib for stage III EGFR-mutant non-small cell lung cancer: a phase II study. Nat Commun 2023;14:4655. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Zhao Y, Guo S, Deng J, et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int J Biol Sci 2022;18:3845-58. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 2019;19:495-509. [Crossref] [PubMed]
- Yan D. Hope and Challenges: Immunotherapy in EGFR-Mutant NSCLC Patients. Biomedicines 2023;11:2916. [Crossref] [PubMed]
- Peng S, Wang R, Zhang X, et al. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer 2019;18:165. [Crossref] [PubMed]
- Bao Y, Gu C, Xie H, et al. Comprehensive study of neoadjuvant targeted therapy for resectable non-small cell lung cancer. Ann Transl Med 2021;9:493. [Crossref] [PubMed]
- Blakely CM, Urisman A, Gubens MA, et al. Neoadjuvant Osimertinib for the Treatment of Stage I-IIIA Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer: A Phase II Multicenter Study. J Clin Oncol 2024;42:3105-14. [Crossref] [PubMed]
- Zhang Y, Fu F, Hu H, et al. Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non-small cell lung cancer: A phase II study. J Thorac Cardiovasc Surg 2021;161:434-442.e2. [Crossref] [PubMed]
- Zhong W, Yang X, Yan H, et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol 2015;8:54. [Crossref] [PubMed]
- Zhong WZ, Yan HH, Chen KN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer: final overall survival analysis of the EMERGING-CTONG 1103 randomised phase II trial. Signal Transduct Target Ther 2023;8:76. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Lisberg A, Cummings A, Goldman JW, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol 2018;13:1138-45. [Crossref] [PubMed]
- Zhang C, Sun YX, Yi DC, et al. Neoadjuvant sintilimab plus chemotherapy in EGFR-mutant NSCLC: Phase 2 trial interim results (NEOTIDE/CTONG2104). Cell Rep Med 2024;5:101615. [Crossref] [PubMed]
- Yang JC, Lee DH, Lee JS, et al. Phase III KEYNOTE-789 Study of Pemetrexed and Platinum With or Without Pembrolizumab for Tyrosine Kinase Inhibitor-Resistant, EGFR-Mutant, Metastatic Nonsquamous Non-Small Cell Lung Cancer. J Clin Oncol 2024;42:4029-39. [Crossref] [PubMed]
- Mok T, Nakagawa K, Park K, et al. Nivolumab Plus Chemotherapy in Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small-Cell Lung Cancer After Disease Progression on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Final Results of CheckMate 722. J Clin Oncol 2024;42:1252-64. [Crossref] [PubMed]
- Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol 2016;49:1360-8. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Khalaf K, Hana D, Chou JT, et al. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front Immunol 2021;12:656364. [Crossref] [PubMed]
- Yang CY, Liao WY, Ho CC, et al. Association between programmed death-ligand 1 expression, immune microenvironments, and clinical outcomes in epidermal growth factor receptor mutant lung adenocarcinoma patients treated with tyrosine kinase inhibitors. Eur J Cancer 2020;124:110-22. [Crossref] [PubMed]
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014;15:1243-53. [Crossref] [PubMed]
- Sun L, Xu G, Liao W, et al. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: a meta-analysis. Oncotarget 2017;8:39658-72. [Crossref] [PubMed]


