
Uniportal video-assisted thoracoscopic surgery does not increase recurrence rates following pneumonectomy in non-small cell lung cancer: a retrospective study from the National Cancer Center of China
Highlight box
Key findings
• Patients undergoing uniportal video-assisted thoracoscopic surgery (U-VATS) pneumonectomy for non-small cell lung cancer (NSCLC) demonstrate non-inferior outcomes compared to those undergoing open thoracotomy in terms of postoperative morbidity, survival prognosis, and recurrence patterns.
What is known and what is new?
• U-VATS can be safely performed for pneumonectomy in patients with centrally located NSCLC without adversely affecting prognosis.
• There is limited research on the safety and feasibility of U-VATS in patients undergoing pneumonectomy after neoadjuvant therapy.
What is the implication, and what should change now?
• U-VATS pneumonectomy for NSCLC is both safe and feasible for patients who have received neoadjuvant systemic therapy.
Introduction
Lung cancer remains the leading cause of cancer morbidity and mortality globally (1). In 2020, there were 2.2 million new cases and 1.8 million deaths, with lung cancer accounting for approximately 11.4% of advanced cancer cases and resulting in 18.0% of cancer-related deaths (2). In China, the 5-year survival rate for lung cancer patients is only 28.7% (3). Surgical intervention is the primary treatment for resectable non-small cell lung cancer (NSCLC) (4). While sleeve lobectomy can be considered for selected patients with central lung cancer to reduce respiratory impact without compromising tumor outcomes, pneumonectomy is necessary in cases where central lung cancer invades major vascular structures or airways, and no parenchyma-sparing surgical options can achieve radical resection (5).
With advancements in surgical equipment and technology, uniportal video-assisted thoracoscopic surgery (U-VATS), which utilizes a single incision for all instruments and cameras, has evolved significantly over the past decade (6). U-VATS is a minimally invasive technique for accessing the pleural cavity through a 3–4 cm incision without the need for rib expansion (7). Several studies have previously reported on multi-portal video-assisted thoracoscopic surgery (VATS) pneumonectomies (8,9). Furthermore, previous research indicates that U-VATS can be safely performed for pneumonectomy in patients with centrally located NSCLC without adversely affecting prognosis (10).
With the advancement of comprehensive treatment approaches, locally advanced cancer can now be managed with neoadjuvant therapy (11). However, there is limited research on the safety and feasibility of U-VATS in patients undergoing pneumonectomy after neoadjuvant therapy. Additionally, the recurrence patterns associated with U-VATS in pneumonectomy patients have yet to be explored.
This study aimed to evaluate the prognosis and recurrence patterns of pneumonectomy patients who underwent U-VATS compared to those who had open thoracotomy. Furthermore, we analyzed the feasibility of U-VATS for pneumonectomy in patients receiving neoadjuvant systemic therapy. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-41/rc).
Methods
Study population
This retrospective, observational study was conducted at the National Cancer Center, Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS). This study was approved by the Medical Ethics Committee of CHCAMS (approval No. 2022030911242202). Due to the retrospective nature of the study and the preoperative consent for data usage, individual consent for data was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
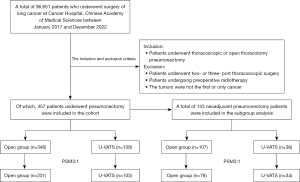
We collected data on 36,651 patients with NSCLC who underwent surgical treatment at the CHCAMS from January 2017 to December 2022. Exclusion criteria, outlined in Figure 1, included patients who underwent two- or three-port VATS, those with tumors that were not the first or only cancer, and patients who received preoperative radiotherapy. All patients underwent preoperative evaluations, which included brain magnetic resonance imaging (MRI), chest computed tomography (CT), and whole-body positron emission tomography-CT (PET-CT). Ultimately, 457 patients who underwent pneumonectomy were included in our study, comprising both open surgery (including those converted from U-VATS to open) and U-VATS groups.
Baseline variables assessed included gender, age, histological subtype, clinical and pathological stage, neoadjuvant systemic therapy, tumor grade, adjuvant therapy, tumor thrombosis, nerve invasion, smoking history, family cancer history, tumor location, tumor diameter, pulmonary function [measured by forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FEV1/FVC)], and Charlson Comorbidity Index (CCI). Postoperative outcomes measured included intensive care unit (ICU) stay, postoperative complications (according to Clavien-Dindo classification), operation time, intraoperative blood loss, blood transfusions, postoperative hospital stay, 30- and 90-day mortality rates, the number of lymph nodes dissected, and dissected lymph node stations. Clinical and pathological staging followed the 8th edition of the lung cancer tumor node metastasis (TNM) staging system.
Follow-up, survival, and recurrence
Recurrence-free survival (RFS) was defined as the duration from the date of surgery to the confirmation of cancer relapse or the last follow-up date. Overall survival (OS) was calculated from the date of surgery to the confirmation of death or the last follow-up date. Loco-regional recurrence (LR) refers to recurrence at the surgical resection margin or ipsilateral mediastinal disease (N2). Distant recurrence (DR) is defined as recurrence occurring in the contralateral lung or extra-thoracic organs such as the brain, liver, bone, or other sites.
Surgical techniques
In U-VATS pneumonectomy, a single 3–5 cm incision was made between the posterior axillary line and midline at the fourth or fifth intercostal space, based on surgeon preference. A wound protector was utilized without rib spreading. A high-definition, 10-mm, 30-degree thoracoscope was introduced through the posterior portion of the incision, while all instruments, including staplers, were operated via the anterior portion of the same incision. An electrocoagulation hook or ultrasonic scalpel and a long-curved suction device were also employed for vascular and bronchial splitting and lymph node clearance.
In open pneumonectomy, a posterolateral incision was made through the fourth or fifth intercostal space to access the thoracic cavity of the patient. A muscle-sparing technique was rigorously applied in all open thoracotomy cases. The mediastinal pleura was incised along the pulmonary hilum and bluntly dissected toward the pulmonary side to expose the hilar vessels. The upper and lower lobes of the lung were retracted posteriorly to visualize the anterior margin of the pulmonary hilum. The veins, arteries, pulmonary ligament, and main bronchus were sequentially ligated and divided. After confirming no air leakage, the thoracic cavity was irrigated. Bronchial stump closure was performed using a stapler technique. A 28-F chest tube was placed posteriorly through the incision.
Statistical analysis and propensity score matching (PSM)
The PSM ratio was set at 3:1, with a caliper value of 0.2. The matched variables included gender, age, histological subtype, clinical stage, pathology stage, neoadjuvant systemic therapy, tumor grade, adjuvant therapy, tumor thrombosis, nerve invasion, smoking history, family cancer history, tumor location, tumor diameter, FEV1, FEV1/FVC, and CCI. Continuous variables were presented as mean [standard deviation (SD)], while categorical variables were expressed as n (percentage). Independent sample t-tests were used to compare parameters, and Mann-Whitney U tests were employed for non-parametric continuous variables. Chi-squared tests were conducted for categorical variable comparisons. Logistic regression analysis was performed to identify independent risk factors for postoperative complications. The Kaplan-Meier curve was utilized to assess survival distribution between the two groups, and a Cox proportional hazards model (using the backward logistic regression method) was applied to compare prognostic risks between the open and U-VATS groups. A P value of less than 0.05 was deemed statistically significant. All analyses were conducted using SPSS software (version 26.0) and R programming software (version 4.0.2).
Results
Patients characteristics
A total of 36,651 patients with NSCLC underwent surgical treatment at the CHCAMS from January 2017 to December 2022. Among these, 457 patients who underwent pneumonectomy fulfilled the inclusion and exclusion criteria for our study (Figure 1). Based on the surgical approach, patients were classified into two groups: the open group (n=348) and the U-VATS group (n=109). After applying a 3:1 PSM, 334 patients were well-matched, comprising 231 patients in the open pneumonectomy group and 103 in the U-VATS group. The baseline characteristics of patients before and after PSM are summarized in Table 1. Following PSM, there were no significant differences in baseline characteristics between the two groups (included gender, age, histological subtype, clinical and pathological stage, neoadjuvant systemic therapy, tumor grade, adjuvant therapy, tumor thrombosis, nerve invasion, smoking history, family cancer history, tumor location, tumor diameter, pulmonary function, and CCI).
Table 1
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| U-VATS (n=109) | Open (n=348) | P value | U-VATS (n=103) | Open (n=231) | P value | ||
| Gender | 0.09 | 0.28 | |||||
| Male | 90 (82.6) | 309 (88.8) | 85 (82.5) | 201 (87.0) | |||
| Female | 19 (17.4) | 39 (11.2) | 18 (17.5) | 30 (13.0) | |||
| Age (years) | 0.40 | 0.99 | |||||
| ≤60 | 62 (56.9) | 182 (52.3) | 57 (55.3) | 128 (55.4) | |||
| >60 | 47 (43.1) | 166 (47.7) | 46 (44.7) | 103 (44.6) | |||
| Neoadjuvant systemic therapy | 0.65 | 0.89 | |||||
| Negative | 73 (67.0) | 241 (69.3) | 69 (67.0) | 153 (66.2) | |||
| Positive | 36 (33.0) | 107 (30.7) | 34 (33.0) | 78 (33.8) | |||
| Histological subtype | 0.005 | 0.57 | |||||
| Adenocarcinoma | 32 (29.4) | 54 (15.5) | 27 (26.2) | 49 (21.2) | |||
| Squamous cell carcinoma | 72 (66.1) | 271 (77.9) | 71 (68.9) | 168 (72.7) | |||
| Other | 5 (4.6) | 23 (6.6) | 5 (4.9) | 14 (6.1) | |||
| Tumor grade | 0.80 | 0.93 | |||||
| G1 | 4 (3.7) | 10 (2.9) | 3 (2.9) | 7 (3.0) | |||
| G2 | 44 (40.4) | 132 (37.9) | 42 (40.8) | 89 (38.5) | |||
| G3 | 61 (56.0) | 206 (59.2) | 58 (56.3) | 135 (58.4) | |||
| Tumor thrombosis | 0.10 | 0.19 | |||||
| Negative | 75 (68.8) | 209 (60.1) | 71 (68.9) | 142 (61.5) | |||
| Positive | 34 (31.2) | 139 (39.9) | 32 (31.1) | 89 (38.5) | |||
| Nerve invasion | 0.54 | 0.70 | |||||
| Negative | 74 (67.9) | 225 (64.7) | 70 (68.0) | 152 (65.8) | |||
| Positive | 35 (32.1) | 123 (35.3) | 33 (32.0) | 79 (34.2) | |||
| Smoking history | <0.001 | 0.11 | |||||
| No | 40 (36.7) | 68 (19.5) | 35 (34.0) | 59 (25.5) | |||
| Yes | 69 (63.3) | 280 (80.5) | 68 (66.0) | 172 (74.5) | |||
| Family cancer history | 0.44 | 0.53 | |||||
| No | 82 (75.2) | 274 (78.7) | 77 (74.8) | 180 (77.9) | |||
| Yes | 27 (24.8) | 74 (21.3) | 26 (25.2) | 51 (22.1) | |||
| Tumor location | 0.59 | 0.41 | |||||
| LUL | 59 (54.1) | 180 (51.7) | 55 (53.4) | 124 (53.7) | |||
| LLL | 41 (37.6) | 115 (33.0) | 39 (37.9) | 71 (30.7) | |||
| LMB | 5 (4.6) | 23 (6.6) | 5 (4.9) | 13 (5.6) | |||
| RUL | 1 (0.9) | 12 (3.4) | 1 (1.0) | 10 (4.3) | |||
| RML | 0 (0) | 4 (1.1) | 0 (0) | 3 (1.3) | |||
| RLL | 2 (1.8) | 10 (2.9) | 2 (1.9) | 9 (3.9) | |||
| RMB | 1 (0.9) | 4 (1.1) | 1 (1.0) | 1 (0.4) | |||
| Tumor diameter (cm) | 3.6 (2.7, 5.1) | 4.3 (3.4, 5.6) | <0.001 | 3.8 (2.75, 5.1) | 4.1 (3.3, 5.1) | 0.07 | |
| Clinical stage | 0.07 | 0.41 | |||||
| I | 33 (30.3) | 69 (19.8) | 29 (28.2) | 50 (21.6) | |||
| II | 40 (36.7) | 141 (40.5) | 34 (33) | 87 (37.7) | |||
| III | 36 (33.0) | 138 (39.7) | 40 (38.8) | 94 (40.7) | |||
| Pathology stage | <0.001 | 0.15 | |||||
| I | 35 (32.1) | 47 (13.5) | 30 (29.1) | 45 (19.5) | |||
| II | 36 (33.0) | 125 (35.9) | 35 (34.0) | 92 (39.8) | |||
| III | 38 (34.9) | 176 (50.6) | 38 (36.9) | 94 (40.7) | |||
| Adjuvant therapy | 0.38 | 0.90 | |||||
| No | 67 (61.5) | 230 (66.1) | 63 (61.2) | 143 (61.9) | |||
| Yes | 42 (38.5) | 118 (33.9) | 40 (38.8) | 88 (38.1) | |||
| CCI score | 0.69 | 0.95 | |||||
| 0 | 5 (4.6) | 10 (2.9) | 5 (4.9) | 7 (3.0) | |||
| 1 | 11 (10.1) | 29 (8.3) | 9 (8.7) | 21 (9.1) | |||
| 2 | 42 (38.5) | 131 (37.6) | 39 (37.9) | 89 (38.5) | |||
| 3 | 36 (33.0) | 141 (40.5) | 36 (35.0) | 88 (38.1) | |||
| 4 | 13 (11.9) | 31 (8.9) | 12 (11.7) | 22 (9.5) | |||
| 5 | 2 (1.8) | 6 (1.7) | 2 (1.9) | 4 (1.7) | |||
| FEV1 (L) | 2.47±0.61 | 2.25±0.59 | 0.001 | 2.43±0.57 | 2.39±0.57 | 0.57 | |
| FEV1/FVC | 0.81 (0.73, 0.87) | 0.81 (0.73, 0.90) | 0.56 | 0.81 (0.73, 0.87) | 0.81 (0.73, 0.87) | 0.73 | |
Data are presented as n (%) or median (interquartile range) or mean ± standard deviation. CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LLL, left lower lobe; LMB, left main bronchus; LUL, left upper lobe; PSM, propensity score matching; RLL, right lower lobe; RMB, right main bronchus; RML, right middle lobe; RUL, right upper lobe; U-VATS, uniportal video-assisted thoracoscopic surgery.
Perioperative outcomes and postoperative complications
After PSM, patients who underwent U-VATS pneumonectomy had significantly shorter postoperative hospital stays compared to the open group (P<0.001). However, there were no significant differences between the two groups regarding ICU stay after surgery (P=0.056), operation time (P=0.07), intraoperative blood loss (P=0.87), blood transfusion status (P=0.34), number of lymph nodes dissected (P=0.37), and lymph node stations dissected (P=0.99) (Table 2). Additionally, postoperative 30-day mortality (P>0.99) and 90-day mortality (P=0.99) showed no significant differences between the open and U-VATS groups, regardless of PSM.
Table 2
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| U-VATS (n=109) | Open (n=348) | P value | U-VATS (n=103) | Open (n=231) | P value | ||
| ICU stay after surgery | 0.03 | 0.056 | |||||
| No | 95 (87.2) | 326 (93.7) | 91 (88.3) | 218 (94.4) | |||
| Yes | 14 (12.8) | 22 (6.3) | 12 (11.7) | 13 (5.6) | |||
| Clavien-Dindo classification | 0.02 | 0.11 | |||||
| 0 | 98 (89.9) | 281 (80.7) | 93 (90.3) | 194 (84.0) | |||
| 1 | 9 (8.3) | 30 (8.6) | 8 (7.8) | 19 (8.2) | |||
| ≥2 | 2 (1.8) | 37 (10.6) | 2 (1.9) | 18 (7.8) | |||
| Complications | |||||||
| Clavien-Dindo I | |||||||
| Chest tube duration (>7 d) | 9 (8.3) | 26 (7.5) | 0.79 | 8 (7.8) | 17 (7.4) | 0.90 | |
| Drainage of infected wounds | 0 (0) | 4 (1.1) | 0.59 | 0 (0) | 2 (0.9) | 0.86 | |
| Clavien-Dindo II | |||||||
| Pulmonary infection | 0 (0) | 6 (1.7) | 0.37 | 0 (0) | 2 (0.9) | 0.86 | |
| Atrial fibrillation | 1 (0.9) | 9 (2.6) | 0.51 | 1 (1.0) | 5 (2.2) | 0.76 | |
| Clavien-Dindo III | |||||||
| Pleural effusion needing intervention | 0 (0) | 4 (1.1) | 0.59 | 0 (0) | 2 (0.9) | 0.86 | |
| Chylothorax | 0 (0) | 2 (0.6) | >0.99 | 0 (0) | 1 (0.4) | >0.99 | |
| Clavien-Dindo IV | |||||||
| Respiratory failure | 1 (0.9) | 2 (0.6) | >0.99 | 1 (1.0) | 1 (0.4) | >0.99 | |
| Heart failure | 0 (0) | 3 (0.9) | 0.77 | 0 (0) | 3 (1.3) | 0.59 | |
| Cardiogenic shock | 0 (0) | 4 (1.1) | 0.59 | 0 (0) | 2 (0.9) | 0.86 | |
| MODS | 0 (0) | 3 (0.9) | 0.77 | 0 (0) | 2 (0.9) | 0.86 | |
| Clavien-Dindo V | |||||||
| Death | 0 (0) | 4 (1.1) | 0.59 | 0 (0) | 0 (0) | – | |
| Operation time (min) | 135 (102, 170) | 120 (100, 155) | 0.12 | 135 (103, 171) | 120 (99, 155) | 0.07 | |
| Intraoperative blood loss (mL) | 10 (10, 20) | 10 (10, 20) | 0.71 | 10 (10, 15) | 10 (10, 10) | 0.87 | |
| Blood transfusion | 0.19 | 0.34 | |||||
| No | 104 (95.4) | 319 (91.7) | 99 (96.1) | 216 (93.5) | |||
| Yes | 5 (4.6) | 29 (8.3) | 4 (3.9) | 15 (6.5) | |||
| Postoperative hospital stay (day) | 6 (4, 7) | 7 (6, 8) | <0.001 | 6 (4, 7) | 7 (6, 8) | <0.001 | |
| Recurrence | |||||||
| LR | 2 (1.8) | 12 (3.4) | 0.59 | 2 (1.9) | 4 (1.7) | 0.99 | |
| DR | 11 (10.1) | 20 (5.7) | 0.12 | 10 (9.7) | 13 (5.6) | 0.17 | |
| 30-day mortality | 0 (0) | 7 (2.0) | 0.39 | 0 (0) | 1 (0.4) | >0.99 | |
| 90-day mortality | 1 (0.9) | 10 (2.9) | 0.42 | 1 (1.0) | 3 (1.3) | 0.99 | |
| Lymph nodes dissected | 25 (19, 32) | 27 (21, 34) | 0.048 | 25 (19, 32) | 26 (21, 33) | 0.37 | |
| Lymph node stations dissected | 9 (8, 11) | 10 (8, 11) | 0.98 | 9 (8, 11) | 10 (8, 11) | 0.99 | |
Data are presented as n (%) or median (interquartile range). DR, distant recurrence; ICU, intensive care unit; LR, loco-regional recurrence; MODS, multiple organ dysfunction syndrome; PSM, propensity score matching; U-VATS, uniportal video-assisted thoracoscopic surgery.
Postoperative complications were classified according to the Clavien-Dindo classification. In the matched cohort, both groups displayed the highest rate of grade 1 complications (8.2% in the open group and 7.8% in the U-VATS group), with similar complication rates observed between the two groups (Table 2).
Risk factors for postoperative complications
In the PSM cohort, 334 patients were classified into two groups, 287 patients without postoperative complications and 47 patients with complications. Logistic regression analysis was performed to identify potential risk factors for postoperative complications. The results of the univariable logistic regression analysis indicated that ICU status and blood transfusion were associated with an increased risk of postoperative complications (Table 3).
Table 3
| Characteristics | Total (N=334) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Group | ||||||
| Open | 231 | Reference | – | – | ||
| U-VATS | 103 | 0.54 (0.26–1.14) | 0.11 | – | – | |
| ICU status | ||||||
| No | 309 | Reference | Reference | |||
| Yes | 25 | 3.16 (1.28–7.81) | 0.01 | 2.03 (0.56–7.35) | 0.28 | |
| Operation time | 334 | 1.00 (0.998–1.01) | 0.16 | – | – | |
| Intraoperative blood loss | 334 | 1.00 (1.00–1.01) | 0.17 | – | – | |
| Blood transfusion | ||||||
| No | 315 | Reference | Reference | |||
| Yes | 19 | 3.00 (1.08–8.32) | 0.04 | 1.69 (0.38–7.59) | 0.49 | |
| Lymph nodes dissected | 334 | 1.01 (0.98–1.04) | 0.44 | – | – | |
| Lymph node stations dissected | 334 | 1.09 (0.92–1.30) | 0.30 | – | – | |
| Gender | ||||||
| Male | 286 | Reference | – | – | ||
| Female | 48 | 0.83 (0.33–2.07) | 0.69 | – | – | |
| Age (years) | ||||||
| ≤60 | 185 | Reference | – | – | ||
| >60 | 149 | 1.28 (0.69–2.37) | 0.42 | – | – | |
| Neoadjuvant systemic therapy | ||||||
| No | 222 | Reference | – | – | ||
| Yes | 112 | 1.10 (0.58–2.09) | 0.77 | – | – | |
| Histological subtype | ||||||
| LUAD | 76 | Reference | Reference | |||
| LSCC | 239 | 0.49 (0.25–0.98) | 0.044 | 0.566 (0.225–1.428) | 0.23 | |
| Other | 19 | 1.00 (0.29–3.43) | >0.99 | 0.525 (0.065–4.272) | 0.55 | |
| Tumor grade | ||||||
| G1 | 10 | Reference | – | – | ||
| G2 | 131 | 1.71 (0.20–14.28) | 0.62 | – | – | |
| G3 | 193 | 1.40 (0.17–11.52) | 0.75 | – | – | |
| Tumor thrombosis | ||||||
| Negative | 213 | Reference | – | – | ||
| Positive | 121 | 0.61 (0.31–1.20) | 0.16 | – | – | |
| Nerve invasion | ||||||
| Negative | 222 | Reference | – | – | ||
| Positive | 112 | 0.62 (0.30–1.24) | 0.18 | – | – | |
| Smoking history | ||||||
| No | 94 | Reference | – | – | ||
| Yes | 240 | 1.06 (0.53–2.11) | 0.86 | – | – | |
| Family cancer history | ||||||
| No | 257 | Reference | – | – | ||
| Yes | 77 | 1.84 (0.95–3.58) | 0.07 | – | – | |
| Tumor location | ||||||
| LUL | 179 | Reference | – | – | ||
| LLL | 110 | 0.85 (0.43–1.67) | 0.64 | – | – | |
| LMB | 18 | 0.67 (0.14–3.09) | 0.61 | – | – | |
| RLL | 11 | 1.19 (0.24–5.84) | 0.82 | – | – | |
| RMB | 2 | – | - | – | – | |
| RUL | 11 | 0.53 (0.06–4.38) | 0.56 | – | – | |
| RML | 3 | – | - | – | – | |
| Tumor diameter (cm) | 334 | 1.05 (0.88–1.26) | 0.53 | – | – | |
| Clinical stage | ||||||
| I | 79 | Reference | – | – | ||
| II | 121 | 0.976 (0.442–2.155) | 0.95 | – | – | |
| III | 134 | 0.866 (0.393–1.909) | 0.72 | – | – | |
| Pathology stage | ||||||
| I | 75 | Reference | – | – | ||
| II | 127 | 0.42 (0.19–1.13) | 0.63 | – | – | |
| III | 132 | 0.62 (0.29–1.29) | 0.20 | – | – | |
| Adjuvant therapy | ||||||
| No | 206 | Reference | – | – | ||
| Yes | 128 | 1.43 (0.77–2.66) | 0.25 | – | – | |
| FEV1 | 334 | 1.11 (0.65–1.89) | 0.70 | – | – | |
| FEV1/FVC | 334 | 1.01 (0.08–12.70) | 0.98 | – | – | |
CI, confidence interval; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; ICU, intensive care unit; LLL, left lower lobe; LMB, left main bronchus; LSCC, lung squamous cell carcinoma; LUAD, lung adenocarcinoma; LUL, left upper lobe; OR, odds ratio; PSM, propensity score matching; RLL, right lower lobe; RMB, right main bronchus; RML, right middle lobe; RUL, right upper lobe; U-VATS, uniportal video-assisted thoracoscopic surgery.
Survival analyses in the matched cohort
The median follow-up for the U-VATS and open groups was 47.2 and 54.3 months, respectively. In the PSM cohort, the 5-year OS rate for the U-VATS group was 69.6%, while the 5-year RFS rate was 57.9%. In contrast, the open group had a 5-year OS rate of 62.7% and a 5-year RFS rate of 49.2%. There were no significant differences in 5-year OS (P=0.19) or 5-year RFS (P=0.37) between the U-VATS and open groups (Figure 2).
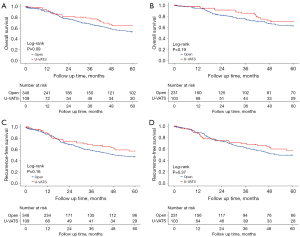
Univariate analysis identified several risk factors for OS, including nerve invasion (P=0.046), tumor diameter (P=0.02), pathology stage (P=0.007), histological subtype (P=0.008), number of lymph node stations dissected (P=0.03), and CCI score 5 (P=0.04). For RFS, tumor diameter (P=0.003) and pathology stage (P=0.01) were also identified as risk factors. In multivariate analysis, pathology stage (P=0.047), histological subtype (P=0.001), and the number of lymph node stations dissected (P=0.007) were identified as risk factors for OS. A significant inverse correlation was observed between tumor diameter and RFS, with larger tumor size associated with a higher risk of disease recurrence [hazard ratio (HR) =1.14, 95% confidence interval (CI): 1.01–1.29, P=0.03] (Table 4).
Table 4
| Characteristics | Total (N=334) | OS | RFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Operation approach | ||||||||||||
| Open | 231 | Reference | – | – | Reference | – | – | |||||
| U-VATS | 103 | 0.68 (0.39–1.20) | 0.19 | – | – | 0.82 (0.53–1.26) | 0.37 | – | – | |||
| Gender | ||||||||||||
| Male | 286 | Reference | – | – | Reference | – | – | |||||
| Female | 48 | 0.71 (0.35–1.44) | 0.35 | – | – | 0.92 (0.55–1.55) | 0.77 | – | – | |||
| Age (years) | ||||||||||||
| ≤60 | 185 | Reference | – | – | Reference | – | – | |||||
| >60 | 149 | 1.23 (0.77–1.96) | 0.37 | – | – | 1.17 (0.80–1.70) | 0.41 | – | – | |||
| Neoadjuvant systemic therapy | ||||||||||||
| No | 222 | Reference | – | – | Reference | – | – | |||||
| Yes | 112 | 1.05 (0.62–1.77) | 0.85 | – | – | 1.41 (0.95–2.09) | 0.09 | – | – | |||
| Tumor thrombosis | ||||||||||||
| Negative | 213 | Reference | – | – | Reference | – | – | |||||
| Positive | 121 | 1.38 (0.87–2.21) | 0.17 | – | – | 1.17 (0.79–1.71) | 0.42 | – | – | |||
| Nerve invasion | ||||||||||||
| Negative | 222 | Reference | Reference | Reference | – | – | ||||||
| Positive | 112 | 1.60 (1.00–2.56) | 0.046 | 1.50 (0.92–2.43) | 0.09 | 1.20 (0.81–1.76) | 0.35 | – | – | |||
| Smoking history | ||||||||||||
| No | 94 | Reference | – | – | Reference | – | – | |||||
| Yes | 240 | 1.06 (0.63–1.78) | 0.81 | – | – | 1.03 (0.68–1.56) | 0.87 | – | – | |||
| Family cancer history | ||||||||||||
| No | 257 | Reference | – | – | Reference | |||||||
| Yes | 77 | 0.83 (0.47–1.45) | 0.52 | – | – | 1.09 (0.72–1.66) | 0.67 | |||||
| Tumor diameter (cm) | 334 | 1.17 (1.02–1.34) | 0.02 | 1.12 (0.96–1.31) | 0.13 | 1.18 (1.05–1.32) | 0.003 | 1.14 (1.01–1.29) | 0.03 | |||
| Clinical stage | ||||||||||||
| I | 79 | Reference | – | – | Reference | – | – | |||||
| II | 134 | 1.90 (0.94–3.84) | 0.07 | – | – | 1.41 (0.85–2.34) | 0.18 | – | – | |||
| III | 121 | 1.85 (0.89–3.82) | 0.09 | – | – | 1.18 (0.69–2.03) | 0.53 | – | – | |||
| Pathology stage | ||||||||||||
| I | 75 | Reference | Reference | Reference | Reference | |||||||
| II | 127 | 2.15 (0.89–5.23) | 0.09 | 1.74 (0.69–4.35) | 0.23 | 1.44 (0.78–2.64) | 0.23 | 1.27 (0.68–2.35) | 0.44 | |||
| III | 132 | 3.27 (1.38–7.72) | 0.007 | 2.55 (1.01–6.46) | 0.047 | 2.09 (1.16–3.76) | 0.01 | 1.68 (0.89–3.17) | 0.10 | |||
| Tumor grade | ||||||||||||
| G1 | 10 | Reference | – | – | Reference | – | – | |||||
| G2 | 131 | 1.64 (0.22–12.13) | 0.63 | – | – | 1.37 (0.33–5.67) | 0.66 | – | – | |||
| G3 | 193 | 2.32 (0.32–16.83) | 0.40 | – | – | 1.79 (0.44–7.32) | 0.41 | – | – | |||
| Histological subtype | ||||||||||||
| LUAD | 76 | Reference | Reference | Reference | – | – | ||||||
| LSCC | 239 | 0.96 (0.54–1.66) | 0.88 | 1.26 (0.70–2.26) | 0.44 | 0.77 (0.50–1.19) | 0.25 | – | – | |||
| Other | 19 | 2.98 (1.32–6.71) | 0.008 | 3.93 (1.69–9.10) | 0.001 | 1.95 (0.97–3.92) | 0.059 | – | – | |||
| Adjuvant therapy | ||||||||||||
| No | 206 | Reference | – | – | Reference | – | – | |||||
| Yes | 128 | 0.64 (0.40–1.04) | 0.08 | – | – | 1.05 (0.72–1.53) | 0.77 | – | – | |||
| CCI score | ||||||||||||
| 0 | 12 | Reference | – | – | Reference | – | – | |||||
| 1 | 30 | 0.84 (0.21–3.36) | 0.81 | – | – | 0.49 (0.17–1.41) | 0.19 | – | – | |||
| 2 | 128 | 0.92 (0.27–3.05) | 0.89 | – | – | 0.65 (0.28–1.54) | 0.33 | – | – | |||
| 3 | 124 | 1.22 (0.37–3.99) | 0.74 | – | – | 0.71 (0.30–1.68) | 0.45 | – | – | |||
| 4 | 34 | 0.67 (0.15–3.02) | 0.61 | – | – | 0.73 (0.26–2.03) | 0.56 | – | – | |||
| 5 | 6 | 6.56 (1.08–39.74) | 0.04 | – | – | 1.12 (0.13–9.36) | 0.92 | – | – | |||
| FEV1 | 334 | 0.91 (0.61–1.35) | 0.65 | – | – | 0.96 (0.70–1.33) | 0.85 | – | – | |||
| FEV1/FVC | 334 | 1.25 (0.21–7.41) | 0.80 | – | – | 0.98 (0.23–4.18) | 0.98 | – | – | |||
| Lymph nodes dissected | 334 | 1.01 (0.98–1.02) | 0.64 | – | – | 0.99 (0.97–1.01) | 0.77 | – | – | |||
| Lymph node stations dissected | 334 | 0.85 (0.75–0.97) | 0.03 | 0.82 (0.71–0.94) | 0.007 | 0.96 (0.86–1.06) | 0.46 | – | – | |||
CCI, Charlson Comorbidity Index; CI, confidence interval; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HR, hazard ratio; LSCC, lung squamous cell carcinoma; LUAD, lung adenocarcinoma; OS, overall survival; RFS, recurrence-free survival; U-VATS, uniportal video-assisted thoracoscopic surgery.
Subgroup analysis for the neoadjuvant systemic therapy patients
We conducted a subgroup analysis for patients who received neoadjuvant systemic therapy to compare perioperative outcomes between the two groups. The baseline characteristics of patients in the neoadjuvant subgroup were presented in Table S1.
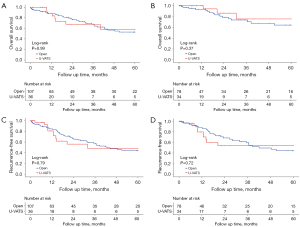
The results of the subgroup analysis indicated that postoperative outcomes did not significantly differ between the two groups (Table S2). Univariate analysis revealed no significant differences in OS (P=0.37) and RFS (P=0.72) between the U-VATS and open groups for patients who received neoadjuvant systemic therapy (Table S3). The 5-year OS and 5-year RFS survival outcomes were displayed in Figure 3.
Recurrence pattern
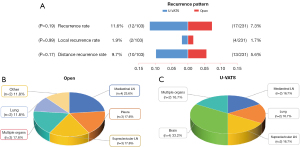
In our study, the rates of LR for the U-VATS and open groups were 1.9% and 1.7%, respectively, demonstrating no significant difference between the groups (P=0.99) (Figure 4). Additionally, the proportion of DR was similar between the U-VATS and open groups (9.7% vs. 5.6%; P=0.17). Among patients in the U-VATS group with LR, ipsilateral mediastinal disease (N2) involvement was the most prevalent, whereas brain metastasis was the most common site for those with DR (Table S4).
Characteristics of patients from U-VATS converted to open approach
A total of 18 patients who were initially scheduled for U-VATS required conversion to the open approach during the procedure. The most common reason for conversion was dense adhesion of surrounding tissues, affecting 66.7% (12/18) of patients. Other reasons included vascular injury (16.7%, 3/18 patients) and a large tumor limiting exposure in U-VATS (16.7%, 3/18 patients). The baseline characteristics of patients undergoing thoracotomy were presented in Table S5.
Univariable logistic regression analysis indicated that neoadjuvant systemic therapy, lung squamous cell carcinoma (LSCC), and smoking history were risk factors associated with conversion from U-VATS to open thoracotomy. Furthermore, multivariate logistic regression analysis revealed that both non-neoadjuvant systemic therapy and smoking history were significant risk factors for this conversion (Table S6).
Furthermore, the thoracotomy conversion rate and reason for the neoadjuvant systemic therapy group and the non-neoadjuvant systemic therapy group were shown in Table S7.
Discussion
Pneumonectomy is a challenging procedure, even with open thoracotomy techniques. While pneumonectomy may be simpler than parenchyma-preserving procedures, it should be avoided unless radical resection cannot be achieved by other means, regardless of the surgical approach. To date, there have been few studies examining pneumonectomy via U-VATS (12,13). Central primary lung cancer is particularly difficult to manage, and pneumonectomy is often considered the treatment of choice, despite ongoing controversies regarding its effectiveness (14). U-VATS is increasingly used in lung cancer surgery and is gaining traction among experienced thoracic surgeons for advanced, complex cases (6). Studies indicate that U-VATS may offer advantages over open thoracotomy and multi-portal VATS, particularly in terms of postoperative recovery (15-17).
Our study found that U-VATS pneumonectomy is safe compared to the open approach, with short-term results indicating that the U-VATS method contributes to shorter postoperative hospital stays. Additionally, the 30- and 90-day mortality rates were similar between the two groups, and propensity score-matched analyses showed no significant differences in OS or RFS. Regarding recurrence patterns, our study demonstrated no significant differences in LR or DR between the two groups. Notably, the U-VATS group exhibited the highest proportion of brain metastases among DRs. Postoperative surveillance was primarily guided by pathological staging and additional risk factors such as minimal residual disease (MRD). Our study further delineated recurrence patterns in pneumonectomy patients, revealing a higher proportion of recurrence in the U-VATS group. These findings provide critical supplemental insights to refine postoperative monitoring strategies in this population.
Previous studies have demonstrated that surgery following induction therapy is both safe and effective (18,19). While neoadjuvant systemic therapy can introduce additional challenges and risks, including increasing the likelihood of conversion to thoracotomy. Our findings demonstrated a higher conversion rate to thoracotomy in non-neoadjuvant patients. However, in the subgroup analysis of 143 pneumonectomies performed after neoadjuvant systemic therapy, only 36 cases (34 after PSM) were completed via U-VATS, indicating a limited sample size. The original cohort of 457 patients suggested that U-VATS is safe and feasible following induction therapy. We found that the perioperative complication rates are comparable to those observed in open thoracotomy patients, indicating that U-VATS pneumonectomy is a reasonable option for patients undergoing neoadjuvant systemic therapy. Neoadjuvant systemic therapy may indirectly influence surgical decision-making (e.g., intraoperative mid-opening) by increasing hilar scar formation. However, U-VATS can still be safely performed in elective patients through rigorous preoperative evaluation and intraoperative operation optimization (e.g., blunt separation of adhesions, and use of reinforcing suture techniques). While previous studies have indicated that thoracoscopic lobectomy is associated with pain relief and reduced length of hospitalization (8,20), our study confirmed the reduced postoperative hospital stay in the U-VATS cohort aligns with the established benefits of minimally invasive techniques in enhancing postoperative recovery. However, no significant differences were observed in oncological outcomes [OS, disease-free survival (DFS)] or postoperative complications, suggesting that U-VATS achieves comparable radicality to open thoracotomy while expediting recovery.
There are several limitations to the present study. It was a retrospective analysis conducted at a single institution, and despite employing multivariate adjustments and PSM to mitigate potential selection bias, unobserved confounding factors may still exist. Although efforts were made to minimize subjectivity in the selection of surgical approaches, some decision-making bias remains, the subjective preferences of the operating surgeon regarding patient selection may also introduce bias. Additionally, further large-scale prospective clinical trials are needed to validate the impact of specific neoadjuvant therapy regimens and treatment cycles on the outcomes of different surgical modalities.
Conclusions
In conclusion, patients undergoing U-VATS pneumonectomy for NSCLC demonstrate non-inferior outcomes compared to those undergoing open thoracotomy in terms of postoperative morbidity, survival prognosis, and recurrence patterns. Furthermore, U-VATS pneumonectomy for NSCLC is both safe and feasible for patients who have received neoadjuvant systemic therapy.
Acknowledgments
The authors thank the National Cancer Center, Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS) for providing the research environment.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-41/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-41/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-41/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of CHCAMS (approval No. 2022030911242202). Due to the retrospective nature of the study and the preoperative consent for data usage, individual consent for data was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zeng H, Zheng R, Sun K, et al. Cancer survival statistics in China 2019-2021: a multicenter, population-based study. J Natl Cancer Cent 2024;4:203-13. [Crossref] [PubMed]
- Li T, Zhang Y, Fu F, et al. The evolution of the treatment of non-small cell lung cancer: A shift in surgical paradigm to a more individualized approach. J Thorac Cardiovasc Surg 2025;169:737-744.e2. [Crossref] [PubMed]
- Luo J, Ji C, Campisi A, et al. Surgical Outcomes of Video-Assisted versus Open Pneumonectomy for Lung Cancer: A Real-World Study. Cancers (Basel) 2022;14:5683. [Crossref] [PubMed]
- Reinersman JM, Passera E, Rocco G. Overview of uniportal video-assisted thoracic surgery (VATS): past and present. Ann Cardiothorac Surg 2016;5:112-7. [Crossref] [PubMed]
- Sachs E, Jackson V, Al-Ameri M, et al. Uniportal video-assisted thoracic surgery: segmentectomy versus lobectomy-early outcomes. Eur J Cardiothorac Surg 2024;65:ezae127. [Crossref] [PubMed]
- Yang CJ, Yendamuri S, Mayne NR, et al. The role of thoracoscopic pneumonectomy in the management of non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg 2019;158:252-264.e2. Erratum in: J Thorac Cardiovasc Surg 2020;159:749. [Crossref] [PubMed]
- Nitsche LJ, Jordan S, Demmy T, et al. Analyzing the impact of minimally invasive surgical approaches on post-operative outcomes of pneumonectomy and sleeve lobectomy patients. J Thorac Dis 2023;15:2497-504. [Crossref] [PubMed]
- Gao J, Zhang L, Li Z, et al. UniPortal thoracoscopic pneumonectomy does not compromise perioperative and long-term survival in patients with NSCLC: A retrospective, multicenter, and propensity score matching study. Lung Cancer 2021;159:135-44. [Crossref] [PubMed]
- Yang X, Yin R, Xu L. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;379:e14. [PubMed]
- Li J, Xue Q, Gao Y, et al. Uniportal video-assisted thoracoscopic left pneumonectomy: Retrospective analysis of eighteen consecutive patients from a single center. Thorac Cancer 2021;12:324-8. [Crossref] [PubMed]
- Halezeroğlu S. Single incision video-assisted thoracic surgery pneumonectomy for centrally located lung cancer. Future Oncol 2018;14:41-5. [Crossref] [PubMed]
- Blanc K, Zaimi R, Dechartres A, et al. Early acute respiratory distress syndrome after pneumonectomy: Presentation, management, and short- and long-term outcomes. J Thorac Cardiovasc Surg 2018;156:1706-1714.e5. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Li J, Qiu B, Scarci M, et al. Uniportal video-assisted thoracic surgery could reduce postoperative thorax drainage for lung cancer patients. Thorac Cancer 2019;10:1334-9. [Crossref] [PubMed]
- Halezeroğlu S. Advantages and disadvantages of single incision VATS in major anatomical resection for lung cancer. J Vis Surg 2017;3:115. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- Ismail M, Nachira D, Swierzy M, et al. Uniportal video-assisted thoracoscopy major lung resections after neoadjuvant chemotherapy. J Thorac Dis 2018;10:S3655-61. [Crossref] [PubMed]
- Liu Y, Gao Y, Zhang H, et al. Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence. J Thorac Dis 2016;8:3537-42. [Crossref] [PubMed]


